Abstract
In this retrospective study, we aimed to clarify the risk of developing a second primary cancer and to determine the periods of high risk of second primary cancers. Subjects were all patients who had been diagnosed with a first primary cancer and registered with the Nagasaki Prefecture Cancer Registry between 1985 and 2007. We calculated the standardized incidence ratio (SIR) of second primary cancer according to site and years after diagnosis of the first primary cancer. A second primary cancer developed in 14 167 of 174 477 subjects (8.1%) during a median follow-up of 1.8 years. The SIR of all cancer was 1.10 (95% confidence interval, 1.08–1.11). Some specific relationships were observed between sites with risk factors in common, such as smoking, drinking, and hormone status. The SIRs were relatively high after approximately 10 years for all sites, and trends differ among cancer sites. We showed that cancer patients are at higher risk of a second primary cancer than the general population. In respect of the risk of a second primary cancer, physicians should be alert for cancers that have risk factors in common with the first primary cancer.
Keywords: Follow-up, Japan, multiple primary cancer, Nagasaki, population-based cancer registry
Cancer is no longer invariably a fatal disease; the survival rate of cancer patients has been improving.(1) In addition, the number of cancer patients in Japan is reportedly increasing;(2) thus, the national burden of cancer has been growing. Therefore, there is a pressing need to determine the optimal duration of follow-up of cancer patients and the implications of cancer screening.(3)
Cancer patients face the possibility of multiple primary cancers. The occurrence of subsequent cancers in cancer patients has become more frequent,(4–6) this being related to late effects of treatment for the first primary cancer or risk factors common to the first and subsequent primary cancers; these include environment, lifestyle factors, and inherited genes.(7–10) Therefore, identifying which second cancers have a high risk of developing in patients with various first primary cancers and determining the period of high risk are valuable starting points for establishing the optimal duration of follow-up.
The objectives of the present study were to clarify the risk of developing a second primary cancer and to provide information about the periods of high risk of second primary cancers.
Material and Methods
Study subjects
Subjects were all patients diagnosed with a first primary cancer and registered with the Nagasaki Prefecture Cancer Registry (Nagasaki, Japan) between 1985 and 2007. The incidence of second primary cancers was assessed until the end of 2008. Third or subsequent primary cancers were not considered in this analysis.
Cancer site was categorized into 16 groups according to the International Classification of Diseases Tenth Revision as described in a previous study.(4) These categories are mouth/pharynx (C00–14), esophagus (C15), stomach (C16), colorectum (C18–20), liver (C22), gallbladder (C23, 24), pancreas (C25), larynx (C32), lung (C33, 34), breast for females (C50), uterus for females (C53–55), ovary for females (C56), prostate for males (C61), kidney/urinary tract/bladder (C64–68), thyroid (C73), and blood (C81–85, 88, 90, 91–96).
Statistical analysis and definition of multiple primary cancers
The standardized incidence ratio (SIR), which is the ratio of the observed to the expected number of second primary cancers, was calculated. In order to obtain the expected number of second primary cancers, each sex-, age group-, and year of diagnosis-specific incidence rates in the general population in Nagasaki Prefecture were calculated using Nagasaki Prefecture Cancer Registry data. Next, person-years at risk for each sex-, age group-, and year in patients diagnosed with a first primary cancer were calculated. They were calculated from the date of diagnosis of the first primary cancer to whichever of the following occurred first: the date of diagnosis of the second primary cancer, the date of death, or 31 December 2008. Incidence rates in the general population and person-years in patients were multiplied, and all sex-, age group-, and year expected incidences were summed to obtain the expected number of second primary cancers. Ninety-five percent confidence intervals (CIs) of SIRs were calculated assuming a Poisson distribution.(11) The SIRs were calculated according to sites of first and second primary cancers and intervals between the first and second primary cancers.
The International Agency for Research on Cancer criteria were used to define multiple primary cancers.(12) These criteria do not accept any cancers in the same site as second primary cancers unless their histological type differs from that of the first primary cancer. Therefore, especially for major cancers, the SIR for all sites will be underestimated. To avoid such underestimation, both the observed and expected numbers of second primary cancers in the same site as the first primary cancer were excluded from the SIR calculations.(4)
Results
The total number of subjects was 174 477. The proportion of patients with second primary cancers during the follow-up period was 8.1% (median, 1.8 years; mean, 4.3 years). The cancers were verified histologically in 81.9% of first primary cancers and 84.4% of second primary cancers.
Table 1 lists the SIRs according to sites of first and second primary cancers; all sites are included. The SIR and 95% CI among cancer patients for all sites was 1.10 (1.08–1.11). According to the first primary cancer site, first esophagus, larynx, ovary, and mouth/pharynx cancers had the highest SIRs (>1.5). The SIRs were >1.0 for most sites except for pancreas, prostate, gallbladder, and lung. According to the second primary cancer site, second thyroid, followed by esophagus, had the highest SIRs. The SIRs were >1.0 for most second sites except for gallbladder, blood, liver, prostate, and pancreas.
Table 1.
Standardized incidence ratios (SIR) of second primary cancers according to sites of first and second primary cancers
| First cancer site | Second cancer site | Observed | Expected | SIR | 95% CI |
|
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| All sites | All sites | 14 167 | 12 928 | 1.10 | 1.08 | 1.11 |
| Mouth/pharynx | All sites | 407 | 260 | 1.56 | 1.42 | 1.72 |
| Esophagus | All sites | 401 | 215 | 1.86 | 1.68 | 2.05 |
| Stomach | All sites | 3225 | 2952 | 1.09 | 1.06 | 1.13 |
| Colorectum | All sites | 2997 | 2742 | 1.09 | 1.05 | 1.13 |
| Liver | All sites | 627 | 626 | 1.00 | 0.92 | 1.08 |
| Gallbladder | All sites | 251 | 276 | 0.91 | 0.80 | 1.03 |
| Pancreas | All sites | 138 | 168 | 0.82 | 0.69 | 0.97 |
| Larynx | All sites | 302 | 177 | 1.71 | 1.52 | 1.91 |
| Lung | All sites | 1062 | 1116 | 0.95 | 0.90 | 1.01 |
| Breast (females) | All sites | 727 | 625 | 1.16 | 1.08 | 1.25 |
| Uterus | All sites | 569 | 429 | 1.33 | 1.22 | 1.44 |
| Ovary | All sites | 148 | 94 | 1.58 | 1.34 | 1.86 |
| Prostate | All sites | 822 | 932 | 0.88 | 0.82 | 0.94 |
| Kidney/urinary tract/bladder | All sites | 1162 | 1018 | 1.14 | 1.08 | 1.21 |
| Thyroid | All sites | 232 | 204 | 1.14 | 0.99 | 1.29 |
| Blood | All sites | 663 | 618 | 1.07 | 0.99 | 1.16 |
| All sites | Mouth/pharynx | 322 | 243 | 1.33 | 1.19 | 1.48 |
| All sites | Esophagus | 458 | 300 | 1.53 | 1.39 | 1.67 |
| All sites | Stomach | 2209 | 1823 | 1.21 | 1.16 | 1.26 |
| All sites | Colorectum | 2411 | 2132 | 1.13 | 1.09 | 1.18 |
| All sites | Liver | 945 | 959 | 0.99 | 0.92 | 1.05 |
| All sites | Gallbladder | 413 | 482 | 0.86 | 0.78 | 0.94 |
| All sites | Pancreas | 480 | 483 | 0.99 | 0.91 | 1.09 |
| All sites | Larynx | 120 | 104 | 1.16 | 0.96 | 1.39 |
| All sites | Lung | 2044 | 1931 | 1.06 | 1.01 | 1.11 |
| All sites | Breast (females) | 395 | 392 | 1.01 | 0.91 | 1.11 |
| All sites | Uterus | 282 | 217 | 1.30 | 1.15 | 1.46 |
| All sites | Ovary | 126 | 95 | 1.32 | 1.10 | 1.57 |
| All sites | Prostate | 961 | 973 | 0.99 | 0.93 | 1.05 |
| All sites | Kidney/urinary tract/bladder | 929 | 775 | 1.20 | 1.12 | 1.28 |
| All sites | Thyroid | 251 | 126 | 1.98 | 1.75 | 2.25 |
| All sites | Blood | 785 | 842 | 0.93 | 0.87 | 1.00 |
CI, confidence interval.
Table 2 lists the SIRs according to selected sites of first and second primary cancers with SIRs that were greater or less than 1 (P < 0.05). We observed some specific relationships between sites of first and second primary cancers. In particular, the SIRs for mouth/pharynx, esophagus, and larynx were high, namely, 12.67 for the first mouth/pharynx to the second esophagus, 12.33 for the first esophagus to the second mouth/pharynx, and 6.74 for the first esophagus to the second larynx. Site relationships between breast, uterus, and ovary were relatively high, as were those between esophagus, stomach, and colorectum. In addition, we identified high SIRs for second thyroid cancers. However, we identified low SIRs for first liver, gallbladder, pancreas, or lung cancers. The SIRs for first or second prostate cancers were also low.
Table 2.
Standardized incidence ratios (SIR) of second primary cancers according to sites of first and second primary cancers arranged by SIRs > or <1
| First cancer site | Second cancer site | Observed | Expected | SIR | 95% CI |
|
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| SIR > 1 (P < 0.05) | ||||||
| Mouth/pharynx | Esophagus | 77 | 6 | 12.67 | 10.00 | 15.84 |
| Mouth/pharynx | Liver | 39 | 19 | 2.02 | 1.44 | 2.76 |
| Mouth/pharynx | Lung | 57 | 38 | 1.51 | 1.14 | 1.95 |
| Mouth/pharynx | Thyroid | 9 | 2 | 4.40 | 2.01 | 8.36 |
| Esophagus | Mouth/pharynx | 51 | 4 | 12.33 | 9.18 | 16.21 |
| Esophagus | Stomach | 148 | 39 | 3.82 | 3.23 | 4.49 |
| Esophagus | Liver | 26 | 17 | 1.55 | 1.01 | 2.27 |
| Esophagus | Larynx | 14 | 2 | 6.74 | 3.68 | 11.31 |
| Esophagus | Lung | 47 | 34 | 1.39 | 1.02 | 1.85 |
| Stomach | Esophagus | 134 | 79 | 1.70 | 1.43 | 2.02 |
| Stomach | Colorectum | 891 | 658 | 1.35 | 1.27 | 1.45 |
| Colorectum | Stomach | 751 | 547 | 1.37 | 1.28 | 1.47 |
| Colorectum | Pancreas | 137 | 113 | 1.21 | 1.02 | 1.43 |
| Colorectum | Ovary | 34 | 19 | 1.83 | 1.27 | 2.56 |
| Colorectum | Kidney/urinary tract/bladder | 235 | 195 | 1.20 | 1.06 | 1.37 |
| Liver | Stomach | 170 | 118 | 1.44 | 1.23 | 1.67 |
| Liver | Thyroid | 15 | 5 | 3.29 | 1.84 | 5.43 |
| Pancreas | Esophagus | 9 | 4 | 2.49 | 1.14 | 4.74 |
| Pancreas | Stomach | 42 | 30 | 1.41 | 1.02 | 1.91 |
| Larynx | Mouth/pharynx | 22 | 3 | 6.69 | 4.19 | 10.13 |
| Larynx | Esophagus | 22 | 5 | 4.67 | 2.93 | 7.07 |
| Larynx | Stomach | 47 | 32 | 1.49 | 1.09 | 1.98 |
| Larynx | Colorectum | 49 | 33 | 1.49 | 1.10 | 1.97 |
| Larynx | Lung | 75 | 29 | 2.60 | 2.04 | 3.25 |
| Larynx | Thyroid | 5 | 1 | 6.66 | 2.15 | 15.54 |
| Lung | Thyroid | 39 | 9 | 4.55 | 3.24 | 6.22 |
| Breast (females) | Lung | 90 | 64 | 1.41 | 1.13 | 1.73 |
| Breast (females) | Uterus | 75 | 53 | 1.41 | 1.11 | 1.77 |
| Breast (females) | Ovary | 34 | 19 | 1.83 | 1.27 | 2.56 |
| Breast (females) | Thyroid | 36 | 17 | 2.07 | 1.45 | 2.87 |
| Uterus | Lung | 77 | 40 | 1.91 | 1.50 | 2.38 |
| Uterus | Kidney/urinary tract/bladder | 28 | 16 | 1.79 | 1.19 | 2.59 |
| Ovary | Colorectum | 32 | 18 | 1.79 | 1.22 | 2.52 |
| Ovary | Uterus | 41 | 8 | 5.08 | 3.64 | 6.89 |
| Prostate | Kidney/urinary tract/bladder | 128 | 72 | 1.78 | 1.49 | 2.12 |
| Kidney/urinary tract/bladder | Lung | 218 | 169 | 1.29 | 1.12 | 1.47 |
| Kidney/urinary tract/bladder | Prostate | 187 | 98 | 1.91 | 1.64 | 2.20 |
| Thyroid | Lung | 40 | 22 | 1.83 | 1.31 | 2.49 |
| Blood | Thyroid | 19 | 6 | 3.15 | 1.89 | 4.91 |
| SIR < 1 (P < 0.05) | ||||||
| Stomach | Prostate | 246 | 284 | 0.87 | 0.76 | 0.98 |
| Liver | Lung | 64 | 99 | 0.65 | 0.50 | 0.82 |
| Liver | Prostate | 36 | 52 | 0.69 | 0.48 | 0.96 |
| Liver | Kidney/urinary tract/bladder | 24 | 39 | 0.61 | 0.39 | 0.91 |
| Gallbladder | Lung | 22 | 40 | 0.55 | 0.34 | 0.83 |
| Gallbladder | Prostate | 8 | 17 | 0.48 | 0.21 | 0.95 |
| Pancreas | Blood | 2 | 10 | 0.19 | 0.02 | 0.70 |
| Lung | Liver | 70 | 92 | 0.76 | 0.59 | 0.96 |
| Lung | Gallbladder | 26 | 43 | 0.60 | 0.39 | 0.88 |
| Lung | Prostate | 79 | 109 | 0.72 | 0.57 | 0.90 |
| Prostate | Esophagus | 9 | 25 | 0.36 | 0.17 | 0.69 |
| Prostate | Stomach | 150 | 178 | 0.84 | 0.71 | 0.99 |
| Prostate | Liver | 48 | 69 | 0.70 | 0.52 | 0.93 |
| Prostate | Lung | 152 | 180 | 0.85 | 0.72 | 0.99 |
| Prostate | Blood | 38 | 60 | 0.63 | 0.45 | 0.87 |
| Thyroid | Gallbladder | 1 | 8 | 0.13 | 0.00 | 0.74 |
CI, confidence interval.
Figure 1 shows the trends in SIRs after diagnosis of first primary cancers of all sites. We calculated tentative SIRs after excluding second primary cancers diagnosed within less than 3 months of the first primary cancer, because these were more likely to be diagnosed as a result of detection bias. Results both including and excluding these cancers are shown in Figure 1. The SIRs were high in the first year, decreased in the second year, continued to increase after the second year and were relatively high in approximately 10 years, and then decreased after 20 years. When we excluded second primary cancers within less than 3 months of the first primary cancer, the SIR for the first year was low.
Figure 1.

Trends in standardized incidence ratio (SIR) after diagnosis of first primary cancers of all sites according to inclusion (filled circles) or exclusion (hollow circles) of second primary cancers diagnosed within 3 months of diagnosis of the first.
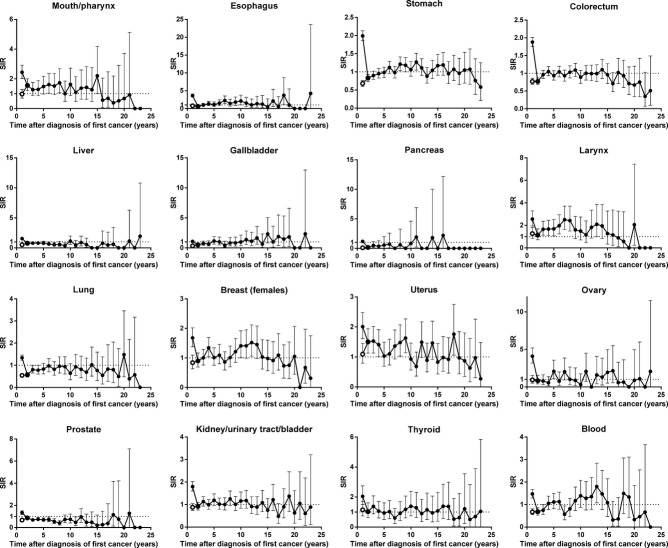
Figure 2 shows the trends in SIRs after diagnosis of first primary cancers according to cancer sites. The SIRs in the first year were high for all sites of cancer, decreasing when we excluded second primary cancers diagnosed within less than 3 months of the first primary cancer. The trends differed among cancer sites. The SIRs for stomach showed the same tendency as all sites combined; namely, decreasing in the second year, continued to increase after the second year, relatively high in approximately 10 years, and decreasing after 20 years. Those for liver, pancreas, lung, or prostate were consistently lower than 1. In some sites, including larynx, breast in female subjects, uterus, or blood, the SIRs were relatively high a few years after diagnosis of the first primary cancer, and even after 10 years.
Figure 2.
Trends in standardized incidence ratio (SIR) after diagnosis of first primary cancers by sites, according to inclusion (filled circles) or exclusion (hollow circles) of second primary cancers diagnosed within less than 3 months of the first.
Discussion
A previous study from data of European cancer registries reported an overall incidence of multiple primary cancers of 6.3% (range, 0.4–12.9%); cancer registries with registration periods of 10 years or less are reporting smaller percentages of multiple primary cancers, depending on the length of the registration.(13) Another study reported fluctuation of the proportions of multiple primary cancers between 6.1% and 10.5%, the percentage apparently stabilizing approximately 10 years after registration.(14) Because the Nagasaki Prefecture Cancer Registry has records dating back to 1985, and the data is of high quality, we consider our finding of 8.1% multiple primary cancers as realistic.
The risk of second primary cancer among cancer patients was higher than the risk of cancer among general population. This finding is consistent with those of previous studies.(4,5,7,15) Esophagus, larynx, ovary, and mouth/pharynx were the most frequent sites for developing second primary cancers in a previous study in Osaka, Japan.(4) However, our findings differ from those of a previous study in Queensland, Australia from 1997 to 2001, in which second primary cancers of esophagus and ovary were not so common, whereas those of head and neck were frequent (P < 0.05).(5) Possible explanations include differences in risk factors for cancer, such as smoking and drinking alcohol; other possibilities include genetic factors and treatment for the first primary cancer.(7–9)
Conversely, we found low SIRs for first liver, gallbladder, pancreas, and lung cancers. It is unlikely that factors related to these first primary cancers protect the patients from developing second primary cancers. One possible explanation for these low SIRs is the short durations of follow-up of patients with these sites of cancer. These four sites of cancer have the lowest survival rates and the shortest duration of follow-up; the median durations of follow-up were 3 months for pancreas, 5 months for gallbladder, and 8 months for liver and lung. Therefore, with these sites of cancer, there were only very short periods of time during which treatment-related second primary cancers could develop.(16) Moreover, the SIRs of these sites remained low, as shown in the results of trends in SIRs. This implies that, in regard to multiple primary cancers, these patients require less follow-up than patients with primary cancers in other sites. Follow-up for these patients is not so important on the issue of the diagnosis of second primary cancer.
The SIRs for first and second prostate cancers were both low. The low SIR for first prostate cancers is consistent with findings of previous studies.(5,7) One possible explanation is that they have no known risk factors in common with other cancers. Prostate cancer has a large genetic risk factor;(17) risk factors shared with other cancers have not yet been identified.(18) In addition, smoking, which is an important risk factor for many cancers, is reportedly associated with a decreased risk of prostate cancer.(19,20) We have to also consider socioeconomic status (SES) such as educational attainment and income, which is correlated with cancer risk. Persons with lower SES have generally higher cancer incidence rates than those with higher SES, whereas prostate cancer incidence increases with higher education and income.(21) Therefore, it is possible that some factors that increase the risk of other cancers actually decrease the risk of developing prostate cancer, and other factors which increase prostate cancer risk protect from developing other cancers. More detailed investigation about risk and protective factors is needed.
We found strong site relationships between first and second primary cancers within the mouth/pharynx, esophagus, and larynx. This finding is consistent with those of previous studies.(4,22,23) The major risk factor for these sites is smoking;(22) a synergetic effect between smoking and drinking may also contribute to the strong relationship between these sites.(17) Breast, uterus, and ovary also showed strong site relationships, as found in previous studies.(4,7) Female-specific causes, such as the hormonal environment, including estrogen concentrations, and dietary factors may affect these relationships.(17) Stomach, esophagus, and colorectum also showed strong site relationships. Drinking of alcohol and smoking may contribute to the development of multiple primary cancers at these sites.(18,24) In addition, diagnosis of esophagus and stomach cancer is mostly confrimed with biopsy by upper endoscopy. Therefore, it is possible that these cancers are more likely to be diagnosed at the same time. Moreover, mouth/pharynx, esophagus, and larynx, breast, uterus, and ovary, and stomach, esophagus, and colorectum are diagnosed in the same medical department. These detection biases would affect the high SIRs in the first year.
The high SIRs of second thyroid cancers may be partly attributable to medical surveillance of cancer patients.(22,25) When a first primary cancer is diagnosed, extensive surveillance is carried out to locate possible metastases. In addition, follow-up surveillance is carried out to locate possible recurrence and metastasis in cancer patients. In Korea, cancer screening is considered to have played a major role in the rapid increase in incidence of thyroid cancer since 2000.(26) Therefore, particularly for thyroid cancer, the effect of surveillance of cancer patients should be considered.
Some previous studies have investigated the SIRs for all sites according to defined intervals after diagnosis of the first primary cancer (i.e., 3 months–1 year, 1–5 years, and 5–10 years), and found no clear trends toward either increasing or decreasing.(4,5,15) In the present study, for first primary cancers of all sites, second primary cancers occurred more frequently in the first year, decreased in the second year, and then showed a late increase after approximately 10 years. Detection bias probably accounts for the high SIRs in the first year. Cancer patients are under close scrutiny, especially shortly after the diagnosis of their first primary cancer.(22) Of second primary cancers, 19.0% were diagnosed within less than 3 months of diagnosis of the first primary cancer. When these second primary cancers are excluded, the SIR of developing a second primary cancer in the first year is 0.89 (0.88–0.91).
A possible explanation for the decreasing SIR after 20 years is lifestyle modification, including smoking, drinking, and dietary habit. The risk of developing most sites of cancer returns to that of never smokers after more than 10 years' cessation of smoking.(27) In particular, in Japan, the risk of developing lung cancer returns to that of never smokers after more than 20 years' cessation of smoking.(28) If cancer patients modify their lifestyles, it is possible that the risk of developing cancer decreases to equal to or lower than the general population.
A previous study reported a significant risk peak for second cancers between the eighth and ninth year after diagnosis of breast cancer; the authors considered this was probably attributable to a late effect of local radiotherapy for the first breast cancer.(29) In the present study, the SIRs for first breast cancers were relatively high after approximately 10 years; however, this increase was not statistically significant (P < 0.05). We observed similar increases for larynx, uterus, and blood cancers. The proportions of subjects in the present study with these cancers who had received radiotherapy were relatively high except for blood cancers; namely, 6.2% for all sites, 54.3% for larynx, 17.8% for uterus, 11.8% for breast, and 6.1% for blood. To confirm a late effect of radiotherapy, we carried out a stratified analysis by treatment history of radiotherapy. The Nagasaki Prefecture Cancer Registry collects information on the first course of treatment. Subjects were classified according to whether or not they had received radiotherapy as part of their initial cancer treatment. Figure 3 shows the trends in SIRs after diagnosis of first primary cancers of all sites and these sites. The SIRs of patients treated without radiotherapy were consistently around 1.00 except for the first year, whereas those of patients treated with radiotherapy increased after the second year and were relatively high in approximately 7 and 12 years. Therefore, a late effect of radiotherapy could partly explain the increased risk. The SIRs according to cancer sites were not stable due to the small number of patients, and the clear differences between the SIRs of patients treated with radiotherapy and those of patients treated without radiotherapy were not observed. Our results, however, implied that follow-up for at least 10 years after diagnosis of the first primary cancer is needed for patients with these cancers, especially those who have received radiotherapy. We have to consider radiation dose, age at exposure to radiation, and subsequent treatment to clarify the effect of radiotherapy.
Figure 3.
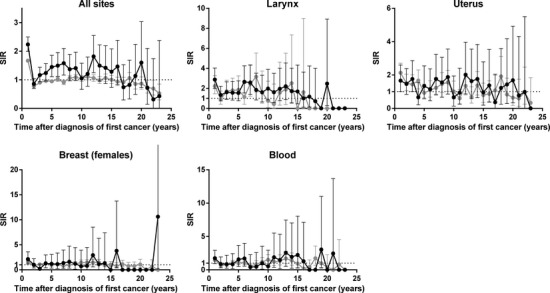
Trends in standardized incidence ratio (SIR) after diagnosis of first primary cancers of all sites according to treatment with or without radiotherapy. Black circles, all second cancers in patients treated with radiotherapy; gray circles, all second cancers in patients treated without radiotherapy.
Further investigation in consideration of recurrence and metastasis is needed to discuss patient's life after diagnosis and determine the optimal duration of follow-up period. We have no information on recurrence and metastasis, but recurrence and metastasis rates are known to differ according to cancer sites and stages. For example, female breast cancer has comparatively favorable prognosis (5-year survival rate of study subjects was 82.8%), but has a high risk of recurrence even 5 years after prognosis. We focused on multiple primary cancers in the present study, and clarified that 47.0% of cancer patients live more than 5 years after diagnosis but they are at equal or higher risk of a second primary cancer than the general population's risk of cancer. However, we have to pay attention to the possibility of detection bias because cancer patients continued to be followed-up to detect possible recurrence and metastasis. More detailed analysis can offer new insight into patients' prognosis.
The present study has some limitations. First, we have no data on patients who moved out of Nagasaki Prefecture and developed second primary cancers while living elsewhere. Therefore, underestimation of our SIRs can result from under-ascertainment of second primary cancers in patients who moved out of Nagasaki Prefecture to an indeterminable degree. Second, we had no data concerning risk factors. Cancer patients could differ from the general population in regard to smoking, drinking of alcohol, and other potential risk factors. Further studies that assessed these risk factors would allow clearer interpretation of the factors contributing to development of multiple primary cancers. Third, some misclassification of cancers may have occurred. The International Agency for Research on Cancer guidelines are conservative compared with those used in clinical trials; we may have underestimated the risk of second primary cancers.(4)
Our study also has some strengths. The Nagasaki Prefecture Cancer Registry has long-term high quality data with follow-up from 1985. The proportion of death certificate only (DCO%) was 8.8% during the period 1985–2008, thereby fulfilling the international criterion (DCO% <10%). A high proportion of cancers have been verified histologically, which is important for distinguishing between second primary cancers and metastases from first primary cancers. Thus, we had the data to carry out detailed analyses and evaluate long-term trends after diagnosis of first primary cancers. In addition, we were able to derive observed and expected numbers of second primary cancers from the same population (in Nagasaki Prefecture). Thus, the population-based nature of the registry from which we obtained our data allowed us to bypass the problems of selection or referral bias that confound some clinical series.(9)
In conclusion, we have shown that cancer patients are at higher risk of second primary cancers than the general population's risk of cancer. Medical scrutiny for second primary cancers that have risk factors in common with the first primary cancer is important. The risk of developing second primary cancer is relatively high after approximately 10 years for all sites, and the trends differ among cancer sites. For patients and clinicians, the identification of strong site relationships and the periods of high risk of second primary cancers may be useful for screening, prevention strategies, and counseling.
Disclosure Statement
The authors have no conflict of interest.
References
- 1.Matsuda T, Ajiki W, Marugame T, et al. Population-based survival of cancer patients diagnosed between 1993 and 1999 in Japan: a chronological and international comparative study. Jpn J Clin Oncol. 2011;41:40–51. doi: 10.1093/jjco/hyq167. [DOI] [PubMed] [Google Scholar]
- 2.Tabata N, Ohno Y, Matsui R, et al. Partial cancer prevalence in Japan up to 2020: estimates based on incidence and survival data from population-based cancer registries. Jpn J Clin Oncol. 2008;38:146–57. doi: 10.1093/jjco/hym156. [DOI] [PubMed] [Google Scholar]
- 3.Mariotto AB, Rowland JH, Ries LA, Scoppa S, Feuer EJ. Multiple cancer prevalence: a growing challenge in long-term survivorship. Cancer Epidemiol Biomarkers Prev. 2007;16:566–71. doi: 10.1158/1055-9965.EPI-06-0782. [DOI] [PubMed] [Google Scholar]
- 4.Tabuchi T, Ito Y, Ioka A, Miyashiro I, Tsukuma H. Incidence of metachronous second primary cancers in Osaka, Japan: update of analyses using population-based cancer registry data. Cancer Sci. 2012;103:1111–20. doi: 10.1111/j.1349-7006.2012.02254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Youlden DR, Baade PD. The relative risk of second primary cancers in Queensland, Australia: a retrospective cohort study. BMC Cancer. 2011;11:83. doi: 10.1186/1471-2407-11-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsukuma H, Fujimoto I, Hanai A, Hiyama T, Kitagawa T, Kinoshita N. Incidence of second primary cancers in Osaka residents, Japan, with special reference to cumulative and relative risks. Jpn J Cancer Res. 1994;85:339–45. doi: 10.1111/j.1349-7006.1994.tb02364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curtis RE, Boice JD, Jr, Kleinerman RA, Flannery JT, Fraumeni JF., Jr Summary: multiple primary cancers in Connecticut, 1935-82. Natl Cancer Inst Monogr. 1985;68:219–42. [PubMed] [Google Scholar]
- 8.Kuligina E, Reiner A, Imyanitov EN, Begg CB. Evaluating cancer epidemiologic risk factors using multiple primary malignancies. Epidemiology. 2010;21:366–72. doi: 10.1097/EDE.0b013e3181cc8871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Travis LB. The epidemiology of second primary cancers. Cancer Epidemiol Biomarkers Prev. 2006;15:2020–6. doi: 10.1158/1055-9965.EPI-06-0414. [DOI] [PubMed] [Google Scholar]
- 10.Travis LB, Rabkin CS, Brown LM, et al. Cancer survivorship–genetic susceptibility and second primary cancers: research strategies and recommendations. J Natl Cancer Inst. 2006;98:15–25. doi: 10.1093/jnci/djj001. [DOI] [PubMed] [Google Scholar]
- 11.Breslow NE, Day NE. Statistical methods in cancer research. Volume II–The Design and Analysis of Cohort Studies. Lyon: IARC Sci Publ; 1987. [PubMed] [Google Scholar]
- 12.International Agency for Research on Cancer (IARC) International Rules for Multiple Primary Cancer (ICD-O 3rd edn) Lyon: IARC; 2004. [Google Scholar]
- 13.Rosso S, De Angelis R, Ciccolallo L, et al. Multiple tumours in survival estimates. Eur J Cancer. 2009;45:1080–94. doi: 10.1016/j.ejca.2008.11.030. [DOI] [PubMed] [Google Scholar]
- 14.Filali K, Hedelin G, Schaffer P, et al. Multiple primary cancers and estimation of the incidence rates and trends. Eur J Cancer. 1996;32A:683–90. doi: 10.1016/0959-8049(95)00621-4. [DOI] [PubMed] [Google Scholar]
- 15.Crocetti E, Buiatti E, Falini P Italian Multiple Primary Cancer Working Group. Multiple primary cancer incidence in Italy. Eur J Cancer. 2001;37:2449–56. doi: 10.1016/s0959-8049(01)00314-8. [DOI] [PubMed] [Google Scholar]
- 16.National Cancer Institute. New Malignancies Among Cancer Survivors: SEER Cancer Registries, 1973–2000. Bethesda: NHI Pibl; 2006. [Google Scholar]
- 17.Adami H-O, Hunter D, Trichopoulos D. Textbook of Cancer Epidemiology. New York: Oxford Universoty Press; 2002. [Google Scholar]
- 18.Japan Epidemiology Association. Handbook of Epidemiology – Epidemiology and Prevention of Major Diseases. Tokyo: Nankodo; 1998. [Google Scholar]
- 19.Kondo H, Soda M, Mine M, Yokota K. Effects of radiation on the incidence of prostate cancer among Nagasaki atomic bomb survivors. Cancer Sci. 2013;104:1368–71. doi: 10.1111/cas.12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watters JL, Park Y, Hollenbeck A, Schatzkin A, Albanes D. Cigarette smoking and prostate cancer in a prospective US cohort study. Cancer Epidemiol Biomarkers Prev. 2009;18:2427–35. doi: 10.1158/1055-9965.EPI-09-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clegg LX, Reichman ME, Miller BA, et al. Impact of socioeconomic status on cancer incidence and stage at diagnosis: selected findings from the surveillance, epidemiology, and end results: National Longitudinal Mortality Study. Cancer Causes Control. 2009;20:417–35. doi: 10.1007/s10552-008-9256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hiyama T, Sato T, Yoshino K, Tsukuma H, Hanai A, Fujimoto I. Second primary cancer following laryngeal cancer with special reference to smoking habits. Jpn J Cancer Res. 1992;83:334–9. doi: 10.1111/j.1349-7006.1992.tb00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobayashi Y, Arimoto H, Ono I, Watanabe S. Multiple primary cancers in patients with initial laryngeal cancer. Jpn J Clin Oncol. 1990;20:128–33. doi: 10.1093/oxfordjournals.jjco.a039376. [DOI] [PubMed] [Google Scholar]
- 24.Botteri E, Iodice S, Bagnardi V, Raimondi S, Lowenfels AB, Maisonneuve P. Smoking and colorectal cancer: a meta-analysis. JAMA. 2008;300:2765–78. doi: 10.1001/jama.2008.839. [DOI] [PubMed] [Google Scholar]
- 25.Engeland A, Bjorge T, Haldorsen T, Tretli S. Use of multiple primary cancers to indicate associations between smoking and cancer incidence: an analysis of 500,000 cancer cases diagnosed in Norway during 1953-93. Int J Cancer. 1997;70:401–7. doi: 10.1002/(sici)1097-0215(19970207)70:4<401::aid-ijc5>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 26.Han MA, Choi KS, Lee HY, Kim Y, Jun JK, Park EC. Current status of thyroid cancer screening in Korea: results from a nationwide interview survey. Asian Pac J Cancer Prev. 2011;12:1657–63. [PubMed] [Google Scholar]
- 27.International Agency for Research on Cancer, World Health Organization. IARC Handbooks of Cancer Prevention, Tobacco Control, Vol. 11: Reversal of Risk After Quitting Smoking. Lyon: IARC Sci Publ; 2007. [Google Scholar]
- 28.Gao CM, Tajima K, Kuroishi T, Hirose K, Inoue M. Protective effects of raw vegetables and fruit against lung cancer among smokers and ex-smokers: a case-control study in the Tokai area of Japan. Jpn J Cancer Res. 1993;84:594–600. doi: 10.1111/j.1349-7006.1993.tb02018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosso S, Terracini L, Ricceri F, Zanetti R. Multiple primary tumours: incidence estimation in the presence of competing risks. Popul Health Metr. 2009;7:5. doi: 10.1186/1478-7954-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]