Abstract
Olaratumab (IMC-3G3) is a fully human IgG1 monoclonal antibody that selectively binds the external domain of human platelet-derived growth factor receptor-α with high affinity and blocks ligand binding. This was a single-center, dose-escalation, phase I trial of olaratumab in Japanese patients with advanced/refractory solid malignancies. Three to six patients were enrolled into each of three cohorts: Patients received i.v. olaratumab: 10 mg/kg on days 1 and 8 every 3 weeks (cohort 1); 20 mg/kg every 2 weeks (cohort 2); and 15 mg/kg on days 1 and 8 every 3 weeks (cohort 3). Doses were escalated from cohort 1 through cohort 3. The primary objective was to establish the safety and pharmacokinetic profile of olaratumab. Sixteen patients were treated across three cohorts. There were no dose-limiting toxicities, so the maximum tolerated dose was not reached. The most common olaratumab-related treatment-emergent adverse events (TEAEs) were proteinuria (25.0%) and elevated aspartate transaminase (12.5%). One patient (cohort 2) had two olaratumab-related Grade 3 TEAEs (increased aspartate aminotransferase and tumor hemorrhage); otherwise, olaratumab-related TEAEs were Grade 1/2. Seven patients (43.8%) had a best response of stable disease. Based on the pharmacokinetic concentration profile of olaratumab, the trough concentrations following single and multiple doses at 15 mg/kg on days 1 and 8 every 3 weeks (cohort 3) and multiple doses at 20 mg/kg every 2 weeks (cohort 2) were above the 155 μg/mL target. Thus, these two doses could represent an acceptable schedule for future trials in Japanese patients. Olaratumab had an acceptable safety profile and was well tolerated.
Keywords: IMC-3G3, monoclonal antibody, olaratumab, phase 1, platelet-derived growth factor receptor
The platelet-derived growth factor receptor family (PDGFR) consists of PDGFRα and PDGFRβ.(1) These receptors and their ligands are involved in normal organ development and function, wound-healing, and the pathogenesis of malignant and non-malignant diseases.(1) The PDGFRα/platelet-derived growth factor (PDGF) axis is required for vascular endothelial growth factor production by tumor stroma and the regulation of tumoral angiogenesis.(2)
Platelet-derived growth factor receptor-α is expressed in several types of cancer on transformed cells and in tumor stroma.(3–6) PDGFRα expression is associated with disease progression, diminished patient survival, and metastases to lymph nodes and bone.(7–10) Due to the effects of the PDGFRα/PDGF axis on tumor growth and tumor-associated vasculature, there is interest in developing therapeutic inhibitors of this pathway.(11,12) Most of these inhibitors are small molecule tyrosine kinase inhibitors (TKIs) that typically inhibit multiple kinases.(11,12)
Olaratumab (IMC-3G3) is a fully human IgG1 monoclonal antibody that selectively binds human PDGFRα with high affinity (approximately 40 pM) and blocks ligand-binding.(13) This antibody inhibits the proliferation and growth of a variety of human tumor cell lines both in vitro and in vivo.(5,6,13) Based on its activity in preclinical models involving human cells,(5,6,13) olaratumab entered clinical development. One phase I trial in patients with advanced tumors is complete (CP15-0601; I5B-IE-JGDC)(14) and several phase II trials are ongoing. Here, we report the results of a phase I trial of olaratumab in a cohort of Japanese patients (CP15-0907; I5B-IE-JGDF) with advanced solid tumors.
Materials and Methods
Patients
Patients (≥20 years old) with advanced primary or recurrent solid tumors not responding to standard therapy, or for whom no standard therapy was available, were eligible. Other enrollment criteria included Eastern Cooperative Oncology Group Performance Status of 0–1; estimated life expectancy >3 months; and adequate hematologic, hepatic, renal, and coagulation function.
Patients with known brain metastases were excluded due to risk of bleeding. Other exclusion criteria included chemotherapy or radiotherapy within 28 days (6 weeks for nitrosoureas or mitomycin C) prior to entering the study or presence of ongoing side effects ≥Grade 2 due to agents administered >28 days prior to study entry; uncontrolled intercurrent illness; participation in clinical trials of unapproved agents within 4 weeks of study entry for small molecules or within 8 weeks for monoclonal antibodies; and hepatitis B virus antigen, hepatitis C virus antibody, or human immunodeficiency virus antibody positivity.
This study was conducted in accordance with the Good Clinical Practices, Japanese Good Clinical Practices, the Declaration of Helsinki, and approval by the medical institution's Ethical Review Board. Patients provided written informed consent prior to inclusion. The ClinicalTrials.gov identifier is NCT01199822.
Study design
This was a single-center, open-label, dose-escalation, phase I trial. The primary objective was to establish the safety and pharmacokinetic (PK) profile of olaratumab administered on day 1 every 2 weeks (q2w) or on days 1 and 8 every 3 weeks (q3w) in this patient population. Exploratory analyses included preliminary assessment of antitumor activity and assessment of the pharmacodynamic effect of olaratumab.
Patients received i.v. olaratumab (infusion rate not exceeding 25 mg/min) q2w or on days 1 and 8 q3w. One cycle was defined as 6 weeks. Tumor response was evaluated radiographically every 6 weeks, starting from the first drug administration and independently from the treatment cycle. After cycle 1, patients experiencing a complete response (CR), partial response (PR), or stable disease (SD) received olaratumab at their cohort dose and schedule until there was evidence of progressive disease (PD) or until other withdrawal criteria were met.
Treatment cohorts
Olaratumab dosing was based on baseline body weight; the dose was recalculated if there was a ≥10% weight change from baseline. A minimum of three patients were enrolled in each cohort. The cohort 1 dose was 10 mg/kg administered on days 1 and 8 q3w. Dose escalation from cohort 1 to cohort 2 (20 mg/kg q2w) occurred after all cohort 1 patients completed the first cycle of therapy or discontinued due to a dose-limiting toxicity (DLT). Enrollment into cohort 3 (15 mg/kg q3w) occurred after all cohort 2 patients completed the first cycle of therapy or discontinued due to a DLT. Intrapatient dose escalations were not permitted. Patients who did not complete the first 6 weeks (one cycle) of treatment for reasons other than a DLT were replaced.
If one DLT was observed in any cohort during cycle 1, 3 additional patients were enrolled into that cohort. If no additional DLTs were observed, dose escalation continued. If a patient did not recover from the DLT to ≤ Grade 1 within 2 weeks, the patient was discontinued from the study.
A DLT was defined as one of the following conditions: if considered by the investigator to be definitely, probably, or possibly related to olaratumab; grade 4 neutropenia lasting >7 days; grade ≥3 thrombocytopenia with bleeding or requiring platelet transfusions; grade ≥3 neutropenia associated with fever; grade 3 or 4 non-hematologic toxicity; grade ≥3 skin toxicity (despite pre-emptive and supportive care); and/or grade ≥3 diarrhea, nausea, or vomiting (despite pre-emptive and supportive care).
Dose adjustments
Dose reductions were not permitted. Dose delays were permitted after cycle 1 for patients with non-life-threatening, reversible grade 3–4 adverse events (AEs) that resolved to grade ≤1 within 2 weeks. For these AEs, treatment could resume within 2 weeks and could continue until PD or other withdrawal criteria were met.
Determination of maximum tolerated dose
This trial used a conventional 3 + 3 design. If ≥2 patients in cohort 1 experienced a DLT, the study was to be discontinued. If ≥2 patients in cohort 2 or 3 experienced a DLT, then the cohort 1 dose was to be the maximum tolerated dose (MTD). If no MTD was determined, both cohorts 2 and 3 were to be expanded to six patients, with the goal of obtaining enough data for a PK analysis.
Pharmacokinetic assessments
Serum olaratumab was quantitated by using a validated ELISA. For the 10 mg/kg (cohort 1) and 15 mg/kg (cohort 3) (dosed on day 1 and day 8 every 3 weeks) q3w groups, PK samples were collected up to 168 h post end of day 1 infusion and 336 h post end of day 8 infusion. For the 20 mg/kg q2w group (cohort 2), PK samples were collected up to 336 h post end of infusion following the first (cycle 1, day 1) and fifth (cycle 2, day 1) infusions. Beginning cycle 3, samples were collected prior to and 1 h after completion of the first infusion in every subsequent cycle. The PK parameters were calculated from individual serum concentrations versus time profiles by noncompartmental analysis method by using winnonlin (Version 5.3; Certara, St. Louis, MO, USA).
Pharmacodynamic assessments
Human PDGF-AA and PDGF-BB in sodium heparin plasma collected at pre-specified time points was quantitatively determined by using an ELISA at Intertek Laboratories (Houston, TX, USA).
For cohorts 1 and 3, PD markers were analyzed using plasma samples (from approximately 7 mL of blood) obtained prior to the first infusion; immediately after the first infusion; and 1, 4, 8, 24, and 168 h following the completion of the first infusion, prior to and 1 h following the completion of the fifth infusion and ninth infusion, and prior to and 1 h following the completion of the infusion every 6 weeks thereafter. A blood sample for PD assessment was also taken at the end of study visit.
For cohort 2, PD markers were analyzed using plasma samples obtained prior to the first infusion; immediately after the end of the first infusion; 1, 4, 8, 24, 168, and 336 h following the completion of the first infusion; prior to and 1 h following the completion of the fourth infusion and seventh infusion; and prior to and 1 h following the completion of the infusion every 6 weeks thereafter. A blood sample for PD assessment was also taken at the end of study visit.
Safety assessments
Adverse events were coded by the Medical Dictionary for Regulatory Activities and graded according to the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events, Version 4.02.(15)
Disease assessment
Baseline tumor burden was assessed within 28 days prior to study registration. Patients were evaluated for response according to the Response Evaluation Criteria in Solid Tumors (v 1.0) after every cycle.(16) Confirmatory scans were obtained ≥4 weeks following initial documentation objective response.
Data and statistical analysis
The anticipated sample size was 18 patients. This sample size was based on cohort size. Data were analyzed using sas® software (Cary, NC, USA), version 9.2.
Analysis populations
The safety population included all enrolled patients who received any olaratumab, regardless of study eligibility, and was based on the actual initial therapy that a patient received, regardless of any other cohort to which the patient was assigned. The safety population was used for the analysis of baseline characteristics, safety data, and efficacy data. The MTD population included all enrolled patients who completed cycle 1 or discontinued during cycle 1 due to a DLT.
Results
Patient characteristics and treatment
Sixteen patients at one Japanese center received olaratumab. One additional patient signed an informed consent form and was enrolled in the study, but was considered a screen failure and was not treated due to pneumonia at the time of study entry. Across all cohorts, the median age was 60.7 years (range 35.6–71.4). The majority of patients were male (62.5%) and had colorectal or gastric type cancers (81.3%); all patients were Asian (Japanese). Table 1 shows the baseline demographics and disease characteristics.
Table 1.
Baseline demographics and disease characteristics
| Number of patients, n (%) (unless otherwise indicated) |
||||
|---|---|---|---|---|
| Cohort 1 (10 mg/kg) n = 3 | Cohort 2 (20 mg/kg) n = 7 | Cohort 3 (15 mg/kg) n = 6 | All cohorts N = 16 | |
| Age, years | ||||
| Median (range) | 69.6 (59.6–71.4) | 60.9 (35.6–70.3) | 59.1 (50.5–69.7) | 60.7 (35.6–71.4) |
| Sex | ||||
| Male | 3 (100.0) | 5 (71.4) | 2 (33.3) | 10 (62.5) |
| Female | 0 | 2 (28.6) | 4 (66.7) | 6 (37.5) |
| Race | ||||
| Asian (Japanese) | 3 (100.0) | 7 (100.0) | 6 (100.0) | 16 (100.0) |
| Type of cancer† | ||||
| Colorectal | 1 (33.3) | 5 (71.4) | 1 (16.7) | 7 (43.8) |
| Gastric | 1 (33.3) | 0 | 1 (16.7) | 2 (12.6) |
| Gastrointestinal stroma | 0 | 2 (28.6) | 2 (33.3) | 4 (25.0) |
| Head and neck | 1 (33.3) | 0 | 1 (16.7) | 2 (12.5) |
| Sarcoma | 0 | 0 | 1 (16.7) | 1 (6.3) |
| Duration of disease, months‡ | ||||
| Median (range) | 45.6 (2.4–61.4) | 66.3 (32.5–90.4) | 48.5 (25.5–102.5) | 49.6 (2.4–102.5) |
| ECOG performance status | ||||
| 0 | 3 (100.0) | 7 (100.0) | 5 (83.3) | 15 (93.8) |
| 1 | 0 | 0 | 1 (16.7) | 1 (6.3) |
| Metastatic site | ||||
| Lung | 2 (66.7) | 2 (28.6) | 3 (50.0) | 7 (43.8) |
| Liver | 1 (33.3) | 6 (85.7) | 3 (50.0) | 10 (62.5) |
| Lymph nodes | 1 (33.3) | 2 (28.6) | 3 (50.0) | 6 (37.5) |
| Peritoneal | 1 (33.3) | 1 (14.3) | 2 (33.3) | 4 (25.0) |
| Pleural | 1 (33.3) | 0 | 0 | 1 (6.3) |
| Other | 0 | 3 (42.9) | 3 (50.0) | 6 (37.5) |
| Prior disease-related therapy | ||||
| Chemotherapy§ | 2 (66.7) | 7 (100.0) | 6 (100.0) | 15 (93.8) |
| Other¶ | 0 | 2 (28.6) | 1 (16.7) | 3 (18.8) |
| Missing | 1 (33.3) | 0 | 0 | 1 (6.3) |
| Prior disease-related radiotherapy | ||||
| Yes | 0 | 0 | 1 (16.7) | 1 (6.3) |
| No | 3 (100.0) | 7 (100.0) | 4 (66.7) | 14 (87.5) |
| Missing | 0 | 0 | 1 (16.7) | 1 (6.3) |
| Prior disease-related surgery | ||||
| Yes | 2 (66.7) | 6 (85.7) | 6 (100.0) | 14 (87.5) |
| No | 0 | 1 (14.3) | 0 | 1 (6.3) |
| Missing | 1 (33.3) | 0 | 0 | 1 (6.3) |
Not coded and was presented as reported.
Duration of disease is time (in months) from date of histologic/cytologic confirmation of advanced solid tumor to date of first dose. If the day of first confirmation of cancer is unknown, it was replaced by 15MMMYYYY.
Includes agents such as cetuximab, sunitinib, imatinib, aflibercept, and bevacizumab.
Other than chemotherapy, hormonal therapy, immunotherapy, and biologic therapy. ECOG, Eastern Cooperative Oncology Group.
Dose
The median duration of treatment was 13.1 (range 7.0–13.6), 6.0 (range 3.0–13.4), and 7.0 (range 7.0–25.1) weeks in cohort 1 (n = 3), cohort 2 (n = 7), and cohort 3 (n = 6), respectively. The median number of infusions was 8.0 (range 4.0–8.0), 3.0 (range 2.0–6.0), and 4.0 (range 4.0–16.0) in cohort 1, cohort 2, and cohort 3, respectively. The median relative dose intensity was >85% in all three cohorts.
Safety
There were no DLTs in this trial; therefore, the MTD was not reached, consistent with the previous phase I trial.(14) One patient experienced an AE that met DLT definitions (grade 3 olaratumab-related tumor hemorrhage), but this event occurred outside the DLT assessment period (patient discontinued treatment prior to the completion of cycle 1 because, in the investigator's opinion, continued treatment was inappropriate); thus, the event was not considered a DLT.
There were four dose delays; two occurring in cohort 2 and 1 each occurring in cohorts 1 and 3. Two dose delays were caused by AEs in cohort 2 (grade 1 olaratumab-related proteinuria) and cohort 3 (fatigue/anorexia/weight loss). There were no infusion interruptions. No AE led to treatment discontinuation.
All patients experienced at least one AE of any grade. Across all cohorts and cycles, the most frequently reported treatment-emergent adverse events (TEAEs) regardless of causality were pyrexia (4 [25.0%]), proteinuria (4 [25.0%]), constipation (3 [18.8%]), and anorexia (3 [18.8%]). During cycle 1, the most frequently reported TEAEs regardless of causality were pyrexia (4 [25.0%]), constipation (3 [18.8%]), and proteinuria (3 [18.8%]).
Table 2 shows TEAEs that were assessed as olaratumab-related occurring through all cycles. The most common olaratumab-related TEAEs were proteinuria (4 [25.0%]) and elevated aspartate aminotransaminase (2 [12.5%]). One patient (cohort 2) had two grade 3 olaratumab-related AEs (i.e., increased aspartate aminotransferase and tumor hemorrhage); both AEs occurred in cycle 1.
Table 2.
| Number of patients, n (%) |
|||
|---|---|---|---|
| Preferred term | Cohort 1 (10 mg/kg) n = 3 | Cohort 2 (20 mg/kg) n = 7 | Cohort 3 (15 mg/kg) n = 6 |
| Patients with any AE | 1 (33.3) | 6 (85.7) | 1 (16.7) |
| Hematologic | |||
| Anemia | 0 | 1 (14.3) | 0 |
| Leukopenia | 0 | 1 (14.3) | 0 |
| Non-hematologic | |||
| Aspartate aminotransferase increased | 0 | 2 (28.6) | 0 |
| Cough | 1 (33.3) | 0 | 0 |
| Dermatitis | 0 | 0 | 1 (16.7) |
| Diarrhea | 0 | 1 (14.3) | 0 |
| Fatigue | 0 | 1 (14.3) | 0 |
| Fibrin D-dimer increased | 0 | 1 (14.3) | 0 |
| Hyperglycemia | 0 | 1 (14.3) | 0 |
| Hypertension | 0 | 1 (14.3) | 0 |
| Proteinuria | 0 | 3 (42.9) | 1 (16.7) |
| Rash | 0 | 1 (14.3) | 0 |
| Tumor hemorrhage | 0 | 1 (14.3) | 0 |
For each preferred term, each patient is counted only once per preferred term.
AEs with missing relationship to study drug were considered as related. AE, adverse event.
Two serious AEs, both occurring in cycle 1, were reported during the trial (malignant neoplasm and tumor hemorrhage). The tumor hemorrhage was considered by the investigator to be olaratumab-related. There were no patient deaths due to AEs on study or within 30 days of the last olaratumab dose. One patient (cohort 3) died due to PD, approximately 2 months after the patient's last olaratumab dose.
Efficacy
The best overall response was SD (Table 3). The disease control rate (CR+PR+SD) was 66.7% in cohort 1, 42.9% in cohort 2, and 33.3% in cohort 3. The median duration of SD was 2.8 months in cohort 1 and cohort 2, and 4.9 months in cohort 3.
Table 3.
Efficacy of olaratumab
| Cohort 1 (10 mg/kg) n = 3 | Cohort 2 (20 mg/kg) n = 7 | Cohort 3 (15 mg/kg) n = 6 | |
|---|---|---|---|
| Best overall tumor response, n (%) | |||
| CR | 0 | 0 | 0 |
| PR | 0 | 0 | 0 |
| SD | 2 (66.7)† | 3 (42.9)‡ | 2 (33.3)§ |
| PD | 1 (33.3) | 3 (42.9) | 4 (66.7) |
| NE | 0 | 1 (14.3) | 0 |
| Objective response rate (CR+PR), % | 0.0 | 0.0 | 0.0 |
| Disease control rate (CR+PR+SD), % | 66.7 | 42.9 | 33.3 |
| 95% CI¶ | 9.4–99.2 | 9.9–81.6 | 4.3–77.7 |
| Duration of SD, n (%) | |||
| Median, months | 2.8 | 2.8 | 4.9 |
| 95% CI | – | 2.8–N/A | 4.2–5.6 |
Carcinoid tumor of rectum; parotid tumor.
Colon cancer; gastrointestinal stromal tumor; rectal.
Hypopharyngeal cancer; leiomyosarcoma of inferior vena cava origin.
Binomial exact confidence interval.
CI, confidence interval; CR, complete response; N/A, not attainable; NE, not evaluable; PD, progressive disease; PR, partial response; SD, stable disease.
Of the seven patients with a best response of SD, two patients in cohort 3 experienced disease stabilization >4 months; these patients had hypopharyngeal cancer (4.2 months) and leiomyosarcoma of inferior vena cava origin (5.6 months). The others experienced disease stabilization that lasted approximately 2.8 months each. Figure 1 shows time on treatment for each patient.
Figure 1.
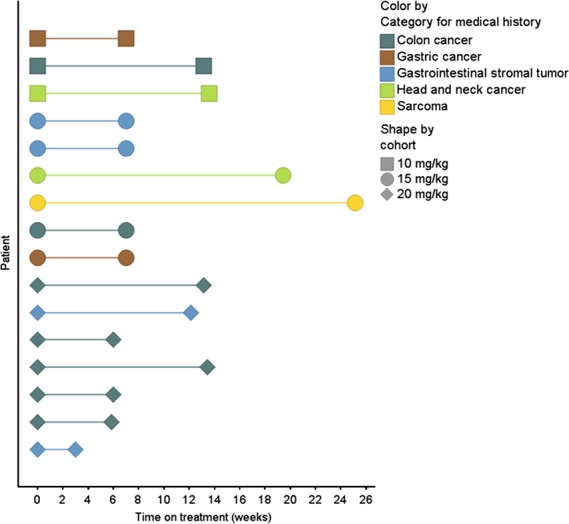
Time on treatment. The duration of treatment for each patient is shown.
Pharmacokinetics
Non-compartmental PK analysis was conducted for three patients from cohort 1, six patients from cohort 2, and six patients from cohort 3; one patient was excluded from PK analysis due to a dosing error (protocol deviation). The mean serum concentration versus time profiles following the first and multiple doses of olaratumab infusion are shown in Figure 2(a,b) respectively. The second peak, occurring at approximately 169 h for the 10 mg/kg (cohort 1) and 15 mg/kg (cohort 3) dose groups, is associated with the second infusion of olaratumab given on day 8 (168 h).
Figure 2.
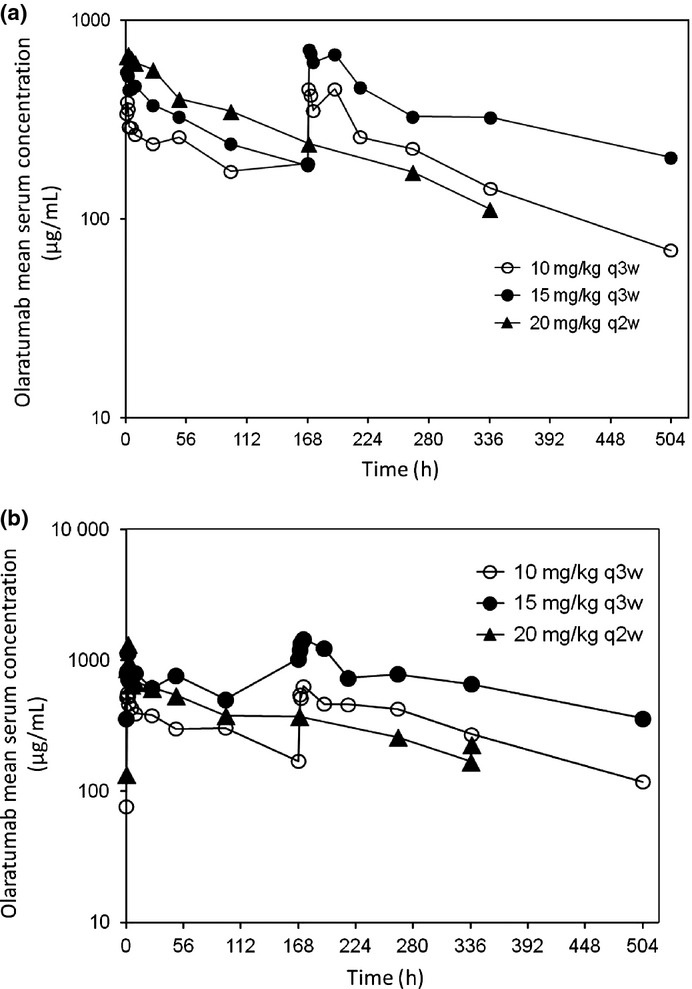
Arithmetic mean olaratumab serum concentration versus time profiles following the first dose (a) and multiple (b) doses of olaratumab. Semi-log scales are shown in each plot. h, hour; q2w, every 2 weeks; q3w, every 3 weeks.
The PK parameters following the first infusion and multiple infusions of olaratumab at 10 mg/kg q3w (cohort 1), 15 mg/kg q3w (cohort 3), and 20 mg/kg q2w (cohort 2) are summarized in Table 4. After a single infusion, PK parameters, including area under the serum concentration versus time curve from zero to infinity (AUC(0–∞)), total body clearance of drug calculated after intravenous administration (CL), and terminal phase volume (Vz), were not calculated for the 10 mg/kg (cohort 1) and 15 mg/kg (cohort 3) dose groups; the terminal elimination t1/2 was calculated following day 8 infusion because of the unique dosing schedules of these cohorts (patients received first infusion on day 1 and second infusion on day 8 q3w). The individual terminal elimination t1/2 following the first and multiple doses ranged from 4.42 to 9.38 days and 4.06 to 8.83 days, respectively, across all dose groups and dosing schedules. Due to the relatively short PK sampling time (336 h) post end of infusion, the true terminal elimination phase may not have been completely captured and accurately estimated. Therefore, t1/2 and its associated parameters, including AUC(0–∞) and CL, should be interpreted with caution. The olaratumab maximum observed serum drug concentration (Cmax) following the first infusion appeared to increase with dose.
Table 4.
Summary of olaratumab pharmacokinetic parameters
| Geometric mean (CV%)† |
|||
|---|---|---|---|
| Regimen | 10 mg/kg (N = 3)‡,§ | 15 mg/kg (N = 6)§ | 20 mg/kg (N = 6) |
| q3w | q3w | q2w | |
| After the first dose | |||
| Cmax (μg/mL) | 362.322; 436.172 | 587 (40) | 735 (29)¶ |
| tmax (h)†† | 1.20; 1.73 | 1.45 (1.18–9.14) | 2.22 (1.27–3.28)¶ |
| Clast (μg/mL) | 203.320; 176.762 | 173 (46) | 110 (19) |
| AUC(0–168) (μg/h/mL) | NC | 48 000 (47)§§ | 63 400 (21) |
| AUC(0–tlast) (μg/h/mL) | 35 500; 35 600 | 43 600 (45) | 92 500 (20)‡‡ |
| AUC(0–∞) (μg/h/mL) | NC | NC | 126 000 (12)¶ |
| t1/2 (days)‡‡ | 5.33; 6.38 | 7.29 (6.04–9.38)¶¶ | 6.42 (4.42–8.00)¶ |
| CL (mL/h/kg) | NC | NC | 0.159 (12)¶ |
| Regimen | Geometric mean (CV%)† |
||
|---|---|---|---|
| 10 mg/kg (N = 3)††† | 15 mg/kg (N = 6)†††,‡‡‡ | 20 mg/kg (N = 6)¶¶ | |
| q3w | q3w | q2w | |
| After multiple doses | |||
| Cmax (μg/mL) | 658.391; 546.854§§§ | 920.832§§§ | 1160 (91) |
| tmax (h)‡‡ | 1.74; 2.21§§§ | 2.18§§§ | 2.21 (1.70–3.30) |
| Clast (μg/mL) | 151.101; 121.188 | 360.948 | 181 (37) |
| AUC(0–168) (μg/h/mL) | 53 500; 44200 | 82 800 | 77 400 (30) |
| AUCτ (μg/h/mL) | NC | NC | 123 000 (29)¶¶¶ |
| t1/2 (days)‡‡‡ | 4.06; 7.33†††† | 8.25†††† | 7.33 (5.42–8.83) |
| CLss (mL/h/kg) | NC | NC | 0.163 (29) |
| RA (AUC)‡‡‡‡ | 1.55 | 1.38 | 1.46 (15) |
The single value is reported when n = 1; values are separated by semicolon when n = 2.
n = 2 for all parameters. One patient, whose samples were not collected for the initial 168 h, was excluded from PK analysis.
Cmax, Clast, AUC0–168, and AUC0–tlast are calculated following the first infusion (day 1) and t1/2 is calculated following the second infusion (day 8) in day-1 and day-8 dosing in 21-day cycles (q3w).
n = 5.
Median (range).
Geometric mean (range).
n = 4.
n = 3.
Patient received first infusion on day 1 and second infusion on day 8 in 21-day cycles (q3w).
n = 1 for all parameters.
Cmax, tmax, and AUC(0–168) are calculated following the first infusion (day 1) in day 1 and day 8 dosing in 21-day cycles (q3w).
Dosing interval (τ) is 336 h.
t1/2 is calculated following the second infusion (day 8) in day 1 and day 8 dosing in 21-day cycles (q3w).
Intercycle accumulation of olaratumab calculated as AUC(0–504) (Cycle 2)/AUC(0–504) (Cycle 1) for 10 and 15 mg/kg (q3w) and AUC(0–336) (Cycle 2)/AUC(0–336) (Cycle 1) for 20 mg/kg (q2w). AUC(0–168), area under the concentration versus time curve from zero to 168 h; AUC(0–336), area under the concentration versus time curve from zero to 336 h; AUC(0–504), area under the concentration versus time curve from zero to 504 h; AUC(0–∞), area under the serum concentration versus time curve from zero to infinity; AUC(0–tlast), area under the concentration versus time curve from zero to time t, where t is the last scheduled sampling time point with a measurable drug concentration; AUCτ, area under the concentration versus time curve during one dosing interval; Clast, last quantifiable serum drug concentration; Cmax, maximum observed serum drug concentration; CL, total body clearance of drug calculated after intravenous administration; CLss, total body clearance of drug calculated after intravenous administration at steady state; CV, coefficient of variation; N, number of patients with assessable PK; NC, not calculated; PK, pharmacokinetic; q2w, every 2 weeks; q3w, every 3 weeks; RA, accumulation ratio; t1/2, terminal elimination half-life; tmax, time of maximal concentration.
Individual serum concentration-time profiles exhibited a multi-phasic decline (data not shown). Following the multiple doses (fifth dose for the 10 mg/kg [cohort 1] and 15 mg/kg [cohort 3] dose groups and fourth dose for the 20 mg/kg [cohort 2] dose group), individual serum concentrations were higher than the first dose, reflecting some accumulation of olaratumab following multiple infusions (individual patient accumulation ratio, calculated using AUC [RA, AUC] ranged from 1.30 to 1.72) (data not shown).
Following multiple infusions of olaratumab at 10 mg/kg q3w and 20 mg/kg q2w, the geometric mean trough concentrations (Clast) were close to or above the target trough concentration (155 μg/mL) associated with antitumor activity in preclinical xenograft studies.(14) However, olaratumab infusion at 15 mg/kg q3w generated geometric mean pre-dose serum concentrations above 155 μg/mL (target trough concentration) throughout the study (Table 4).
Comparative analyses of clearance (steady state clearance; CLss) and exposure (area under the concentration versus time curve during one dosing interval; AUCτ), following multiple infusions of olaratumab 20 mg/kg q2w, were conducted between this study of Asian patients and the US phase 1 study of non-Asian patients.(14) The results of this analysis are presented in Figure 3. As shown in the figure, the PK parameters CLss and AUCτ appear to be comparable between Asian and non-Asian patients. However, due to the small sample size, a statistical analysis was not conducted.
Figure 3.
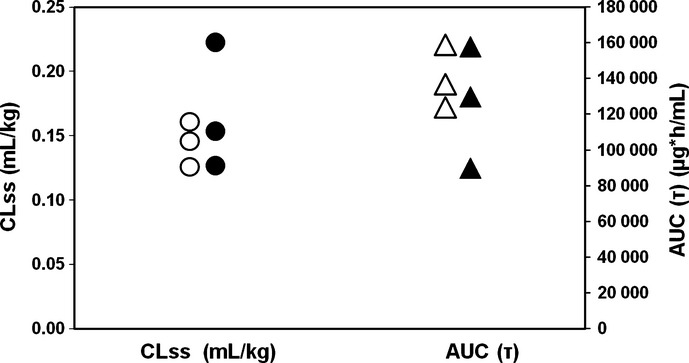
Comparison of PK parameters clearance and exposure between non-Asian and Asian patients following multiple infusions of olaratumab. Shown are CLss (circles) and AUCτ (triangles) for the 20 mg/kg-every-2-weeks groups. White circles (○) = CLss, non-Asian patients; black circles (•) = CLss, Asian patients; white triangles (▵) = AUCτ, non-Asian patients; black triangles (▴) = AUCτ Asian patients. AUCτ, area under the concentration versus time curve during one dosing interval; CLss, total body clearance of drug calculated after intravenous administration at steady state.
Circulating biomarkers
For PDGF-BB, all samples were below the limit of quantitation, so no further analysis was performed.
Prior to the initial olaratumab dose, the median PDGF-AA expression was 11.30 ng/mL for cohort 1, 11.00 ng/mL for cohort 2, and 17.35 ng/mL for cohort 3. Until 24 h following the first infusion, the median PDGF-AA expression increased to 42.15, 62.35, and 48.18 ng/mL for cohort 1, cohort 2, and cohort 3, respectively. However, no trend was identified for the biomarker level change over time for any cohort at later time points. When analyzed by patient, the best overall responses did not seem to be related to the largest change from baseline in PDGF-AA (data not shown).
Discussion
Inhibitors of the PDGF/PDGFR axis are being sought as anti-cancer agents.(11,12) Most of these agents are small molecule TKIs that inhibit multiple kinases and have complex toxicities.(11,12,17,18) Monoclonal antibodies specifically targeting PDGFR are expected to offer an advantage in terms of specificity and minimizing AEs.
This is the first report of the use of olaratumab, a fully human IgG1 monoclonal antibody that selectively binds human PDGFRα,(13) in Japanese cancer patients. As an IgG1 antibody, olaratumab has the potential to induce antibody-dependent cellular cytotoxicity;(19) however, this has not been experimentally tested. This report follows an earlier report of a phase I trial conducted in the United States.(14) In the current report, 16 Japanese patients with advanced solid tumors, who had not responded to standard therapy or for whom no standard therapy was available, were treated with olaratumab in an open-label, dose-escalation, phase 1 trial. This study met its objectives to establish the safety and PK profile of olaratumab.
In this trial, most AEs were mild to moderate in severity. The most frequently reported olaratumab-related AEs were proteinuria (25.0%) and increased aspartate aminotransferase (12.5%). These AEs were distributed across the three cohorts, and thus did not appear to be dose-related. There were only two grade 3 olaratumab-related non-laboratory AEs (elevated aspartate transaminase and tumor hemorrhage) during the trial, both occurring in cycle 1 and in the same patient. No infusion reactions or interruptions were reported and the majority of patients in the safety population received a relative dose intensity of at least 80%.
In this trial, no fluid retention, ascites, or edemas were reported. The PDGFR may be involved in the control of interstitial fluid pressure through PDGF-BB.(20) The use of small molecule multi-kinase TKIs that inhibit PDGFR is sometimes associated with fluid retention,(17,18) and blockade of PDGFRβ in cancer patients by a humanized, pegylated di-Fab was associated with ascites and fluid retention.(21) Fluid retention was not observed in our trial with selective PDGFRα blockade, even in patients with prolonged exposure (up to 25 weeks). This observation supports the hypothesis that PDGFRα is less likely to be involved in the fluid retention observed with nonspecific PDGFR blockade by small molecules or with a selective PDGFRβ blockade.
In this trial, there were no DLTs and the MTD was not reached, which is consistent with the previous US trial.(14) Over the three dose ranges, olaratumab had an acceptable safety profile and was well tolerated in this patient population.
Based on the PK concentration profile of olaratumab, the trough concentrations following single and multiple doses of olaratumab at 15 mg/kg on days 1 and 8 every 3 weeks (cohort 3), and multiple doses at 20 mg/kg every 2 weeks (cohort 2), were above 155 μg/mL, the concentration that was efficacious in preclinical xenograft studies.(14) Thus, olaratumab dosed at 15 mg/kg on days 1 and 8 every 3 weeks and at 20 mg/kg every 2 weeks could represent an acceptable schedule for future trials in Japanese patients. Based on the comparative analysis of both the clearance (CLss) and exposure (AUCτ), the observed PK in the Asian patient population appears to be similar to the non-Asian patient population observed in the previous US phase I trial.(14)
The best overall response in this trial was SD, achieved by 7 of 16 patients (43.8%). Of these seven patients, four had tumors that were located in the gastrointestinal tract; the remaining three patients had tumors of diverse origins. Two patients, both in cohort 3, experienced disease stabilization >4 months (hypopharyngeal cancer [SD = 4.2 months]) and leiomyosarcoma of inferior vena cava origin [SD = 5.6 months]), which indicates some preliminary antitumor activity.
In nude mice, treatment with olaratumab inhibited the growth of human glioblastoma (U118) and leiomyosarcoma (SKLMS-1) xenografts and decreased the amount of tumor-associated phosphotyrosyl-PDGFRα in the glioblastoma model.(13) In cultured cells, olaratumab inhibited PDGF-induced mitogenesis, PDGFRα autophosphorylation, and the phosphorylation of downstream signaling molecules. At this time, it is not known if the same changes occur in patient tumors or whether pharmacologically active concentrations can be achieved in human tumor tissue. Our trial showed that the median plasma PDGF-AA expression increased for 24 h after the first infusion in all three cohorts, but no trend was noted at later time points and best overall responses seemed unrelated to the largest change in PDGF-AA from baseline. Because biomarker studies were not performed in the US trial, comparisons cannot be made with this trial; nonetheless, an increase in PDGF-AA may be a compensatory mechanism that results from PDGFRα inhibition and/or sequestration.
Based on its safety and preliminary efficacy in this trial and the previous phase 1 trial,(14) olaratumab has advanced to phase II trials. Olaratumab is being tested as monotherapy and in combination with other agents in several tumor types.
Acknowledgments
This trial was supported by ImClone Systems, a wholly-owned subsidiary of Eli Lilly and Company. The authors wish to acknowledge the patients, their families, and the study personnel who participated in this clinical trial. The authors thank Lori Kornberg (inVentiv Health Clinical) and Susan Johnson (ImClone Systems, a wholly-owned subsidiary of Eli Lilly and Company) for medical writing assistance, and Joseph Durrant (inVentiv Health Clinical) for editorial support.
Disclosure Statement
Aruna Dontabhaktuni is an employee of ImClone Systems, a wholly-owned subsidiary of Eli Lilly and Company and owns stock in Eli Lilly and Company. Cornelia Nippgen is an employee of Eli Lilly and Company. Johannes Nippgen and Yan Ma were employed by ImClone Systems, a wholly-owned subsidiary of Eli Lilly and Company, during the conduct of this trial. Toshihiko Doi and Atsushi Ohtsu report no conflicts of interest.
References
- 1.Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev. 1999;79:1283–316. doi: 10.1152/physrev.1999.79.4.1283. [DOI] [PubMed] [Google Scholar]
- 2.Dong J, Grunstein J, Tejada M, et al. VEGF-null cells require PDGFR alpha signaling-mediated stromal fibroblast recruitment for tumorigenesis. EMBO J. 2004;23:2800–10. doi: 10.1038/sj.emboj.7600289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams SF, Hickson JA, Hutto JY, Montag AG, Lengyel E, Yamada SD. PDGFR-alpha as a potential therapeutic target in uterine sarcomas. Gynecol Oncol. 2007;104:524–8. doi: 10.1016/j.ygyno.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 4.Fleming TP, Saxena A, Clark WC, et al. Amplification and/or overexpression of platelet-derived growth factor receptors and epidermal growth factor receptor in human glial tumors. Cancer Res. 1992;52:4550–3. [PubMed] [Google Scholar]
- 5.Matei D, Emerson RE, Lai YC, et al. Autocrine activation of PDGFRalpha promotes the progression of ovarian cancer. Oncogene. 2006;25:2060–9. doi: 10.1038/sj.onc.1209232. [DOI] [PubMed] [Google Scholar]
- 6.Stock P, Monga D, Tan X, Micsenyi A, Loizos N, Monga SP. Platelet-derived growth factor receptor-alpha: a novel therapeutic target in human hepatocellular cancer. Mol Cancer Ther. 2007;6:1932–41. doi: 10.1158/1535-7163.MCT-06-0720. [DOI] [PubMed] [Google Scholar]
- 7.Carvalho I, Milanezi F, Martins A, Reis RM, Schmitt F. Overexpression of platelet-derived growth factor receptor alpha in breast cancer is associated with tumour progression. Breast Cancer Res. 2005;7:R788–95. doi: 10.1186/bcr1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chott A, Sun Z, Morganstern D, et al. Tyrosine kinases expressed in vivo by human prostate cancer bone marrow metastases and loss of the type 1 insulin-like growth factor receptor. Am J Pathol. 1999;155:1271–9. doi: 10.1016/S0002-9440(10)65229-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henriksen R, Funa K, Wilander E, Backstrom T, Ridderheim M, Oberg K. Expression and prognostic significance of platelet-derived growth factor and its receptors in epithelial ovarian neoplasms. Cancer Res. 1993;53:4550–4. [PubMed] [Google Scholar]
- 10.Sulzbacher I, Birner P, Traxler M, Marberger M, Haitel A. Expression of platelet-derived growth factor-alpha alpha receptor is associated with tumor progression in clear cell renal cell carcinoma. Am J Clin Pathol. 2003;120:107–12. doi: 10.1309/LQ9E-MK8Q-KE75-NGGX. [DOI] [PubMed] [Google Scholar]
- 11.Dai Y. Platelet-derived growth factor receptor tyrosine kinase inhibitors: a review of the recent patent literature. Expert Opin Ther Pat. 2010;20:885–97. doi: 10.1517/13543776.2010.493559. [DOI] [PubMed] [Google Scholar]
- 12.Levitzki A. PDGF receptor kinase inhibitors for the treatment of PDGF driven diseases. Cytokine Growth Factor Rev. 2004;15:229–35. doi: 10.1016/j.cytogfr.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 13.Loizos N, Xu Y, Huber J, et al. Targeting the platelet-derived growth factor receptor alpha with a neutralizing human monoclonal antibody inhibits the growth of tumor xenografts: implications as a potential therapeutic target. Mol Cancer Ther. 2005;4:369–79. doi: 10.1158/1535-7163.MCT-04-0114. [DOI] [PubMed] [Google Scholar]
- 14.Chiorean EG, Sweeney C, Youssoufian H, et al. A phase I study of olaratumab, an anti-platelet–derived growth factor receptor alpha (PDGFRα) monoclonal antibody, in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2014;73:595–604. doi: 10.1007/s00280-014-2389-9. [DOI] [PubMed] [Google Scholar]
- 15.National Cancer Institute. Cancer therapy evaluation program common terminology criteria for adverse events, version 4.0. Available from URL: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf.
- 16.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 17.Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–80. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 18.Faivre S, Delbaldo C, Vera K, et al. Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol. 2006;24:25–35. doi: 10.1200/JCO.2005.02.2194. [DOI] [PubMed] [Google Scholar]
- 19.Adams GP, Weiner LM. Monoclonal antibody therapy of cancer. Nat Biotechnol. 2005;23:1147–57. doi: 10.1038/nbt1137. [DOI] [PubMed] [Google Scholar]
- 20.Rodt SA, Ahlen K, Berg A, Rubin K, Reed RK. A novel physiological function for platelet-derived growth factor-BB in rat dermis. J Physiol. 1996;495:193–200. doi: 10.1113/jphysiol.1996.sp021584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jayson GC, Parker GJ, Mullamitha S, et al. Blockade of platelet-derived growth factor receptor-beta by CDP860, a humanized, PEGylated di-Fab', leads to fluid accumulation and is associated with increased tumor vascularized volume. J Clin Oncol. 2005;23:973–81. doi: 10.1200/JCO.2005.01.032. [DOI] [PubMed] [Google Scholar]


