Abstract
Lung cancer is one of the most common cancers worldwide and is the leading cause of cancer-induced death in the USA. Although much attention has been focused on the anti-carcinogenic effect of consuming carotenoid-containing food or supplements, the results have been inconsistent. We investigated whether serum carotenoid levels were associated with the mortality risk of lung cancer in US adults using data from a nationally representative sample. The data were obtained from the Third Nutrition and Health Examination Survey (NHANES III) database and the NHANES III Linked Mortality File. A total of 10 382 participants aged over 20 years with available serum carotenoid levels and no other missing information on questionnaires and biomarkers at baseline (NHANES III) were included in the present study. Of the 10 382 participants, 161 subjects died due to lung cancer. We found that high serum levels of alpha-carotene and beta-cryptoxanthin at baseline were significantly associated with a lower risk of lung cancer death. When we stratified the risk by current smoking status, the risk of death of current smokers was significantly decreased to 46% (95% confidence interval, 31–94%) for alpha-carotene and 61% (95% confidence interval, 19–80%) for beta-cryptoxanthin. By contrast, no association was observed among never/former smokers at baseline. High serum levels of alpha-carotene and beta-cryptoxanthin are associated with a lower risk of lung cancer death in US adults.
Keywords: Alpha-carotene, beta-cryptoxanthin, lung cancer, oxidative stress, smoking
Lung cancer is one of the most common cancers worldwide, with 1.35 million new cases diagnosed each year.1 In the USA, lung cancer incidence and death rates have been steadily declining; however, it is still a common, lethal disease, accounting for approximately 27% of all cancer deaths.2 Although smoking is the primary cause of lung cancer, a combination of many causes (e.g. radon, asbestos and environmental tobacco smoke), rather than a single cause, has been implicated in the etiology of lung cancer.
Preventing lung cancer is an important public health concern for both smokers and non-smokers. Much attention has been paid to the cancer-protective effect of consuming carotenoid-containing foods or supplements.3,4 Carotenoids are naturally occurring red, orange or yellow plant pigments. They have potent anticarcinogenic properties, including antioxidant activity, stimulation of gap junction intercellular communication, induction of detoxifying enzymes and inhibition of cellular proliferation.4,5 To date, several studies have been undertaken to reveal the association between carotenoids and lung cancer. The results from existing studies such as observational studies and randomized trials are inconsistent. Some studies have suggested a strong inverse association between dietary consumption or supplements of specific carotenoids and lung cancer risk,6–11 whereas null or weak inverse associations have been observed in other studies.12–14
The aim of the present study was to investigate prospectively the association of serum carotenoids, namely alpha-carotene, beta-carotene, beta-cryptoxanthin, lycopene and lutein + zeaxanthin, with lung cancer death in a nationally representative US sample to determine whether circulating serum carotenoid levels may reduce the risk of death from lung cancer.
Materials and Methods
Study population
The data used in this study were obtained from the Third Nutrition and Health Examination Survey (NHANES III) database and the NHANES III Linked Mortality File. The NHANES III, which was conducted between 1988 and 1994, was a stratified, multistage sampling designed to represent the non-institutionalized civilian US population,15 and it consisted of an interview, a physical examination and laboratory testing. The NHANES III Linked Mortality File was a mortality follow-up study matching the NHANES III records with data available in the National Death Index as of 31 December 2006. The date of death and cause of death in the National Death Index were derived from death certificates.16
We limited the study population to subjects ≥20 years of age at the time of the examination. Of the 18 800 subjects, 15 954 (85%) had serum carotenoid levels available. Among them, we excluded 5572 patients who had a history of cancer (n = 1237) or who were missing data on smoking and alcohol consumption (n = 3769) or other questionnaires (n = 566). The cohort analysis presented in this study was thus based on 10 382 NHANES III participants.
Baseline data collection
The participants were interviewed in NHANES III to obtain information on age (20–29, 30–39, 40–49, 50–59 or ≥60 years), gender (male or female), race/ethnicity (White, Black, Hispanic or other), education (less than high school, high school graduate, or college or more) and alcohol consumption (drinker or non-drinker). Exercise was assessed using the question, “Compared with most (men/women) your age, would you say that you are more active, less active, or about the same?” The response was categorized as more, less or about the same. Smoking-related variables included current smoking status (current, former or never) and pack-year history of smoking (<10 pack-year or ≥10 pack-year). A pack year is defined as smoking 20 cigarettes (1 pack) per day for 1 year. The body mass index (BMI) was calculated by dividing the weight in kilograms by the height in meters squared. The BMI were categorized into three groups (<18.5 kg/m2, 18.5–24.9 kg/m2 and ≤25.1 kg/m2). Total cholesterol levels were divided into three groups (≤200 mg/dL, 220–239.9 mg/dL and ≥240 mg/dL). Fat intake (<65 g/day or ≥65 g/day) was defined as total fat calculated from the dietary interview. Intake of vegetables or fruit was determined using the question, “How often did you have ten types of vegetables per month?” or “How often did you have six types of fruit per month?” Participants answered “yes” or “no” to each of the questions, and the responses were divided into two groups: vegetables (<7 types of vegetables or ≥7 types of vegetables) and fruits (<3 types of fruit or ≥3 types of fruit).
Serum carotenoid measurements
Carotenoid levels were measured in blood because serum carotenoid levels are useful biomarkers of the total dietary intake of vegetables and fruit. The most common types of carotenoids in humans, alpha-carotene, beta-carotene, beta-cryptoxanthin, lycopene and lutein + zeaxanthin, were assayed in NHANES III. Detailed procedures are described elsewhere.17 Isocratic HPLC-based methods were used to quantify these assays. The median interassay coefficients of variation were 9.4% for alpha-carotene, 7.0% for beta-carotene, 8.7% for beta-cryptoxanthin, 7.7% for lycopene and 11.0% for lutein + zeaxanthin.18
Mortality follow up
The International Classification of Diseases, Ninth Revision was used for deaths occurring from 1988 through 1998, and the International Statistical Classification of Diseases, Tenth Revision (ICD-10), was used for deaths occurring from 1999 to 2000. The underlying causes of death were grouped according to the National Center for Health Statistics for each coding system, and all deaths from 1988 to 1998 that were coded under the International Classification of Diseases, Ninth Revision, Clinical Modification guidelines were classified into comparable groups based on the ICD-10 underlying causes of death.19 Here, we focused on death from lung cancer (ICD-10 codes C33–34).
Statistical analysis
Current smokers and non-smokers (including those that have never smoked and former smokers) had dissimilar distributions of each carotenoid level; thus, we divided the five carotenoids into quartiles by smoking status. The overall risk analysis of lung cancer using the serum carotenoid levels was calculated by Cox proportional hazards regression. The hazard ratios (HR) and 95% confidence intervals (CI) of lung cancer death associated with each quartile level were compared with the first quartile. The proportional hazards assumption for the model was confirmed. All models for the five carotenoids with lung cancer mortality were fitted with increasing degrees of adjustment. First, we adjusted for age and gender. Second, each model was adjusted for ethnicity, education, alcohol consumption, exercise, smoking status, pack-year of smoking, obesity, total cholesterol, daily fat intake, and vegetable and fruit consumption. The risk analyses were also conducted after stratifying for smoking status (current smokers and never/former smokers) across the quartiles of serum carotenoid levels. The cumulative risks of lung cancer based on the lowest quartile (Q1) and the highest quartile (Q4) were compared between current smokers and never/former smokers.
The weighted estimates of the population parameters were computed by the National Center for Health Statistics to account for the complex sampling design. All the analyses were performed using sas 9.2 (SAS Institute, Cary, NC, USA) and R (R Foundation for Statistical Computing, Vienna, Austria).
Results
Of 10 382 participants, 161 subjects died due to lung cancer. Table1 compares the characteristics of the study participants who did and did not die from lung cancer. Participants who died from lung cancer were significantly more likely to be older, male, White, current smokers and non-drinkers at baseline compared with those without lung cancer mortality. Participants with lung cancer mortality were also more likely to have significantly less than a high school education, total cholesterol <200 mg/dL and a lower fruit intake than the control participants. No baseline differences between participants who did or did not die from lung cancer were detected in BMI, dietary fat intake, exercise or vegetable intake.
Table 1.
Baseline characteristics of the participants based on lung cancer mortality
| Participants without lung cancer mortality (n = 10 221) | Participants with lung cancer mortality (n = 161) | P-value | |
|---|---|---|---|
| Discrete variables, number (%) of participants | |||
| Age (years) | |||
| 20–29 | 2299 (22.5) | 0 (0.0) | <0.0001 |
| 30–39 | 2371 (23.2) | 8 (5.0) | |
| 40–49 | 1799 (17.6) | 17 (10.6) | |
| 50–59 | 1181 (11.6) | 34 (21.1) | |
| ≥60 | 2571 (25.2) | 102 (63.4) | |
| Gender | |||
| Male | 5323 (52.1) | 104 (64.6) | 0.0016 |
| Female | 4898 (47.9) | 57 (35.4) | |
| Race/Ethnicity | |||
| White | 4133 (40.4) | 89 (55.3) | <0.0001 |
| Black | 2865 (28.0) | 51 (31.7) | |
| Hispanic | 2867 (28.1) | 16 (9.9) | |
| Other | 356 (3.5) | 5 (3.1) | |
| Education | |||
| Less than high school | 2073 (20.3) | 55 (34.2) | <0.0001 |
| High school | 4963 (48.6) | 74 (46.0) | |
| College or more | 3185 (31.2) | 32 (19.9) | |
| Smoking status | |||
| Current smoker | 2866 (28.0) | 91 (56.5) | <0.0001 |
| Former smoker | 2660 (26.0) | 57 (35.4) | |
| Never smoker | 4695 (45.9) | 13 (8.1) | |
| Pack-year of smoking (pack/year) | |||
| <10 | 7787 (76.2) | 47 (29.2) | <0.0001 |
| ≥10 | 2434 (23.8) | 114 (70.8) | |
| Alcohol drinking | |||
| Drinker | 5769 (56.4) | 78 (48.5) | 0.0424 |
| Non-drinker | 4452 (43.6) | 83 (51.6) | |
| Body mass index (kg/m2) | |||
| <18.5 | 203 (2.0) | 3 (1.9) | 0.2155 |
| 18.5–24.9 | 3881 (38.0) | 72 (44.7) | |
| ≥25.1 | 6137 (60.0) | 86 (53.4) | |
| Total cholesterol (mg/dL) | |||
| <200 | 5009 (49.0) | 54 (33.5) | 0.0004 |
| 200–239.9 | 3220 (31.5) | 63 (39.1) | |
| ≥240 | 1992 (19.5) | 44 (27.3) | |
| Dietary fat intake (g/day) | |||
| <65 | 4375 (42.8) | 78 (48.5) | 0.1511 |
| ≥65 | 5846 (57.2) | 83 (51.6) | |
| ExerCIe | |||
| More | 3268 (32.0) | 62 (38.5) | 0.1129 |
| Less | 2280 (22.3) | 38 (23.6) | |
| About same | 4673 (45.7) | 61 (37.9) | |
| Vegetable intake (kinds of vegetables) | |||
| <7 | 4849 (47.4) | 81 (50.3) | 0.4695 |
| ≥7 | 5372 (52.6) | 80 (49.7) | |
| Fruit intake (kinds of fruits) | |||
| <3 | 4261 (41.7) | 88 (54.7) | 0.0009 |
| ≥3 | 5960 (58.3) | 73 (45.3) | |
| Continuous variables, mean (SD) of serum levels | |||
| Alpha-carotene (μg/dL) | 4.4 (4.3–4.5) | 3.5 (2.7–4.3) | 0.0004 |
| Beta-carotene (μg/dL) | 18.7 (18.3–19.2) | 17.6 (14.3–20.9) | 0.2929 |
| Beta-cryptoxanthin (μg/dL) | 10.5 (10.3–10.7) | 7.4 (6.1–8.7) | <0.0001 |
| Lutein/zeaxanthin (μg/dL) | 22.9 (22.6–23.1) | 22.2 (20.1–24.3) | 0.4837 |
| Lycopene (μg/dL) | 22.5 (22.2–22.7) | 17.4 (15.7–19.2) | <0.0001 |
Figure1 presents the distribution of serum carotenoid levels according to smoking status (current smokers vs never/former smokers) at baseline. Compared with current non-smokers, including never and former smokers, current smokers had relatively lower serum carotenoid levels. There were significant differences between current smokers and never/former smokers in the median levels of alpha-carotene (4.4 μg/dL vs 3.5 μg/dL, respectively), beta-cryptoxanthin (10.5 μg/dL vs 7.4 μg/dL, respectively) and lycopene (22.5 μg/dL vs 17.4 μg/dL, respectively).
Figure 1.
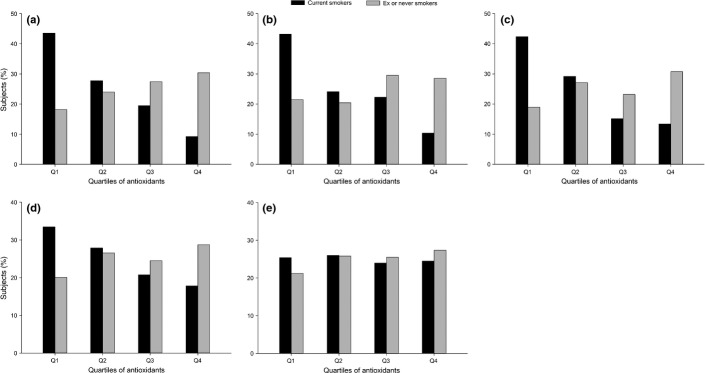
Distribution of serum carotenoids by current smoking status (current smokers vs never/former smokers): (a) Alpha-carotene; (b) beta-carotene; (c) beta-cryptoxanthin, (d) lycopene and (e) lutein/zeaxanthin.
Table2 shows the HR of lung cancer death across the quartiles of the five serum carotenoid levels among the overall participants. Baseline serum levels of beta-carotene, lycopene and lutein + zeaxanthin were not associated with the risk of lung cancer death. By contrast, we observed significant inverse associations between serum alpha-carotene and beta-cryptoxanthin, and lung cancer death. When adjustments were made for age and gender, the risk of lung cancer death was reduced in participants with higher serum alpha-carotene (HR = 0.24, 95% CI, 0.16–0.38) and beta-cryptoxanthin (HR = 0.21, 95% CI, 0.13–0.35) levels at baseline than in participants with the lowest levels. Additional adjustments for other covariates (ethnicity, education, alcohol consumption, exercise, pack-year of smoking, obesity, total cholesterol, daily fat intake, and vegetable and fruit consumption) did not change the results. The adjusted HR for the highest versus the lowest quartile was 0.53 (95% CI, 0.32–0.88) for serum alpha-carotene and 0.56 (95% CI, 0.33–0.96) for beta-cryptoxanthin.
Table 2.
Adjusted hazard ratio of lung cancer mortality across quartiles of serum carotenoids
| Quartile of serum carotenoids |
||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Alpha-carotene | ||||
| Serum concentration (μg/dL) | ≤1 | 2–3 | 4–5 | ≥6 |
| Number of cases | 62 | 36 | 34 | 29 |
| Model 1, HR (95% CI) | Reference | 0.49 (0.33–0.71) | 0.34 (0.23–0.51) | 0.24 (0.16–0.38) |
| Model 2, HR (95% CI) | Reference | 0.59 (0.39–0.91) | 0.59 (0.37–0.92) | 0.53 (0.32–0.88) |
| Beta-carotene | ||||
| Serum concentration (μg/dL) | ≤8 | 9–13 | 14–23 | ≥24 |
| Number of cases | 49 | 26 | 43 | 43 |
| Model 1, HR (95% CI) | Reference | 0.59 (0.38–0.92) | 0.58 (0.39–0.86) | 0.55 (0.37–0.83) |
| Model 2, HR (95% CI) | Reference | 0.64 (0.39–1.04) | 0.76 (0.49–1.17) | 0.76 (0.48–1.20) |
| Beta–cryptoxanthin | ||||
| Serum concentration (μg/dL) | ≤5 | 6–8 | 9–12 | ≥13 |
| Number of cases | 77 | 44 | 20 | 20 |
| Model 1, HR (95% CI) | Reference | 0.60 (0.43–0.85) | 0.31 (0.19–0.50) | 0.21 (0.13–0.35) |
| Model 2, HR (95% CI) | Reference | 0.84 (0.57–1.23) | 0.51 (0.31–0.84) | 0.56 (0.33–0.96) |
| Lycopene | ||||
| Serum concentration (μg/dL) | ≤13 | 14–20 | 21–28 | ≥29 |
| Number of cases | 64 | 50 | 23 | 24 |
| Model 1, HR (95% CI) | Reference | 0.71 (0.47–1.05) | 0.58 (0.38–0.88) | 0.49 (0.33–0.74) |
| Model 2, HR (95% CI) | Reference | 0.87 (0.56–1.33) | 0.81 (0.51–1.28) | 0.67 (0.42–1.07) |
| Lutein/zeaxanthin | ||||
| Serum concentration (μg/dL) | ≤14 | 15–20 | 21–27 | ≥28 |
| Number of cases | 44 | 42 | 36 | 39 |
| Model 1, HR (95% CI) | Reference | 0.93 (0.65–1.31) | 0.49 (0.30–0.77) | 0.54 (0.34–0.85) |
| Model 2, HR (95% CI) | Reference | 1.12 (0.76–1.65) | 0.67 (0.41–1.11) | 0.73 (0.44–1.22) |
Model 1 was adjusted by age and gender; Model 2 was adjusted by model 1 plus ethnicity, education, alcohol consumption, exerCIe, smoking status, pack-year of smoking, obesity, total cholesterol, daily fat intake and vegetable and fruit consumption.
There were no significant impacts of antioxidant levels on risk between never-smokers and ever smokers: alpha-carotene (P = 0.4124), beta-carotene (P = 0.7347), beta-cryptoxanthin (P = 0.5558), lutein/zeaxanthin (P = 0.5094) and lycopene (P = 0.6665).
Table3 shows the adjusted HR of lung cancer death across the quartiles of the five serum carotenoid levels by current smoking status. Current smokers with the highest serum alpha-carotene and beta-cryptoxanthin levels had 46% (95% CI, 0.31–0.94) and 61% (95% CI, 0.19–0.80) lower lung cancer mortalities, respectively, than current non-smokers in the lowest quartile.
Table 3.
Adjusted hazard ratio of lung cancer mortality across quartiles of serum carotenoids by smoking status
| Current smokers (n = 2957) |
Never/Former smokers (n = 7425) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Quartile of serum carotenoids |
Quartile of serum carotenoids |
|||||||
| Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | Q3 | Q4 | |
| Alpha-carotene | ||||||||
| Serum concentration (μg/dL) | ≤1 | 2–2 | 3–3 | ≥4 | ≤2 | 3–4 | 5–6 | ≥7 |
| Number of cases | 49 | 7 | 13 | 22 | 19 | 24 | 10 | 17 |
| Model 1 | Reference | 0.42 (0.21–0.85) | 0.45 (0.25–0.82) | 0.37 (0.23–0.60) | 1.00 | 0.76 (0.44–1.33) | 0.40 (0.19–0.84) | 0.61 (0.33 –1.13) |
| Model 2 | Reference | 0.50 (0.22–1.12) | 0.58 (0.31–1.10) | 0.54 (0.31–0.94) | 1.00 | 0.98 (0.53–1.82) | 0.53 (0.24–1.17) | 0.87 (0.42 –1.78) |
| Beta-carotene | ||||||||
| Serum concentraion (μg/dL) | ≤6 | 7–9 | 10–16 | ≥17 | ≤9 | 10–15 | 16–25 | ≥26 |
| Number of cases | 25 | 16 | 26 | 24 | 14 | 14 | 15 | 27 |
| Model 1 | Reference | 0.71 (0.38–1.31) | 0.84 (0.50–1.43) | 0.62 (0.36–1.08) | 1.00 | 0.82 (0.41–1.62) | 0.64 (0.33–1.27) | 0.92 (0.49 –1.71) |
| Model 2 | Reference | 0.81 (0.43–1.53) | 0.79 (0.45–1.41) | 0.57 (0.30–1.07) | 1.00 | 0.91 (0.43–1.92) | 0.72 (0.34–1.51) | 1.08 (0.54 –2.17) |
| Beta-cryptoxanthin | ||||||||
| Serum concentraion (μg/dL) | ≤4 | 5–6 | 7–9 | ≥10 | ≤6 | 7–8 | 9–13 | ≥14 |
| Number of cases | 41 | 24 | 15 | 11 | 30 | 14 | 12 | 14 |
| Model 1 | Reference | 0.64 (0.40–1.02) | 0.48 (0.28–0.84) | 0.22 (0.12–0.43) | 1.00 | 0.96 (0.53–1.71) | 0.47 (0.25–0.89) | 0.49 (0.26–0.92) |
| Model 2 | Reference | 0.66 (0.39–1.10) | 0.62 (0.33–1.13) | 0.39 (0.19–0.80) | 1.00 | 1.07 (0.56–2.04) | 0.59 (0.30–1.17) | 0.84 (0.42–1.67) |
| Lutein/zeaxanthin | ||||||||
| Serum concentraion (μg/dL) | ≤13 | 14–19 | 20–28 | 29–86 | ≤14 | 15–20 | 21–29 | ≥30 |
| Number of cases | 40 | 19 | 18 | 14 | 27 | 25 | 8 | 10 |
| Model 1 | Reference | 0.73 (0.42–1.27) | 0.52 (0.30–0.91) | 0.60 (0.36–1.00) | 1.00 | 1.16 (0.62–2.19) | 0.96 (0.51–1.82) | 0.71 (0.36–1.37) |
| Model 2 | Reference | 0.89 (0.49–1.61) | 0.62 (0.34–1.13) | 0.68 (0.38–1.22) | 1.00 | 1.35 (0.67–2.71) | 1.31 (0.64–2.66) | 0.86 (0.40–1.85) |
| Lycopene | ||||||||
| Serum concentraion (μg/dL) | ≤13 | 14–17 | 18–24 | ≥25 | ≤15 | 16–21 | 22–29 | ≥30 |
| Number of cases | 28 | 19 | 19 | 25 | 14 | 20 | 20 | 16 |
| Model 1 | Reference | 0.87 (0.53–1.42) | 0.67 (0.38–1.18) | 0.71 (0.40–1.28) | 1.00 | 1.45 (0.86–2.43) | 0.48 (0.23–1.01) | 0.75 (0.36–1.55) |
| Model 2 | Reference | 0.88 (0.50–1.56) | 0.81 (0.45–1.47) | 0.80 (0.41–1.56) | 1.00 | 1.60 (0.91–2.80) | 0.53 (0.24–1.19) | 0.99 (0.46–2.17) |
Model 1 was adjusted by age and gender; Model 2 was adjusted by model 1+ ethnicity, education, alcohol consumption, exerCIe, pack-year of smoking, obesity, total cholesterol, daily fat intake, and vegetable and fruit consumption.
Figure2 presents the cumulative risks of lung cancer death separately for current smokers and never/former smokers based on the lowest (Q1) and the highest (Q4) quartile carotenoids. Among current smokers, the cumulative risk ranged from 5.3% for current smokers with Q1 carotenoid levels to 3.5% for current smokers with Q4 levels. The corresponding risks for never/former smokers with Q1 and Q4 carotenoid levels were the same, at 1.5%. Compared with never/former smokers, the lung cancer mortality risk of current smokers, specifically smokers with the lowest (Q1) carotenoid levels, was dramatically increased with age.
Figure 2.
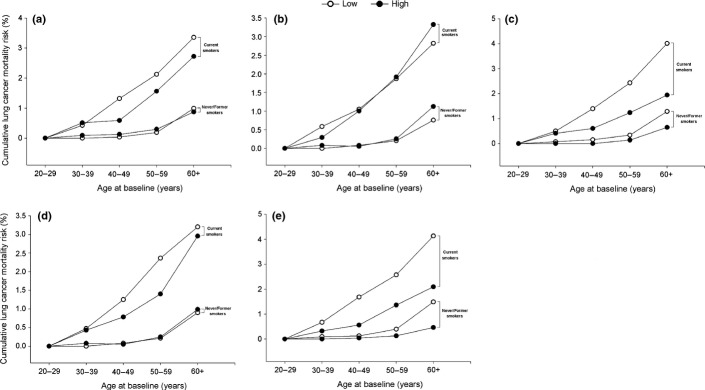
Cumulative risks of lung cancer death among current smokers versus never/former smokers. “Q1” indicates the lowest serum carotenoid levels and “Q4” indicates the highest serum carotenoid levels. (a) Alpha-carotene; (b) beta-carotene; (c) beta-cryptoxanthin, (d) lycopene and (e) lutein/zeaxanthin.
Discussion
In this prospective cohort study of a representative sample of the US population, we found that high serum levels of alpha-carotene and beta-cryptoxanthin at baseline were significantly associated with a lower risk of lung cancer death after adjustment for cigarette smoking and other potential covariates. Among current smokers, the mortality risk progressively decreased with increasing alpha-carotene and beta-cryptoxanthin levels; no association was observed among never/former smokers. Our findings extend the previous evidence on the potential anticarcinogenic effect of carotenoids in lung cancer5 and suggest that a high intake of food rich in alpha-carotene or beta-cryptoxanthin may prevent the development or worsening lung cancer, especially in smokers.
Carotenoids are a class of phytochemicals that are synthesized by plants and microorganisms. They have potent singlet molecular oxygen quenching activity and free radical-scavenging activity,3 and protect biological systems from oxidative damage. The main focus of studies, from observational studies to randomized trials, of carotenoids and cancer prevention has been on beta-carotenoid intake or supplements. However, the protective effect of beta-carotenoids on lung cancer is not confirmed,12,13 and the present study also failed to support the association. Consistent with our results, evidence is accumulating that the intake of or circulating levels of alpha-carotene and beta-cryptoxanthin are inversely associated with lung cancer risk.6–11,20–23 Population-based studies conducted in Hawaii, New Jersey and Finland report that the intake of alpha-carotene is significantly associated with reduced lung cancer risk.20,21,23 The results from two prospective cohort studies (the Nurses' Health Study and the Health Professionals Follow-Up Study) revealed that among never smokers, a significant 63% (relative risk [RR], 0.37; 95% CI, 0.18–0.77) lower risk of lung cancer was observed for the highest quintile compared with the lowest quintile of alpha-carotene intake; no associations were observed among current smokers.7 A study from the Netherlands Cohort Study on Diet and Cancer reports protective effects of beta-cryptoxanthin intake on lung cancer incidence.22 The inverse associations were stronger among current smokers and weaker for former smokers at baseline. In the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study, Holick et al. (2002) observed a significantly lower risk of any type of lung cancer with dietary beta-cryptoxanthin, but not alpha-carotene, in male smokers.6 A pooled analysis from seven cohort studies in North America and Europe showed that a high intake of dietary beta-cryptoxanthin significantly reduced lung cancer risk (RR, 0.76; 95% CI, 0.67–0.86; highest vs lowest quintile).14 The Japan Collaborative Cohort Study showed that men in the highest quartile of serum alpha-carotene and beta-cryptoxanthin intakes were at significantly lower risk of lung cancer death compared with men in the lowest quartiles.10,11 In a cohort study of Chinese men in Shanghai, Yuan et al. (2001) demonstrate a significant inverse association between the pre-diagnostic serum level of beta-cryptoxanthin and lung cancer development that was independent of cigarette smoking.8 Their subsequent study observed an approximate 15–40% reduction in risk of lung cancer for subjects in the highest versus the lowest 10th percentile of dietary beta-cryptoxanthin intake.9 Thus, alpha-carotene and beta-cryptoxanthin may be associated with reduced lung cancer mortality risk, even though null or weak inverse associations have been observed.
Interestingly, we found that the beneficial effect of alpha-carotene and beta-cryptoxanthin on lung cancer death was only present in current smokers. Cigarette smoking is a major cause of lung cancer and is strongly associated with unhealthy diets, including low intake of fruit and vegetables.24 Smokers often have lower circulating antioxidant levels compared with non-smokers.25,26 Whether the phenomenon is attributed to low dietary intake of antioxidant-rich foods27 or to the enhanced degradation of circulating antioxidants from the increased oxidative stress associated with smoking remains unclear.28,29 However, studies have demonstrated no significant differences in the intake of antioxidant-rich foods between smokers and non-smokers after adjusting for various factors.26,30 This result supports the hypothesis that the differences observed between smokers and non-smokers could be due primarily to the elevated oxidative stress levels caused by the toxicity of the smoke and not only to dietary differences in antioxidant nutrients.28 Based on the results, smokers would require higher intake of fruit and vegetables than non-smokers, and antioxidants, especially alpha-carotene and beta-cryptoxanthin, could be effective in reducing the risk of lung cancer that is associated with smoking.
Studies on the mechanisms that underlie the inverse associations between lung cancer mortality risk and serum alpha-carotene and beta-cryptoxanthin are limited. However, because carotenoids have antioxidant properties,4,5 a possible biological mechanism may lie in their antitumor-promoting activity and their protection against oxidative stress. For example, in experimental carcinogenesis studies, alpha-carotene-treated groups showed much higher suppressive activity than beta-carotene-treated groups,31,32 suggesting the potent preventive action of alpha-carotene against carcinogenesis. The administration of mandarin juice, which is rich in beta-cryptoxanthin, inhibits chemically induced mouse lung tumorigenesis.33 In addition, human intervention studies suggest that alpha-carotene exerts a cancer protective effect through a decrease in oxidative and other damage to DNA.34,35 Haegele et al. (2000) also report significant inverse associations of beta-cryptoxanthin and/or lutein with 8-oxodGuo in DNA. A recent study in a cell culture model suggests that beta-cryptoxanthin protects against DNA oxidation and improves DNA repair,36 implying that carotenoids have a cancer-protective role.
Important limitations of the present study include the single measurement of serum carotenoid levels at baseline and the long interval between the measurement and follow-up period. As a result, serum carotenoids were not measured at the time point defined as “death from lung cancer.” We did not address the influence of changes in serum carotenoid levels, such as antioxidant supplements, on death. In addition, because cigarette smoking is strongly related to lung cancer, to minimize confounding by smoking, the statistical model included smoking habits (current smoker, former smoker and never smoker) and pack-year of smoking. However, we cannot completely exclude some residual confounding factors related to smoking; for example, cigarette brand (which may lead to different carcinogenic exposures) or the use of filtered versus unfiltered cigarettes. In addition, several variables were dependent on self-reported data and, therefore, are not free from bias. Due to the observational nature of this investigation, we cannot exclude the possibility of residual confounding effects by unmeasured confounders. Finally, because the NHANES III Linked Mortality File was constructed by causes of death through the National Death Index, there is potential error in classifying the cause of death.
In conclusion, the results of the present study show that high serum levels of alpha-carotene and beta-cryptoxanthin are associated with a lower risk of lung cancer death in US adults. Our finding suggests that a high intake of foods that are rich in alpha-carotene and beta-cryptoxanthin has the potential to reduce lung cancer death and to lower the risk in current smokers.
Acknowledgments
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (grant numbers 2012R1A1A1041318 and 2012R1A1A3017058).
Disclosure Statement
The authors have no conflict of interest.
Funding information
Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2012R1A1A1041318 and 2012R1A1A3017058).
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.Stahl W, Sies H. Antioxidant activity of carotenoids. Mol Aspects Med. 2003;24:345–51. doi: 10.1016/s0098-2997(03)00030-x. [DOI] [PubMed] [Google Scholar]
- 4.Yeum KJ, Russell RM. Carotenoid bioavailability and bioconversion. Annu Rev Nutr. 2002;22:483–504. doi: 10.1146/annurev.nutr.22.010402.102834. [DOI] [PubMed] [Google Scholar]
- 5.Cooper DA, Eldridge AL, Peters JC. Dietary carotenoids and lung cancer: a review of recent research. Nutr Rev. 1999;57:133–45. doi: 10.1111/j.1753-4887.1999.tb01794.x. [DOI] [PubMed] [Google Scholar]
- 6.Holick CN, Michaud DS, Stolzenberg-Solomon R, et al. Dietary carotenoids, serum beta-carotene, and retinol and risk of lung cancer in the alpha-tocopherol, beta-carotene cohort study. Am J Epidemiol. 2002;156:536–47. doi: 10.1093/aje/kwf072. [DOI] [PubMed] [Google Scholar]
- 7.Michaud DS, Feskanich D, Rimm EB, et al. Intake of specific carotenoids and risk of lung cancer in 2 prospective US cohorts. Am J Clin Nutr. 2000;72:990–7. doi: 10.1093/ajcn/72.4.990. [DOI] [PubMed] [Google Scholar]
- 8.Yuan JM, Ross RK, Chu XD, Gao YT, Yu MC. Prediagnostic levels of serum beta-cryptoxanthin and retinol predict smoking-related lung cancer risk in Shanghai, China. Cancer Epidemiol Biomark Prev. 2001;10:767–73. [PubMed] [Google Scholar]
- 9.Yuan JM, Stram DO, Arakawa K, Lee HP, Yu MC. Dietary cryptoxanthin and reduced risk of lung cancer: the Singapore Chinese Health Study. Cancer Epidemiol Biomark Prev. 2003;12:890–8. [PubMed] [Google Scholar]
- 10.Ito Y, Wakai K, Suzuki K, et al. Lung cancer mortality and serum levels of carotenoids, retinol, tocopherols, and folic acid in men and women: a case-control study nested in the JACC Study. J Epidemiol. 2005;15(Suppl 2):S140–9. doi: 10.2188/jea.15.S140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ito Y, Kurata M, Hioki R, Suzuki K, Ochiai J, Aoki K. Cancer mortality and serum levels of carotenoids, retinol, and tocopherol: a population-based follow-up study of inhabitants of a rural area of Japan. Asian Pac J Cancer Prev. 2005;6:10–5. [PubMed] [Google Scholar]
- 12.Druesne-Pecollo N, Latino-Martel P, Norat T, et al. Beta-carotene supplementation and cancer risk: a systematic review and metaanalysis of randomized controlled trials. Int J Cancer. 2010;127:172–84. doi: 10.1002/ijc.25008. [DOI] [PubMed] [Google Scholar]
- 13.Gallicchio L, Boyd K, Matanoski G, et al. Carotenoids and the risk of developing lung cancer: a systematic review. Am J Clin Nutr. 2008;88:372–83. doi: 10.1093/ajcn/88.2.372. [DOI] [PubMed] [Google Scholar]
- 14.Mannisto S, Smith-Warner SA, Spiegelman D, et al. Dietary carotenoids and risk of lung cancer in a pooled analysis of seven cohort studies. Cancer Epidemiol Biomark Prev. 2004;13:40–8. doi: 10.1158/1055-9965.epi-038-3. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease C, Prevention. 1994. Plan and Operation of the Third National Health and Nutrition Examination Survey, 1988–94. Vital Health Stat 1(32). [cited 30 Apr 2013.] Available from URL: http://www.cdc.gov/nchs/data/series/sr_01/sr01_032.pdf.
- 16.National Center for Health S. 2006. NHANES III linked mortality file. [cited 30 Apr 2013.] Available from URL: http://www.cdc.gov/nchs/data_access/data_linkage/mortality/nhanes3_linkage.htm.
- 17.Gunter EW, Lewis BL, Koncikowski SM. Laboratory Methods used for the Third National Health and Nutrition Examination Survey (NHANES III), 1988–1994. Atlanta, GA: US Department of Health and Human Services; 1996. [Google Scholar]
- 18.Shardell MD, Alley DE, Hicks GE, et al. Low-serum carotenoid concentrations and carotenoid interactions predict mortality in US adults: the Third National Health and Nutrition Examination Survey. Nutr Res. 2011;31:178–89. doi: 10.1016/j.nutres.2011.03.003. (New York, NY) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson RN, Minino AM, Hoyert DL, Rosenberg HM, Hyattsville MD National Center for Health S. 2001. Comparability of cause of death between ICD-9 and ICD-10: preliminary estimates. (National vital statistics reports, vol 49, no. 2) [PubMed]
- 20.Knekt P, Jarvinen R, Teppo L, Aromaa A, Seppanen R. Role of various carotenoids in lung cancer prevention. J Natl Cancer Inst. 1999;91:182–4. doi: 10.1093/jnci/91.2.182. [DOI] [PubMed] [Google Scholar]
- 21.Le Marchand L, Hankin JH, Kolonel LN, Beecher GR, Wilkens LR, Zhao LP. Intake of specific carotenoids and lung cancer risk. Cancer Epidemiol Biomark Prev. 1993;2:183–7. [PubMed] [Google Scholar]
- 22.Voorrips LE, Goldbohm RA, Brants HA, et al. A prospective cohort study on antioxidant and folate intake and male lung cancer risk. Cancer Epidemiol Biomark Prev. 2000;9:357–65. [PubMed] [Google Scholar]
- 23.Ziegler RG, Colavito EA, Hartge P, et al. Importance of alpha-carotene, beta-carotene, and other phytochemicals in the etiology of lung cancer. J Natl Cancer Inst. 1996;88:612–5. doi: 10.1093/jnci/88.9.612. [DOI] [PubMed] [Google Scholar]
- 24.Dallongeville J, Marecaux N, Fruchart JC, Amouyel P. Cigarette smoking is associated with unhealthy patterns of nutrient intake: a meta-analysis. J Nutr. 1998;128:1450–7. doi: 10.1093/jn/128.9.1450. [DOI] [PubMed] [Google Scholar]
- 25.Dietrich M, Block G, Norkus EP, et al. Smoking and exposure to environmental tobacco smoke decrease some plasma antioxidants and increase gamma-tocopherol in vivo after adjustment for dietary antioxidant intakes. Am J Clin Nutr. 2003;77:160–6. doi: 10.1093/ajcn/77.1.160. [DOI] [PubMed] [Google Scholar]
- 26.Lykkesfeldt J, Christen S, Wallock LM, Chang HH, Jacob RA, Ames BN. Ascorbate is depleted by smoking and repleted by moderate supplementation: a study in male smokers and nonsmokers with matched dietary antioxidant intakes. Am J Clin Nutr. 2000;71:530–6. doi: 10.1093/ajcn/71.2.530. [DOI] [PubMed] [Google Scholar]
- 27.Ma J, Hampl JS, Betts NM. Antioxidant intakes and smoking status: data from the continuing survey of food intakes by individuals 1994–1996. Am J Clin Nutr. 2000;71:774–80. doi: 10.1093/ajcn/71.3.774. [DOI] [PubMed] [Google Scholar]
- 28.Alberg A. The influence of cigarette smoking on circulating concentrations of antioxidant micronutrients. Toxicology. 2002;180:121–37. doi: 10.1016/s0300-483x(02)00386-4. [DOI] [PubMed] [Google Scholar]
- 29.Zhou JF, Yan XF, Guo FZ, Sun NY, Qian ZJ, Ding DY. Effects of cigarette smoking and smoking cessation on plasma constituents and enzyme activities related to oxidative stress. Biomed Environ Sci. 2000;13:44–55. [PubMed] [Google Scholar]
- 30.Bloomer RJ. Decreased blood antioxidant capacity and increased lipid peroxidation in young cigarette smokers compared to nonsmokers: impact of dietary intake. Nutr J. 2007;6:39. doi: 10.1186/1475-2891-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murakoshi M, Nishino H, Satomi Y, et al. Potent preventive action of alpha-carotene against carcinogenesis: spontaneous liver carcinogenesis and promoting stage of lung and skin carcinogenesis in mice are suppressed more effectively by alpha-carotene than by beta-carotene. Cancer Res. 1992;52:6583–7. [PubMed] [Google Scholar]
- 32.Nishino H, Tokuda H, Murakoshi M, et al. Cancer prevention by natural carotenoids. BioFactors. 2000;13:89–94. doi: 10.1002/biof.5520130115. (Oxford, England) [DOI] [PubMed] [Google Scholar]
- 33.Kohno H, Taima M, Sumida T, Azuma Y, Ogawa H, Tanaka T. Inhibitory effect of mandarin juice rich in beta-cryptoxanthin and hesperidin on 4-(methylnitrosamino)-1-(3-pyridyl)–1-butanone-induced pulmonary tumorigenesis in mice. Cancer Lett. 2001;174:141–50. doi: 10.1016/s0304-3835(01)00713-3. [DOI] [PubMed] [Google Scholar]
- 34.Pool-Zobel BL, Bub A, Muller H, Wollowski I, Rechkemmer G. Consumption of vegetables reduces genetic damage in humans: first results of a human intervention trial with carotenoid-rich foods. Carcinogenesis. 1997;18:1847–50. doi: 10.1093/carcin/18.9.1847. [DOI] [PubMed] [Google Scholar]
- 35.Thompson HJ, Heimendinger J, Haegele A, et al. Effect of increased vegetable and fruit consumption on markers of oxidative cellular damage. Carcinogenesis. 1999;20:2261–6. doi: 10.1093/carcin/20.12.2261. [DOI] [PubMed] [Google Scholar]
- 36.Lorenzo Y, Azqueta A, Luna L, Bonilla F, Dominguez G, Collins AR. The carotenoid beta-cryptoxanthin stimulates the repair of DNA oxidation damage in addition to acting as an antioxidant in human cells. Carcinogenesis. 2009;30:308–14. doi: 10.1093/carcin/bgn270. [DOI] [PubMed] [Google Scholar]


