Abstract
Primary cutaneous γδ T-cell lymphoma (PCGD-TCL) is an aggressive lymphoma consisting of clonal proliferation of mature activated γδ T-cells of a cytotoxic phenotype. Because primary cutaneous γδ T-cell lymphoma is a rare disease, there are few clinicopathological studies. In addition, T-cell receptor (TCR) γδ cells are typically immunostained in frozen sections or determined by TCRβ negativity. We retrospectively analyzed 17 primary cutaneous T-cell lymphomas of the γδ phenotype (CTCL-γδ) in a clinicopathological and molecular study using paraffin-embedded sections. Among 17 patients, 11 had CTCL-γδ without subcutaneous panniculitis-like T-cell lymphoma (SPTCL) features and six had CTCL-γδ with SPTCL features. Immunophenotypically, some significant differences were found in CD8 and CD56 positivity between our patient series of CTCL-γδ patients with SPTCL features and SPTCL-γδ patients described in the previous literature. A univariate analysis of 17 CTCL-γδ patients showed that being more than 60 years old, presence of visceral organ involvement, and small-to-medium cell size were poor prognostic factors. In addition, the 5-year overall survival rate was 42.4% for the CTCL-γδ patients without SPTCL features and 80.0% for those with SPTCL features. Consequently, there was a strikingly significant difference in overall survival among SPTCL, CTCL-γδ with SPTCL features and CTCL-γδ without SPTCL features (P = 0.0005). Our data suggests that an indolent subgroup may exist in CTCL-γδ. Studies on more cases, including those from other countries, are warranted to delineate the clinicopathological features and the significance in these rare lymphomas.
Keywords: Cutaneous γδ T-cell lymphoma, indolent clinical behavior, subcutaneous panniculitis-like T-cell features, T-cell receptor αβ, T-cell receptor γδ
Primary cutaneous T-cell lymphoma (CTCL) is currently defined as a heterogeneous group of lymphoproliferative disorders characterized by localization of neoplastic T-cells in skin. Primary cutaneous γδ T-cell lymphoma (PCGD-TCL) is derived from mature activated cytotoxic γδ T-cells.(1) Whereas CTCL of the γδ phenotype (CTCL-γδ) have poorer prognosis and follow a more aggressive clinical course than those for CTCL of the αβ phenotype (CTCL-αβ),(2) the World Health Organization–European Organization for Research and Treatment of Cancer 2005 classified CTCL-γδ as “provisional,”(3) and in 2008 PCGD-TCL was categorized by the WHO as a rare subtype of CTCL.(1) This group includes subcutaneous panniculitis-like T-cell lymphoma (SPTCL) of the γδ phenotype for the same reason. This feature is very specific to cutaneous tissue because hepatosplenic T-cell lymphoma and enteropathy-associated T-cell lymphoma (EATL) have poor prognosis irrespective of T-cell receptor (TCR) phenotype.(4–6) Therefore, it is becoming increasingly important to classify CTCL as either αβ T-cells or γδ T-cells.
Although TCRγδ is a strong prognostic factor of decreased survival,(2) testing of tumor cells for TCRβ negativity and expression of CD56, or sometimes additional testing for TCRδ1 positivity in frozen sections has helped to identify the TCRγδ phenotype in paraffin-embedded tissue.(2,7) Recently, it was found that staining of γδ T-cells could be performed in formalin-fixed paraffin-embedded sections.(8,9) One report described cases of indolent CTCL-γδ localized to the subcutis,(10) but only TCRβ negativity was checked to determine TCRγδ phenotype. Hence, it is still unknown whether indolent CTCL-γδ actually exists. Because few clinicopathological studies have reported on PCGD-TCL, we conducted a retrospective study of 17 CTCL-γδ patients from Japan and examined further studies for clinicopathological, immunophenotypical and immunogenotypical analysis.
Materials and Methods
Patient selection and clinical data
Histological samples for 47 patients were available from 76 consecutive patients (from 1997 to 2010) selected from the consultation files of the Department of Clinical Pathophysiology/Clinical Pathology, Nagoya University Graduate School of Medicine. Histological samples for 55 patients were available from 89 consecutive patients (from 1989 to 2011) selected from the consultation files of the Department of Pathology, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences. Four faculty pathologists (TY, SN, KT and SK) practicing in an active lymphoma pathology service who had varying degrees of experience in lymphoma pathology reviewed the records. Of the 102 CTCL cases, 68 were classified as CTCL-αβ, 17 were classified as CTCL-γδ as per TCRαβ and TCRγδ immunostaining, and 17 were indeterminable because of exhausted tissue samples. In addition, of the 68 patients classified as CTCL-αβ, 15 had SPTCL; of these 15, clinical data records were available for 13 and these 13 patients were enrolled in the study.
Data on age, sex, clinical presentation, staging, therapy and clinical outcome were obtained by review of the medical records and from follow-up information provided by the patients’ physicians and pathologists. The present study was conducted the with approval of the Institutional Review Board of Okayama University, Okayama, Japan and the Institutional Review Board of Nagoya University, Nagoya, Japan. Written informed consent in accordance with the Helsinki protocol was obtained from each individual. We also statistically compared clinical and immunohistological data records of six CTCL-γδ patients with SPTCL features in the present series with those of 20 SPTCL-γδ patients described in the previous literature.(11)
Immunohistochemistry and in situ hybridization studies
Tissue samples were fixed in 10% formalin and embedded in paraffin. An automated BOND-MAX stainer (Leica Biosystems, Melbourne, Vic., Australia) was used to stain the paraffin sections. The following primary antibodies were used (clone, dilutions): CD20 (L26, 1:200), CD3 (LN10, 1:200), CD5 (4C7, 1:100), CD7 (56C6, 1:50), CD8 (C8/144B, 1:100), CD4 (1F6, 1:40), TIA-1 (2G9, 1:500; Beckman Coulter, Brea, CA, USA) and GranzymeB (GrB-7, ready to use) (Nichirei, Tokyo, Japan). Primary monoclonal antibodies raised against the human TCRβ (βF1; TCR1151, 8A3, 1:50 [Thermo Scientific, Waltham, MA, USA]) and TCR-γ chain constant region (TCR1153, γ3.20, 1:80 [Thermo Scientific]) were used to detect the αβ and γδ TCR, respectively.(12) In situ hybridization with Epstein–Barr virus (EBV)-encoded small RNA (EBER) probes (INFORM EBER; Leica Biosystems) was used to detect EBV.
The samples of CD20, CD3, CD10, CD5, CD7, CD8, CD4, TIA-1,(13) granzyme B(14,15) and EBER antigens were scored as positive when ≥30% of the lymphoma cells were positively stained. The samples of TCR γ, TCR δ and TCR β antigens were scored as positive when ≥50% of the lymphoma cells were positively stained.
DNA extraction, PCR amplification and analysis of PCR products
A sample 5 mm in diameter was scratched from the tumor region of each specimen and dissolved in PCR Golden Buffer (Applied Biosystems, Foster City, CA, USA). An automated thermocycler GeneAmp PCR system 9700 (Applied Biosystems) was used at 94°C for 45 min to extract DNA. PCR reactions were performed according to the BIOMED-2 Concerted Action protocol.(16) Extracted DNA was denatured at 95°C for 7 min and followed by 35 cycles of denaturing at 95°C for 30 s, annealing at 60°C for 30 s, and extension at 72°C for 30 s, with a final extension at 72°C for 10 min. The DNA of the skin biopsy samples was amplified by 2 BIOMED-2 TCRγ PCR (sets A and B) and 3 TCRβ PCR (sets A, B and C) according to the original Biomed-2 report. Next, the ABI PRISM 310 Genetic Analyzer and Gene Mapper software version 3.7 (Applied Biosystems) were used to analyze 1 μL of each PCR product, as previously described. The peaks obtained were regarded as clonal bands when ≥1 discrete peaks within the intended size range were obtained. Peaks with a height of ≥3 times the third highest peak were considered to be positive.(16)
Statistical analysis
Overall survival was calculated from the date of histological diagnosis until the patient's death or last follow-up. The Kaplan–Meier method was used to estimate the survival curves, and the log rank test was used to perform statistical comparisons. Univariate analysis of prognostic factors, clinical features and immunohistological analyses were performed using Fisher's exact test. The Mann–Whitney U-test was used to analyze age and tumor size differences among the groups. A P-value of <0.05 was considered statistically significant. Statistical Product and Services Solution software (version 14.0; SPSS, Chicago, IL, USA) was used to perform all statistical analyses.
Results
Pathological findings
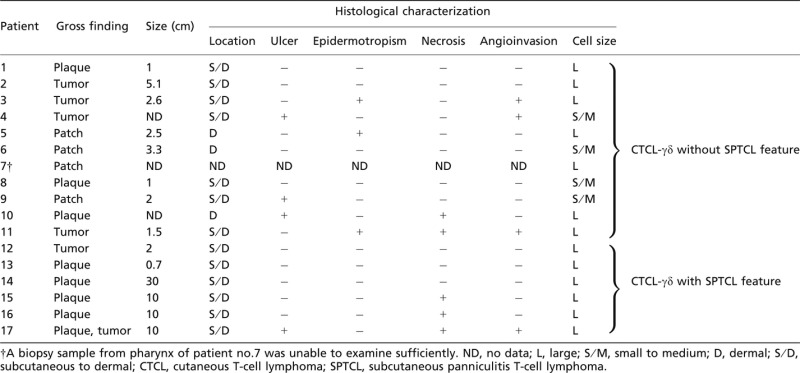
Of all 17 CTCL-γδ patient samples, six presented histological patterns similar to those of SPTCL and 11 presented histologically non-SPTCL features (Figs 1a,b, 2a). The macroscopic and microscopic features of 17 CTCL-γδ patient lesions are shown in Table 1. All six CTCL-γδ with SPTCL patients present as plaques while 11 CTCL-γδ without SPTCL patients present as patches, plaques and tumors. Of the six CTCL-γδ with STPCL features, karyorrhexis was observed in all patient samples, necrosis in three, and ulcer and vascular invasion of small blood vessels in one. Of the 11 samples from CTCL-γδ patients without SPTCL features, three presented both superficial and deep dermal lesions, and seven samples showed partial subcutaneous involvement. Ulcer, epidermotropism and angioinvasion were observed in three patient samples, and necrosis in two. The neoplastic cells were medium to large in size, with coarsely clumped chromatin deposition (Fig. 2a).
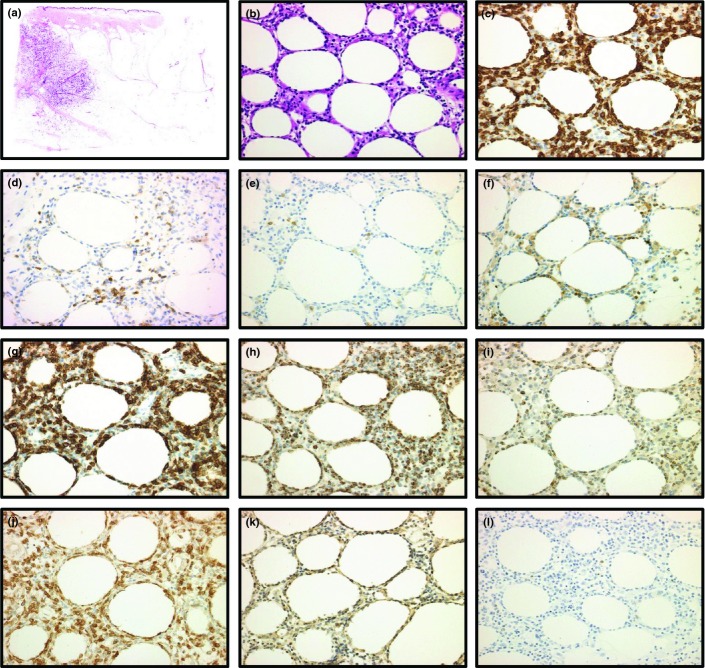
Figure 1.
Immunostainings of primary cutaneous T-cell lymphoma of the γδ phenotype with subcutanous panniculitis-like T-cell lymphoma (SPTCL) feature in patient no. 14 (a–l) (b–l: original magnification ×20 objective). (a) Low-power view showing subcutaneous infiltrates as in SPTCL (H&E). (b) High-power field of subcutaneous infiltrate reveals medium to large sized lymphoid cells with rimming of neoplastic cells (H&E). (c–l) Immunohistochemical results. The lymphoid cells were positive for CD3 (c), down expression of CD5 (d), negative for CD56 (e), negative for CD4 (f), positive for CD8 (g), positive for TIA-1 (h), positive for Granzyme B (i), positive for T-cell receptor (TCR) γ (j), positive for TCRδ (k) and negative for TCRβ (l).
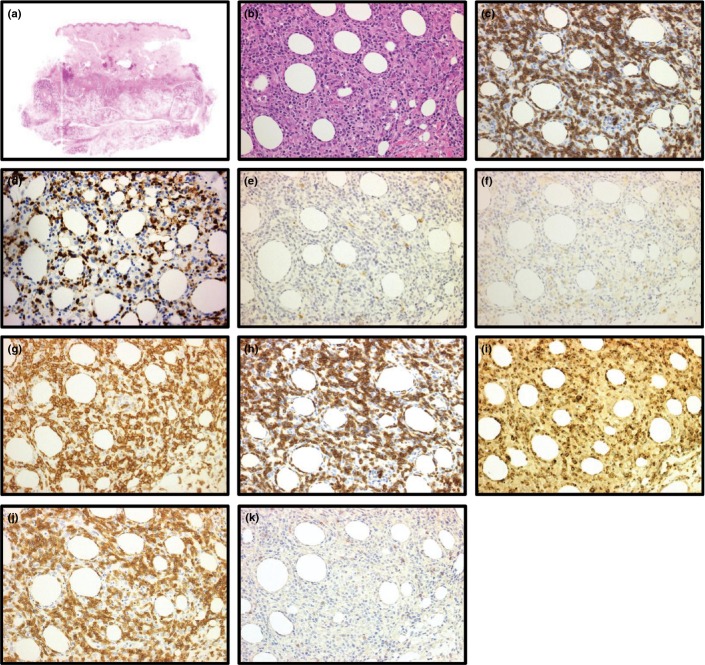
Figure 2.
Immunostainings of primary cutaneous T-cell lymphoma of the γδ phenotype without subcutanous panniculitis-like T-cell lymphoma feature in patient no. 4 (b–e: original magnification ×20 objective). (a) High-power field of cutaneous tumor reveals medium to large sized neoplastic cells infiltrating diffusely (H&E). (b–d) Immunohistochemical results. The lymphoid cells were positive for CD3 (b), positive for T-cell receptor (TCR) γ (c) and negative for TCRβ (d).
Table 1.
Macroscopic and microscopic feature of CTCL-γδ
 |
Immunohistochemical findings
A summary of the immunohistochemical results is shown in Table 2. On the basis of TCRαβ and TCRγδ immunostaining, 17 patients were classified as CTCL-γδ. One patient expressed both TCRαβ and TCRγδ. Figures 1j,k,l and 2c,d show the immunohistochemical results for TCRγ, TCRδ and TCRβ immunostaining. Among all 17 patient samples, CD3 expression was positive in all but one sample (Figs 1c, 2b). Ten CTCL-γδ samples showed decreased or negative CD5 expression (Fig. 1d). Cytotoxic molecules (TIA-1 or granzyme B) were positive in all patient samples except 1 (Fig. 1 h,i). CD56 expression was negative except in two patient samples (Fig. 1e). In addition, we demonstrated SPTCL, which was positive for TCRαβ and negative for TCRγδ (Fig. 3).
Table 2.
Immunophenotypic features of patients with CTCL-γδ without SPTCL features, CTCL-γδ with SPTCL features and SPTCL
| Patient no. | CD3 | CD4 | CD5 | CD7 | CD8 | CD20 | CD56 | CD79a | TIA-1 | GranzymeB | TCRαβ | TCRγδ | EBER-ISH |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | + | ND | + | ND | − | − | − | ND | + | ND | − | + | − |
| 2 | + | ND | − | ND | + | − | p+ | ND | + | ND | − | + | − |
| 3 | + | + | − | ND | − | − | − | ND | − | ND | − | + | − |
| 4 | + | ND | + | ND | p+ | − | − | − | + | ND | − | + | − |
| 5 | − | ± | − | ND | − | − | − | − | + | ND | − | + | − |
| 6 | + | + | + | − | − | − | − | ND | + | ND | − | + | − |
| 7 | + | ND | + | ND | p+ | − | − | ND | − | ND | − | + | − |
| 8 | + | ND | − | ND | − | − | − | ND | + | ND | − | + | − |
| 9 | + | − | − | − | + | − | − | − | + | − | − | + | − |
| 10 | + | + | + | − | − | − | − | ND | − | + | − | + | − |
| 11 | + | + | + | − | − | − | − | ND | − | + | − | + | − |
| 12 | + | − | Down | ND | p+ | − | − | − | + | + | − | + | − |
| 13 | + | − | Down | ND | + | − | − | − | + | + | + | + | − |
| 14 | + | − | Down | ND | + | − | − | − | + | + | − | + | − |
| 15 | + | p+ | Down | ND | p+ | − | p+ | ND | + | + | − | + | − |
| 16 | + | − | + | ND | − | − | − | ND | + | + | − | + | − |
| 17 | + | − | Down | ND | − | − | − | ND | + | + | − | + | − |
| 18 | + | UD | + | ND | − | − | − | − | ND | + | + | − | − |
| 19 | + | − | + | ND | p+ | − | UD | − | p+ | p+ | + | − | − |
| 20 | + | − | + | ND | + | − | − | − | − | + | + | − | − |
| 21 | + | UD | + | ND | + | − | − | − | + | + | + | − | − |
| 22 | + | − | + | ND | + | − | − | − | + | + | + | − | − |
| 23 | + | − | + | ND | + | − | − | ND | + | + | + | − | − |
| 24 | + | + | + | + | p+ | − | p+ | ND | + | p+ | + | − | − |
| 25 | + | + | + | ND | − | − | − | ND | + | + | + | − | − |
| 26 | + | − | + | ND | + | − | − | ND | + | + | + | − | − |
| 27 | + | UD | + | ND | p+ | − | p+ | ND | + | + | + | − | − |
| 28 | + | − | + | ND | + | − | − | ND | + | + | + | − | − |
| 29 | + | − | + | + | + | − | − | ND | + | + | + | − | − |
| 30 | + | − | + | ND | − | − | − | ND | + | − | + | − | − |
| 31 | + | − | + | ND | + | − | − | ND | + | + | + | − | − |
| 32 | + | − | + | ND | p+ | − | p+ | ND | + | + | + | − | − |
Down, down expression; ND, not done; p+, partially positive; UD, undetectable.
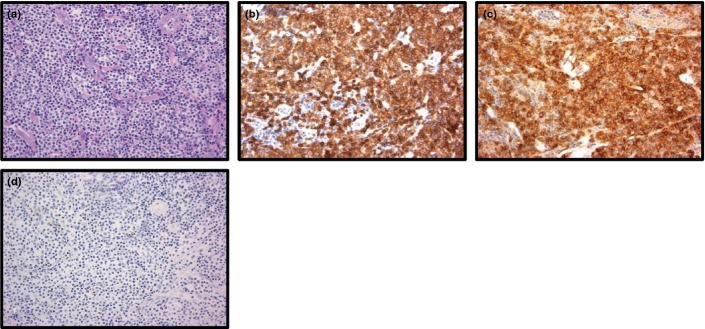
Figure 3.
Immunostainings of subcutanous panniculitis-like T-cell lymphoma in patient no. 18 (a–k) (b–k: original magnification ×20 objective). (a) Low-power view of a punch biopsy. Subcutaneous infiltration of neoplastic cells are seen (H&E). (b) High-power field reveals medium to large sized atypical lymphoid cells with rimming of neoplastic cells (H&E). (c–k) Immunohistochemical results. The lymphoid cells were positive for CD3 (c), positive for CD5 (d), negative for CD56 (e), negative for CD4 (f), positive for CD8 (g), positive for TIA-1 (h), positive for Granzyme B (i), positive for T-cell receptor (TCR) β (j) and negative for TCRγ (k).
Molecular studies
T-cell receptor gene rearrangement analyses were performed in 20/32 cases. PCR was used to analyze TCRγ and/or TCRβ gene rearrangements in 19 cases. TCRγ gene rearrangement was observed in eight cases, and TCRβ gene rearrangement in 10 (see Tables 3 and 4 and Table S1).
Table 3.
Clinical features and TCR rearrangements of patients with CTCL-γδ without SPTCL features
| Patient | Age/gender | Primary cutaneous/subsutaneous sites | Extracutaneous sites | Recurrent/relapse sites | TNM | Stage | B symptoms | HPS | Therapy | Follow-up (mo) | TCRγ gene rearrangement | TCRβ gene rearrangement |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 64/M | Head and neck, chest, abdomen, back, R arm, hand (bilateral), leg (bilateral) | Pharynx, L. axillary LN | R inguinal LN | T3bN0M1 | IV | + | − | CHOP, PR→THP-COP for relapse, PD →MINE+ESHAP, PD | DOD, 70 | ND | ND |
| 2 | 22/M | Head, R upper arm, leg(bilateral) | Pharynx | − | T3bN2M1 | IV | + | − | CHOP, steroid, MEPP, NC | DOD, 3 | ND | ND |
| 3 | 23/M | Neck, arm(bilateral), R leg | − | Skin/subcutis (submandible) | T3bN0M0 | IV | − | − | BACOP, CR→CHOP+RT for recurrence, CR→RT, CHOP-B+PBSCT for recurrence, CR→VP-16 for repeating recurrence | AWR, 259 | ND | ND |
| 4 | 79/M | Head and neck | L auricular LN, cervial and supraclavicular, axillary LN (bilateral), R inguinal LN | − | T3bN2M0 | IV | − | − | VP-16, PD | DOD, 8 | ND | ND |
| 5 | 75/M | L face, arm | − | − | T3bN0M0 | IV | − | − | VP-16, INF-γ, PUVA, PD→RT, PD→ACNU, clinical trial(Panobinostat), PD→THP-COP, PD | DOD, 65 | Clonal | Clonal |
| 6 | 72/M | Chest, abdomen and genital, back, arm and leg (bilateral) | Axillary and inguinal LN (bilateral), BM | − | T3bN2M1 | IV | − | − | CPA+UVB, PR | Died of B lymphoblastic leukemia/lymphoma, 28 | UD | Clonal |
| 7 | 54/F | Back, buttocks | R inguinal LN | − | T2N1M0 | II | − | − | Steroid, IFN-γ, PUVA, ACNU, NC→Etretinate, PUVA, NC→RT, CHOP, PUVA, PD→RT, NC→UVB, PD→CHOP, RT, PD→MTX, THP-COP, RT | DOD, 121 | UD | Clonal |
| 8 | 64/M | Head and neck | − | − | T1bN0M0 | I | − | − | No treatment | Died of cerebral hemorrhage, 2 | Clonal | Polyclonal |
| 9 | 50/F | Head | − | Nasal cavity | T2bN0M0 | I | − | − | CHOP, NC→VP-16 for recurrence, PR | AWR, 20 | UD | Polyclonal |
| 10 | 33/M | Trunk and extremities | − | − | ND | IV | + | − | IFN, RT, Steroid, NC | DOD, 51 | Clonal | ND |
| 11 | 25/F | L axilla | L axillary LN | − | T1aN1M0 | II | − | − | CHASE, PD | DOD, 8 | UD | Clonal |
CTCL-γδ, primary cutaneous T-cell lymphoma with T-cell receptor gamma-delta phenotype; SPTCL, subcutaneous panniculitis-like T-cell lymphoma; L, left; R, right; LN, Lymph node; BM, bone marrow; HPS, hemophagocytic sydrome; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisolone; THP-COP, cyclophosphamide, pirarubicin, vincristine, prednisolone; MINE, mesna, ifosfamide, mitoxantrone, etoposide; ESHAP, etoposide, methylprednisolone, cytarabine, cisplatin; MEPP, mitoxantrone, etoposide, cisplatin and prednisolone, BACOP, bleomycin, adriamycin, cyclophosphamide, vincristine, prednisone; CHASE, cyclophosphamide, cytosine arabinoside, etoposide, and dexamethasone; RT, radiotherapy; CHOP-B, cytoxan, adriamycin, vincristine, prednisone, bleomycin; VP-16, etoposide; INF-γ, interferon-gamma; PUVA, Psoralen Ultra-Violet A; ACNU, nimustine hydrochloride; CPA, cyclophosphamide; UVB, ultraviolet B; MTX, methotrexate; PD, progressive disease; NC, no change; CR, complete response; AWD, alive with disease; DOD, died of disease, PCR; polymerase chain reaction, TCR; T-cell receptor, ND; not done, UD; undetected.
Table 4.
Clinical features and TCR rearrangements of patients with CTCL-γδ with SPTCL features
| Patient | Age/gender | Primary cutaneous/subsutaneous site | Extracutaneous sites | Recurrent/relapse sites | TNM | Stage | B symptoms | HPS | Therapy | Follow-up (mo) | TCRγ gene rearrangement | TCRβ gene rearrangement |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 12 | 26/M | R jaw, shoulder, L axilla | − | Skin/subcutis (R chest, upper arm) | ND | ND | − | − | No treatment | AIR, 137 | UD | Polyclonal |
| 13 | 18/F | R thigh | − | Skin/subcutis (forearm, thigh) | T1aN0M0 | IE | + | − | Steroid pulse, PR→PSL+Cyclosporin A or chemotherapy for relapse→? | Alive, 42 | Clonal | Clonal |
| 14 | 44/F | R lower leg | Inguinal LN | Skin/subcutis (R lower arm, R chest) | T2bN1M0 | IIE | + | − | CHOP+PSL, PR→CHOP, HD-ETP for relapse, CR | AIR, 20 | Clonal | Clonal |
| 15 | 17/F | L upper arm, upper back, lower back, thighs (bilateral) | − | Same as primary sites | T3N0M0 | IIIE | − | + | CHOP-E, PR→LASP, HD-AraC+L-ASP+auto PBSCT, HD-AraC, Cy-TBI+allo PBSCT for recurrence, CR | AIR, 136 | Clonal | Polyclonal |
| 16 | 65/F | L thigh | − | − | T1bN0M0 | IE | − | − | PSL, CR | AIR, 16 | Clonal | Clonal† |
| 17 | 81/M | Head and neck, upper back, thigh (bilateral), abdomen and genital, L lower arm and hand | Testis | Skin/subcutis (thigh, [bilateral], testis) | T3N0M0 | IV | − | − | MTX, PR→VP-16, excision for recurrence, PR | Died of cerebral infarction, 17 | Clonal | ND†† |
TCRCβ1 rearrangement was positive by southern blotting analysis.
TCRCβ1 rearrangement was negative by southern blotting analysis. CTCL-γδ, primary cutaneous T-cell lymphoma with T-cell receptor gamma-delta phenotype; SPTCL, subcutaneous panniculitis-like T-cell lymphoma; L, left; R, right; LN, Lymph node; ND, no data; HPS, hemophagocytic sydrome; CR, complete response; PR, partial response; NC, no change; PD, progressive disease; PSL, predonisolone; CyA, cyclosporin A; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisolone; HD-ETP, high-dose etoposide; CHOP-E, cyclophosphamide, doxorubicin, vincristine, prednisolone and etoposide; HD-AraC, high dose cytrabine; L-ASP, l-asparaginase; PBSCT, peripheral blood stem cell transplant; MTX, methotrexate; VP-16, etopaside; AIR, alive in remission; PCR, polymerase chain reaction; TCR, T-cell receptor; UD, undetected.
Clinical features and prognostic factors in 17 cutaneus T-cell lymphoma of the γδ phenotype patients
The various clinical features of CTCL-γδ patients with or without SPTCL features are summarized in Tables 3 and 4. The 11 CTCL-γδ patients without SPTCL features comprised nine men and two women aged 22–79 years (median, 54 years). A median follow-up period of 28 months was recorded (range, 2–259 months). Clinical data and follow-up data were available for all 11 patients. As a result of the initial treatment, three patients achieved complete remission or partial response, three exhibited no change, four suffered progressive disease and one died of cerebral hemorrhage without any treatment. In addition, five patients experienced relapse or recurrence. All three patients who responded well to the initial treatments subsequently experienced relapse or recurrence. Of the 11 patients, nine died of disease within 121 months after diagnosis.
The six CTCL-γδ patients with SPTCL features comprised two men and four women aged 17–81 years (median, 33 years). The median follow-up period was 35.5 months (range, 17–136 months). Clinical and follow-up data were available for all six patients. Four patients achieved complete remission and survived in remission (67%); of these patients, one achieved spontaneous regression. One patient is alive, but details are unknown, and one died of cerebral infarction 17 months after diagnosis.
The results of tests for significance of survival from the univariate analysis are summarized in Table 5. More than 60 years old age, presence of visceral organ involvement, and small-to-medium cell size were poor prognostic factors. Although the presence or absence of SPTCL features was not statistically significant as an indicator of survival, there was a tendency towards a higher 5-year overall survival rate for the CTCL-γδ patients with SPTCL features than for the CTCL-γδ patients without SPTCL features (P = 0.065). Of these four clinicopathological prognostic factors of survival for the CTCL-γδ patients, we then examined the impact of SPTCL features.
Table 5.
Univariate analysis of CTCL-γδ
| Clinicopathological characteristics | N | 5-year OS | P |
|---|---|---|---|
| Clinical profile | |||
| Age | |||
| <60 | 10 | 64.0 | 0.036 |
| ≧60 | 7 | 48.0 | |
| Gender | |||
| Male | 10 | 40.0 | 0.179 |
| Female | 7 | 85.7 | |
| Lesion | |||
| Single | 3 | 66.7 | 0.962 |
| Multiple or general | 14 | 53.6 | |
| Tumor size | |||
| <5 | 5 | 75.0 | 0.525 |
| ≧5 | 5 | 53.3 | |
| Lymph node involvement | |||
| + | 11 | 44.4 | 0.301 |
| − | 6 | 57.3 | |
| Visceral involvement | |||
| + | 3 | 0 | 0.016 |
| − | 14 | 67.3 | |
| LDH above normal | |||
| + | 10 | 48.0 | 0.824 |
| − | 5 | 50.0 | |
| B symptoms | |||
| + | 5 | 40.0 | 0.681 |
| − | 12 | 54.7 | |
| Clinical stage | |||
| I | 4 | 75.0 | 0.546 |
| II | 3 | 65.7 | |
| III | 1 | ND | |
| IV | 8 | 37.5 | |
| TNM | |||
| T1 | 4 | 50.0 | 0.534 |
| T2 | 3 | 0 | |
| T3 | 8 | 50.0 | |
| Histological profile | |||
| Location | |||
| Dermal | 2 | 50.0 | 0.534 |
| Dermal to subcutaneous | 14 | 56.8 | |
| Ulceration | |||
| + | 4 | 0 | 0.166 |
| − | 13 | 67.3 | |
| Epidermotropism | |||
| + | 3 | 66.7 | 0.971 |
| − | 13 | 65.3 | |
| Tumor necrosis | |||
| + | 5 | 26.7 | 0.618 |
| − | 11 | 70.1 | |
| Angioinvasion | |||
| + | 4 | 25.0 | 0.318 |
| − | 12 | 66.8 | |
| Cell size | |||
| Small to medium | 4 | 0 | 0.041 |
| Large | 13 | 65.3 | |
| SPTCL feature | |||
| + | 6 | 80.0 | 0.065 |
| − | 11 | 42.4 | |
CTCL, cutaneous T-cell lymphoma; ND, no data; OS, overall survival; SPTCL, subcutaneous panniculitis-like T-cell lymphoma.
The clinical features of our SPTCL patient series are summarized in Table S1. No significant differences in clinical severity (e.g. occurrence of bone marrow involvement or hemophagocytic syndrome) were observed between the SPTCL and CTCL-γδ with SPTCL groups (Table S2). In addition, the log rank test revealed no significant differences in the overall survival distributions by TCR phenotypes (P = 0.6). Moreover, there was no significant difference in relapse-free survival between SPTCL and CTCL-γδ with SPTCL (P = 0.666). On the other hand, the CTCL-γδ without SPTCL patients revealed aggressive clinical behavior. As for clinical features, there was statistical significance in abnormal CT scan result (P = 0.03), initial therapeutic effect (P = 0.01) and status of latest follow up (P = 0.0175) (Table S2). The 5-year overall survival rates of the patients with SPTCL, CTCL-γδ with SPTCL features and CTCL-γδ without SPTCL features were 85.7%, 80.0% and 42.4%, respectively. A statistically significant difference was observed in overall survival among the SPTCL, CTCL-γδ with SPTCL features and CTCL-γδ without SPTCL features groups (P = 0.005; Fig. 4).
Figure 4.
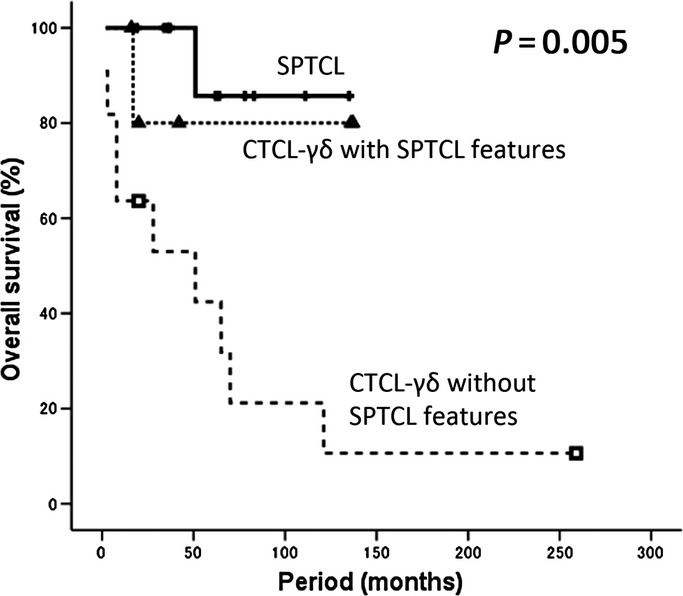
Overall survival of primary cutaneus T-cell lymphoma of the γδ phenotype (CTCL-γδ) without subcutaneous panniculitis-like T-cell lymphoma (SPTCL) features, CTCL-γδ with SPTCL features and SPTCL. A statistically significant difference was observed in overall survival among the SPTCL, CTCL-γδ with SPTCL features and CTCL-γδ without SPTCL features groups (P = 0.005).
Discussion
T-cell lymphomas (TCL) expressing the TCRγδ phenotype are rare; the best known types are hepatosplenic TCL and PCGD-TCL, although sporadic examples of γδ TCL, often extranodal, are recognized as well.(7,17,18) Among hepatosplenic TCL, the TCRγδ phenotype is much more common than the TCRαβ phenotype. A study of the clinical features of hepatosplenic TCL patients revealed that those with the TCRαβ phenotype died within 1 year of diagnosis, which suggested an aggressive behavior similar to that of the TCRγδ patients.(4,19) In addition, the small and large intestines have been predicted to be important origin sites of γδ TCL. Approximately 80% of monomorphic variants of EATL (Type II EATL) have expressed TCRγδ.(5) Nevertheless, patients with either the TCRαβ or TCRγδ phenotype have unfavorable prognoses.(5,6)
The characteristics of CTCL are unique, and the clinical behavior is not like that of other TCL. The prognosis is significantly poorer for patients with CTCL-γδ than for those with αβ cutaneous lymphoma.(2) Furthermore, because TCL patients with SPTCL features who express the TCRγδ phenotype reportedly exhibit poorer prognosis than do those who express the TCRαβ phenotype,(2) this disease variant was classified by the WHO in 2008 as PCGD-TCL. In Willemze et al. (2008) the 5-year overall survival rates of SPTCL and CTCL-γδ with SPTCL features were 81% and 11%, respectively.(11) Previous research has shown that other primary cutaneous disorders, such as mycosis fungoides (MF) and lymphomatoid papulosis type D as well as PCGD-TCL show TCRγ expression.(20) The prognosis of MF is not influenced by TCR subtypes.(21) In the present study, one patient of 17 CTCL-γδ patient series was clinically suspected to have MF; however, only a pharyngeal biopsy specimen was available from this patient, and the histological details were unknown. This patient survived for 121 months after diagnosis, demonstrating long-term clinical course.
The clinicopathological study of 17 CTCL-γδ patients showed that older age, presence of visceral organ involvement, small-to-medium cell size and non-SPTCL features were predictors of worse prognosis. Although age and visceral organ involvement were reasonable prognostic factors, the finding that the presence of SPTCL features was a predictor is interesting because previous reports have stated that CTCL-γδ with SPTCL features has an unfavorable prognosis, similar to that of CTCL-γδ without SPTCL features.(11,22)
We confirmed that CTCL-γδ patients without SPTCL features exhibited extremely poor prognosis, similar to those reported previously.(23) We found only one report of CTCL-γδ that described spontaneous regression;(24) generally, CTCL-γδ progresses unfavorably. Toro et al.(2) report that individuals suffering from CTCL-γδ with subcutaneous involvement exhibited apparently poorer prognosis than did those with epidermal or dermal involvement.(2) However, CTCL-γδ which had lesions in subcutaneous tissue and had SPTCL features were found to have better prognosis in the present study.
In the present study, all six CTCL-γδ patients presented tumors with SPTCL features showed remarkably indolent behavior, and there were no differences in the basic clinical features, overall survival and relapse-free survival between the SPTCL patients and those with CTCL-γδ with SPTCL features. A recent report from the USA describes five cases of CTCL-γδ with SPTCL features that presented indolent clinical behavior,(10) which supports the findings of our study. Bone marrow involvement and hemophagocytic syndrome were significantly less frequent among our six patients than among the patients reported in the literature (data not shown).(11) Furthermore, CTCL-γδ with SPTCL showed a tendency towards better response to initial treatment: five of six CTCL-γδ patients with SPTCL were alive at the time of last follow up, and four of them showed complete responses. These results suggest that the characteristics of CTCL-γδ with SPTCL are different from those previously reported for CTCL-γδ. According to Rodrígues–Pinilla et al. (2013), expression of TCRγ is not a poor prognostic factor by itself, which might support our finding.(20) However, there might have been some differences in natural history and treatment response between the patients in the present study and the CTCL-γδ patients. We need to examine further multinational studies in this disease category. One CTCL-γδ patient sample with SPTCL features expressed both TCRαβ and TCRγδ. Such TCRαβ/γδ double-positive cases have been reported previously, including PCGD-TCL, MF, lymphomatoid papulosis and extranodal NK/T cell lymphoma.(20,25) It is known that 4% of γδ T-cells in peripheral blood have TCRβ rearrangement.(20) However, it is not clear whether double-positive tumor cells are derived from these γδ T-cells in peripheral blood or suffer cytogenetic abberation.
T-cell receptors with a cytotoxic phenotype often express NK cell-associated antigens, such as CD56.(26) Gene expression profiling revealed that peripheral TCL-γδ is very similar to NK-cell lymphoma, but it is distinct from peripheral TCL-αβ and hepatosplenic TCL.(27) CD56 expression is reported to be helpful for the differential diagnosis of SPTCL and CTCL-γδ with SPTCL features when the appropriate staining method for TCRγδ is not known.(7,23) Willemze et al. (2008) report the clinicopathological factors that distinguish between SPTCL and CTCL-γδ with SPTCL features. Most CTCL-γδ patients with SPTCL features are CD56 positive, but SPTCL patients are CD56 negative.(11) In the present study, only one of the six CTCL-γδ patients with SPTCL features was CD56 positive, and CD56 positivity was not significantly different between SPTCL and CTCL-γδ with SPTCL features (Table S3). There was some statistical significance in CD8 and CD56 positivity between six CTCL-γδ patients with SPTCL features in the present series and 20 SPTCL-γδ patients described in the previous literature (data not shown).(11) In our present series of CTCL-γδ with SPTCL features, five of six samples revealed downregulated CD5 expression, which is an important immunophenotypical feature of γδ TCL (Table S3). These results suggest that CD5 downregulation is a common feature of CTCL-γδ with SPTCL features.
In Japan, treatment regimens and guidelines are now being established for different subtypes of CTCL. According to the clinical practice guidelines for cutaneous lymphoma, after the pathological diagnosis of SPTCL is established, the clinical stage is then determined as per TNM classification,(28) revised Ann Arbor classification and Cotswolds classification. When the lesion is localized to a small region without any clinical symptoms, radiotherapy can be considered. Oral steroids or immunosuppressive therapy can be considered as the first choice when there is no poor prognostic factor involved, such as low white blood cell count, high lactate dehydrogenase levels or complication of hemophagocytic syndrome.(11,29,30) However, in the present study, none of the patients with SPTCL or CTCL-γδ with SPTCL features who presented with the aforementioned parameters died. At present, the preferred treatment for aggressive CTCL is multiple combination chemotherapy. Another choice is high-dose chemotherapy followed by hematopoietic stem cell transplantation. Clinicians should carefully follow up when the clinical progression of CTCL-γδ with SPTCL features is indolent. We need to accumulate more cases of CTCL-γδ by using the immunohistochemical technique on paraffin-embedded sections and conduct further clinicopathological studies to determine the real frequency of CTCL-γδ and its clinical behavior.
In conclusion, although a few case reports have described indolent γδ TCL, we unexpectedly observed that the patients of the tumors with SPTCL features reveal indolent clinical behavior. Some cases of CTCL-γδ with SPTCL features might have different characteristics from those of CTCL-γδ without SPTCL features and have more indolent clinical courses than those previously described for SPTCL-γδ. In addition, there may have been interracial differences between the Asian and European/American CTCL-γδ patients with SPTCL features. Future studies on more cases including molecular genetic investigations are required to further elucidate the pathogenesis and behavior of these categories.
Acknowledgments
We thank Yuka Gion and Mutsumi Okabe for their technical assistance. This work was supported in part by a Grant-in-Aid for Cancer Research (No. 21-6-3) from the Ministry of Health, Labour and Welfare, Tokyo, Japan.
Disclosure Statement
The authors have no conflict of interest.
Supporting Information
Additional supporting information may be found in the online version of this article:
Table S1. Clinical features and T-cell receptor (TCR) rearrangements of patients with subcutaneous panniculitis-like T-cell lymphoma (SPTCL).
Table S2. Comparison of clinical features with primary cutaneous T-cell lymphoma (CTCL)-γδ with subcutaneous panniculitis-like T-cell lymphoma (SPTCL) features, CTCL-γδ without SPTCL features and SPTCL.
Table S3. Comparison of immunophenotype with clinical features with primary cutaneous T-cell lymphoma (CTCL)-γδ with subcutaneous panniculitis-like T-cell lymphoma (SPTCL), CTCL-γδ without SPTCL features and SPTCL.
References
- 1.Swerdlow SH, Campo E, Harris HL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC; 2008. [Google Scholar]
- 2.Toro JR, Liewehr DJ, Pabby N, et al. Gamma-delta T-cell phenotype is associated with significantly decreased survival in cutaneous T-cell lymphoma. Blood. 2003;101:3407–12. doi: 10.1182/blood-2002-05-1597. [DOI] [PubMed] [Google Scholar]
- 3.Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768–85. doi: 10.1182/blood-2004-09-3502. [DOI] [PubMed] [Google Scholar]
- 4.Macon WR, Levy NB, Kurtin PJ, et al. Hepatosplenic alpha beta T-cell lymphomas – a report of 14 cases and comparison with hepatosplenic gamma delta T-cell lymphomas. Am J Surg Pathol. 2001;25:285–96. doi: 10.1097/00000478-200103000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Chan JKC, Chan ACL, Cheuk W, et al. Type II enteropathy-associated T-cell lymphoma: a distinct aggressive lymphoma with frequent gamma delta T-cell receptor expression. Am J Surg Pathol. 2011;35:1557–69. doi: 10.1097/PAS.0b013e318222dfcd. [DOI] [PubMed] [Google Scholar]
- 6.Katoh A, Ohshima K, Kanda M, et al. Gastrointestinal T cell lymphoma: predominant cytotoxic phenotypes, including alpha/beta, gamma/delta T cell and natural killer cells. Leuk Lymphoma. 2000;39:97–111. doi: 10.3109/10428190009053543. [DOI] [PubMed] [Google Scholar]
- 7.Arnulf B, Copie-Bergman C, Delfau-Larue MH, et al. Nonhepatosplenic gamma delta T-cell lymphoma: a subset of cytotoxic lymphomas with mucosal or skin localization. Blood. 1998;91:1723–31. [PubMed] [Google Scholar]
- 8.Wertheim GBW, Roullet M, Jones D, et al. Sensitive and specific detection of gamma T-cell receptor in paraffin-embedded T-cell lymphomas. Mod Pathol. 2010;23:328a. [Google Scholar]
- 9.Garcia-Herrera A, Song JY, Chuang SS, et al. Nonhepatosplenic gamma delta T-cell lymphomas represent a spectrum of aggressive cytotoxic T-cell lymphomas with a mainly extranodal presentation. Am J Surg Pathol. 2011;35:1214–25. doi: 10.1097/PAS.0b013e31822067d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Magro CM, Wang X. Indolent primary cutaneous gamma/delta T-cell lymphoma localized to the subcutaneous panniculus and its association with atypical lymphocytic lobular panniculitis. Am J Surg Pathol. 2012;138:50–6. doi: 10.1309/AJCPQGVLTZQ77VFF. [DOI] [PubMed] [Google Scholar]
- 11.Willemze R, Jansen PM, Cerroni L, et al. Subcutaneous panniculitis-like T-cell lymphoma: definition, classification, and prognostic factors: an EORTC Cutaneous Lymphoma Group Study of 83 cases. Blood. 2008;111:838–45. doi: 10.1182/blood-2007-04-087288. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi E, Takata K, Sato Y, et al. Distinct morphologic, phenotypic, and clinical-course characteristics of indolent peripheral T-cell lymphoma. Hum Pathol. 2013;44:1927–36. doi: 10.1016/j.humpath.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Felgar RE, Macon WR, Kinney MC, Roberts S, Pasha T, Salhany KE. TIA-1 expression in lymphoid neoplasms – Identification of subsets with cytotoxic T lymphocyte or natural killer cell differentiation. Am J Pathol. 1997;150:1893–900. [PMC free article] [PubMed] [Google Scholar]
- 14.Debruin PC, Kummer JA, Vandervalk P, et al. Granzyme B-expressing peripheral T-cell lymphomas: neoplastic equivalents of activated cytotoxic T cells with preference for mucosa-associated lymphoid tissue localization. Blood. 1994;84:3785–91. [PubMed] [Google Scholar]
- 15.Yamashita Y, Yatabe Y, Tsuzuki T, et al. Perforin and granzyme expression in cytotoxic T-cell lymphomas. Mod Pathol. 1998;11:313–23. [PubMed] [Google Scholar]
- 16.Miyata-Takata T, Takata K, Yamanouchi S, et al. Detection of T-cell receptor gamma gene rearrangement in paraffin-embedded T or natural killer/T-cell lymphoma samples using the BIOMED-2 protocol. Leuk Lymphoma. 2014 doi: 10.3109/10428194.2013.871634. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 17.Charton-Bain MC, Brousset P, Bouabdallah R, et al. Variation in the histological pattern of nodal involvement by gamma/delta T-cell lymphoma. Histopathology. 2000;36:233–9. doi: 10.1046/j.1365-2559.2000.00772.x. [DOI] [PubMed] [Google Scholar]
- 18.Farcet JP, Gaulard P, Marolleau JP, et al. Hepatosplenic T-cell lymphoma: sinusal/sinusoidal localization of malignant cells expressing the T-cell receptor gamma delta. Blood. 1990;75:2213–9. [PubMed] [Google Scholar]
- 19.Suarez F, Wlodarska I, Rigal-Huguet F, et al. Hepatosplenic alpha beta T-cell lymphoma – An unusual case with clinical, histologic, and cytogenetic features of gamma delta hepatosplenic T-cell lymphoma. Am J Surg Pathol. 2000;24:1027–32. doi: 10.1097/00000478-200007000-00016. [DOI] [PubMed] [Google Scholar]
- 20.Rodríguez-Pinilla SM, Ortiz-Romero PL, Monsalvez V, et al. TCR-γ expression in primary cutaneous T-cell lymphomas. Am J Surg Pathol. 2013;37:375–84. doi: 10.1097/PAS.0b013e318275d1a2. [DOI] [PubMed] [Google Scholar]
- 21.Massone C, Crisman G, Kerl H, Cerroni L. The prognosis of early mycosis fungoides is not influenced by phenotype and T-cell clonality. Br J Dermatol. 2008;159:881–6. doi: 10.1111/j.1365-2133.2008.08761.x. [DOI] [PubMed] [Google Scholar]
- 22.Kao GF, Resh B, McMahon C, et al. Fatal subcutaneous panniculitis-like T-cell lymphoma gamma/delta subtype (cutaneous gamma/delta T-cell lymphoma): report of a case and review of the literature. Am J Dermatopathol. 2008;30:593–9. doi: 10.1097/DAD.0b013e318182c7bf. [DOI] [PubMed] [Google Scholar]
- 23.Toro JR, Beaty M, Sorbara L, et al. gamma delta T-cell lymphoma of the skin – A clinical, microscopic, and molecular study. Arch Dermatol. 2000;136:1024–32. doi: 10.1001/archderm.136.8.1024. [DOI] [PubMed] [Google Scholar]
- 24.Hagiwara M, Takata K, Shimoyama Y, et al. Primary cutaneous T-cell lymphoma of unspecified type with cytotoxic phenotype: clinicopathological analysis of 27 patients. Cancer Sci. 2009;100:33–41. doi: 10.1111/j.1349-7006.2008.01000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pongpruttipan T, Sukpanichnant S, Assanasen T, et al. Extranodal NK/T-cell lymphoma, nasal type, includes cases of natural killer cell and alpha beta, gamma delta, and alpha beta/gamma delta T-cell origin: a comprehensive clinicopathologic and phenotypic study. Am J Surg Pathol. 2012;36:481–99. doi: 10.1097/PAS.0b013e31824433d8. [DOI] [PubMed] [Google Scholar]
- 26.Morice WG. The immunophenotypic attributes of NK cells and NK-cell lineage lymphoproliferative disorders. Am J Clin Pathol. 2007;127:881–6. doi: 10.1309/Q49CRJ030L22MHLF. [DOI] [PubMed] [Google Scholar]
- 27.Iqbal J, Weisenburger DD, Chowdhury A, et al. Natural killer cell lymphoma shares strikingly similar molecular features with a group of non-hepatosplenic gamma delta T-cell lymphoma and is highly sensitive to a novel aurora kinase A inhibitor in vitro. Leukemia. 2011;25:348–58. doi: 10.1038/leu.2010.255. [DOI] [PubMed] [Google Scholar]
- 28.Kim YH, Willemze R, Pimpinelli N, et al. TNM classification system for primary cutaneous lymphomas other than mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the Cutaneous Lymphoma Task Force of the European Organization of Research and Treatment of Cancer (EORTC) Blood. 2007;110:479–84. doi: 10.1182/blood-2006-10-054601. [DOI] [PubMed] [Google Scholar]
- 29.Go RS, Wester SM. Immunophenotypic and molecular features, clinical outcome, treatment, and prognostic factors in subcutaneous panniculitis-like T-cell lymphoma (SPTCL): systematic analysis of 156 cases reported in the literature. Cancer. 2004;101:1404–13. doi: 10.1002/cncr.20502. [DOI] [PubMed] [Google Scholar]
- 30.Ghobrial IM, Weenig RH, Pittlekow MR, et al. Clinical outcome of patients with subcutaneous panniculitis-like T-cell lymphoma. Leuk Lymphoma. 2005;46:703–8. doi: 10.1080/10428190500051380. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Clinical features and T-cell receptor (TCR) rearrangements of patients with subcutaneous panniculitis-like T-cell lymphoma (SPTCL).
Table S2. Comparison of clinical features with primary cutaneous T-cell lymphoma (CTCL)-γδ with subcutaneous panniculitis-like T-cell lymphoma (SPTCL) features, CTCL-γδ without SPTCL features and SPTCL.
Table S3. Comparison of immunophenotype with clinical features with primary cutaneous T-cell lymphoma (CTCL)-γδ with subcutaneous panniculitis-like T-cell lymphoma (SPTCL), CTCL-γδ without SPTCL features and SPTCL.