Abstract
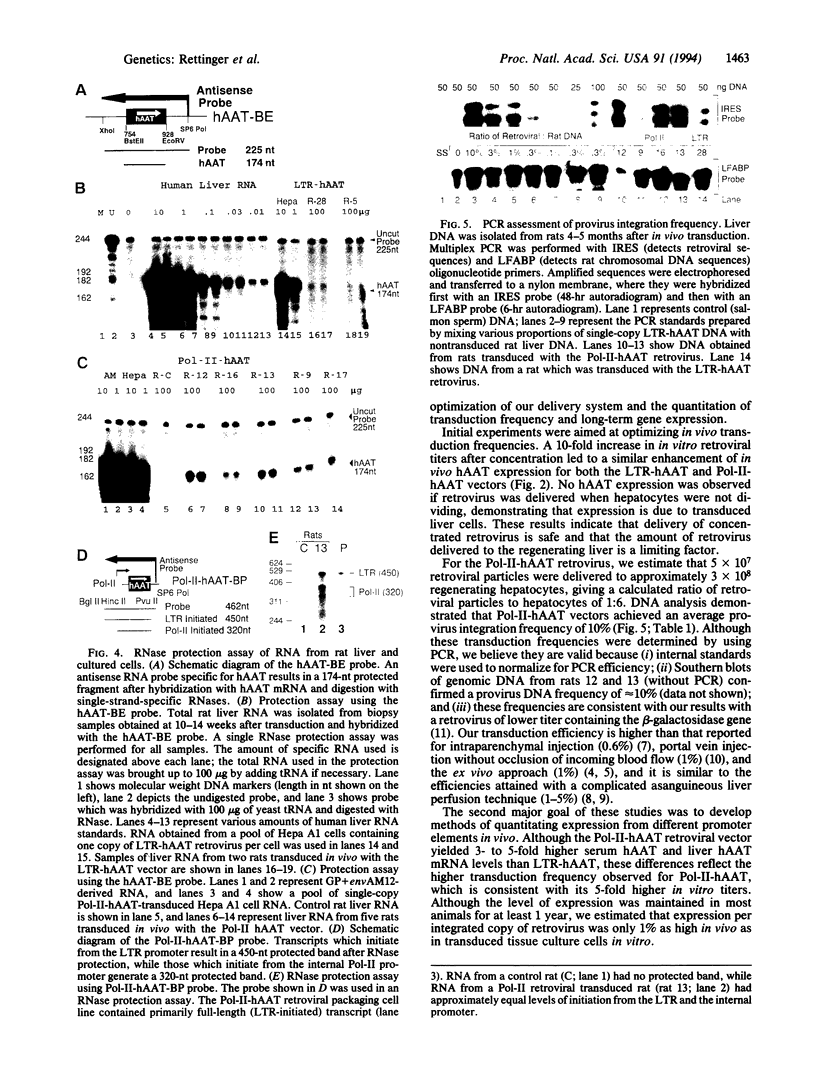
Liver-directed gene therapy will be applicable to many inherited diseases. Although various protocols have been devised for in vivo delivery of retrovirus, comparison of hepatocyte transduction frequencies has been difficult due to variations in retroviral titer and a paucity of DNA data. We have previously reported an in vivo rat hepatocyte transduction technique which involves 70% hepatectomy followed 24 hr later by portal vein injection of retrovirus during hepatic in-flow occlusion. In this study, we employed this method and concentrated retroviral preparations to achieve transduction of up to 15% of hepatocytes as determined by a quantitative PCR assay. As an initial step toward identifying promoters which lead to high-level long-term expression of retroviral transduced genes, we used our in vivo delivery system to compare the Moloney murine leukemia virus long terminal repeat (LTR) promoter with the promoter for the large subunit of murine RNA polymerase II (Pol-II). Human alpha 1-antitrypsin (hAAT) was used as the reporter gene to facilitate long-term analysis of expression. Serum hAAT levels were higher for the Pol-II promoter (143 ng/ml) than for the LTR promoter (50 ng/ml). This difference was consistent with the higher transduction frequency observed for the Pol-II-hAAT vector. Although serum hAAT expression was sustained for up to 1 year in six of eight Pol-II-hAAT-transduced rats and three of five LTR-hAAT-transduced rats and was proportional to hAAT mRNA level and proviral DNA frequency, in vivo expression was significantly lower than in transduced tissue culture cells. We conclude that a high frequency of in vivo transduction can be achieved by using retroviral vectors and our rapid transduction protocol, but transduced gene expression remains a serious problem. The quantitative assays described herein will facilitate in vivo comparisons of gene regulatory elements.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahearn J. M., Jr, Bartolomei M. S., West M. L., Cisek L. J., Corden J. L. Cloning and sequence analysis of the mouse genomic locus encoding the largest subunit of RNA polymerase II. J Biol Chem. 1987 Aug 5;262(22):10695–10705. [PubMed] [Google Scholar]
- Anderson W. F. Human gene therapy. Science. 1992 May 8;256(5058):808–813. doi: 10.1126/science.1589762. [DOI] [PubMed] [Google Scholar]
- Cheng L., Ziegelhoffer P. R., Yang N. S. In vivo promoter activity and transgene expression in mammalian somatic tissues evaluated by using particle bombardment. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4455–4459. doi: 10.1073/pnas.90.10.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chowdhury J. R., Grossman M., Gupta S., Chowdhury N. R., Baker J. R., Jr, Wilson J. M. Long-term improvement of hypercholesterolemia after ex vivo gene therapy in LDLR-deficient rabbits. Science. 1991 Dec 20;254(5039):1802–1805. doi: 10.1126/science.1722351. [DOI] [PubMed] [Google Scholar]
- Ferry N., Duplessis O., Houssin D., Danos O., Heard J. M. Retroviral-mediated gene transfer into hepatocytes in vivo. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8377–8381. doi: 10.1073/pnas.88.19.8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghattas I. R., Sanes J. R., Majors J. E. The encephalomyocarditis virus internal ribosome entry site allows efficient coexpression of two genes from a recombinant provirus in cultured cells and in embryos. Mol Cell Biol. 1991 Dec;11(12):5848–5859. doi: 10.1128/mcb.11.12.5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzoglou M., Lamers W., Bosch F., Wynshaw-Boris A., Clapp D. W., Hanson R. W. Hepatic gene transfer in animals using retroviruses containing the promoter from the gene for phosphoenolpyruvate carboxykinase. J Biol Chem. 1990 Oct 5;265(28):17285–17293. [PubMed] [Google Scholar]
- Herz J., Gerard R. D. Adenovirus-mediated transfer of low density lipoprotein receptor gene acutely accelerates cholesterol clearance in normal mice. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2812–2816. doi: 10.1073/pnas.90.7.2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe H. A., Danel C., Longenecker G., Metzger M., Setoguchi Y., Rosenfeld M. A., Gant T. W., Thorgeirsson S. S., Stratford-Perricaudet L. D., Perricaudet M. Adenovirus-mediated in vivo gene transfer and expression in normal rat liver. Nat Genet. 1992 Aug;1(5):372–378. doi: 10.1038/ng0892-372. [DOI] [PubMed] [Google Scholar]
- Kaleko M., Garcia J. V., Miller A. D. Persistent gene expression after retroviral gene transfer into liver cells in vivo. Hum Gene Ther. 1991 Spring;2(1):27–32. doi: 10.1089/hum.1991.2.1-27. [DOI] [PubMed] [Google Scholar]
- Kaneda Y., Iwai K., Uchida T. Introduction and expression of the human insulin gene in adult rat liver. J Biol Chem. 1989 Jul 25;264(21):12126–12129. [PubMed] [Google Scholar]
- Kay M. A., Baley P., Rothenberg S., Leland F., Fleming L., Ponder K. P., Liu T., Finegold M., Darlington G., Pokorny W. Expression of human alpha 1-antitrypsin in dogs after autologous transplantation of retroviral transduced hepatocytes. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):89–93. doi: 10.1073/pnas.89.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay M. A., Li Q., Liu T. J., Leland F., Toman C., Finegold M., Woo S. L. Hepatic gene therapy: persistent expression of human alpha 1-antitrypsin in mice after direct gene delivery in vivo. Hum Gene Ther. 1992 Dec;3(6):641–647. doi: 10.1089/hum.1992.3.6-641. [DOI] [PubMed] [Google Scholar]
- Kim Y. J., Sah R. L., Doong J. Y., Grodzinsky A. J. Fluorometric assay of DNA in cartilage explants using Hoechst 33258. Anal Biochem. 1988 Oct;174(1):168–176. doi: 10.1016/0003-2697(88)90532-5. [DOI] [PubMed] [Google Scholar]
- Kozak S. L., Kabat D. Ping-pong amplification of a retroviral vector achieves high-level gene expression: human growth hormone production. J Virol. 1990 Jul;64(7):3500–3508. doi: 10.1128/jvi.64.7.3500-3508.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Kay M. A., Finegold M., Stratford-Perricaudet L. D., Woo S. L. Assessment of recombinant adenoviral vectors for hepatic gene therapy. Hum Gene Ther. 1993 Aug;4(4):403–409. doi: 10.1089/hum.1993.4.4-403. [DOI] [PubMed] [Google Scholar]
- Long G. L., Chandra T., Woo S. L., Davie E. W., Kurachi K. Complete sequence of the cDNA for human alpha 1-antitrypsin and the gene for the S variant. Biochemistry. 1984 Oct 9;23(21):4828–4837. doi: 10.1021/bi00316a003. [DOI] [PubMed] [Google Scholar]
- Markowitz D., Goff S., Bank A. A safe packaging line for gene transfer: separating viral genes on two different plasmids. J Virol. 1988 Apr;62(4):1120–1124. doi: 10.1128/jvi.62.4.1120-1124.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz D., Goff S., Bank A. Construction and use of a safe and efficient amphotropic packaging cell line. Virology. 1988 Dec;167(2):400–406. [PubMed] [Google Scholar]
- Miller A. D. Human gene therapy comes of age. Nature. 1992 Jun 11;357(6378):455–460. doi: 10.1038/357455a0. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Rosman G. J. Improved retroviral vectors for gene transfer and expression. Biotechniques. 1989 Oct;7(9):980-2, 984-6, 989-90. [PMC free article] [PubMed] [Google Scholar]
- Mulligan R. C. The basic science of gene therapy. Science. 1993 May 14;260(5110):926–932. doi: 10.1126/science.8493530. [DOI] [PubMed] [Google Scholar]
- Palmer T. D., Rosman G. J., Osborne W. R., Miller A. D. Genetically modified skin fibroblasts persist long after transplantation but gradually inactivate introduced genes. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1330–1334. doi: 10.1073/pnas.88.4.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettinger S. D., Ponder K. P., Saylors R. L., Kennedy S. C., Hafenrichter D. G., Flye M. W. In vivo hepatocyte transduction with retrovirus during in-flow occlusion. J Surg Res. 1993 May;54(5):418–425. doi: 10.1006/jsre.1993.1066. [DOI] [PubMed] [Google Scholar]
- Rozga J., Moscioni A. D., Neuzil D., Demetriou A. A. A model for directed foreign gene delivery to rat liver cells in vivo. J Surg Res. 1992 Mar;52(3):209–213. doi: 10.1016/0022-4804(92)90075-b. [DOI] [PubMed] [Google Scholar]
- Scharfmann R., Axelrod J. H., Verma I. M. Long-term in vivo expression of retrovirus-mediated gene transfer in mouse fibroblast implants. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4626–4630. doi: 10.1073/pnas.88.11.4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen C. C., Levinson A. D. Isolation and expression of an altered mouse dihydrofolate reductase cDNA. Proc Natl Acad Sci U S A. 1983 May;80(9):2495–2499. doi: 10.1073/pnas.80.9.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P., Friedrich G., Lawinger P. Promoter interactions in retrovirus vectors introduced into fibroblasts and embryonic stem cells. J Virol. 1991 May;65(5):2314–2319. doi: 10.1128/jvi.65.5.2314-2319.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweetser D. A., Birkenmeier E. H., Klisak I. J., Zollman S., Sparkes R. S., Mohandas T., Lusis A. J., Gordon J. I. The human and rodent intestinal fatty acid binding protein genes. A comparative analysis of their structure, expression, and linkage relationships. J Biol Chem. 1987 Nov 25;262(33):16060–16071. [PubMed] [Google Scholar]
- Wu G. Y., Wu C. H. Receptor-mediated gene delivery and expression in vivo. J Biol Chem. 1988 Oct 15;263(29):14621–14624. [PubMed] [Google Scholar]