Abstract
The aim of this study was to determine whether pretreatment status of human epidermal growth factor receptor-2 (HER-2) could predict pathologic response to neoadjuvant chemoradiotherapy (nCRT) and outcomes for patients with locally advanced rectal cancer (LARC). A total of 119 patients diagnosed with LARC received standardized multimodal treatment. Their HER-2 status was determined in pretreatment biopsies by immunohistochemistry (IHC) and FISH. Tumor response was assessed in resected regimens using the tumor regression grade system and TNM staging system. Twenty-two cases in 119 patients assessed as IHC3+ or IHC2+ plus gene-amplified were determined as HER-2 positive. Positive HER-2 status was not associated with any pretreatment clinicopathologic parameters (P > 0.05). HER-2 status could not predict pathologic response to nCRT based on downstaging (P = 0.210) and tumor regression grade (P = 0.085) but it provides us with a trend that HER-2-positive tumors may be resistant to nCRT. Positive HER-2 status was significantly associated with poor 5-year disease-free survival (P = 0.015) and 5-year overall survival (P = 0.026). It can act as a worse prognostic factor for LARC patients.
Keywords: HER-2, neoadjuvant chemoradiotherapy, rectal cancer, survival, tumor response
Rectal cancer is one of the leading causes of cancer-related deaths in the world.(1) The implementation of neoadjuvant chemoradiotherapy (nCRT) following surgery reduced the local recurrence risk and increased the sphincter preservation rate of rectal cancer.(2,3) For the above benefits, nCRT combined with surgery has been implemented as a standard treatment strategy of locally advanced rectal cancer (LARC).(4,5) The pathological response is an important prognostic factor for LARC.(6) But there is a wide spectrum of response to nCRT, ranging from none to complete. The variety of tumor response has increased the need to find a useful predictive model for the response of rectal cancer to nCRT, which may be helpful in the design of individualized treatment or early surgery in non-responders.
The HER-2 (c-erbB-2) oncoprotein is a 185-kDa transmembrane cell surface receptor of the human epidermal growth factor family and usually expresses on epithelial cells.(7) The tyrosine kinase activity of HER-2 intracellular domain triggers signal transduction that has important roles in cell proliferation, differentiation, and survival.(8) As a member of the epidermal growth factor receptor (EGFR) tyrosine kinase receptor superfamily, the HER-2 protein is extensively homologous and related to EGFR(9). Expression of EGFR has been proved an indicator for tumor response to radiotherapy in head and neck carcinoma,(10) as well as an indicator for poor response to nCRT in LARC.(11) Kim et al.(12) found a low level of EGFR expression may be a significant predictive molecular marker for increased tumor downstaging after CRT. Therefore, we raised the question whether HER-2 may play a role in predicting the response to nCRT in LARC as well.
Originally, HER-2 was widely studied in breast cancer and its overexpression has been documented in 10–34% of invasive cancer cases.(13) Overexpression of HER-2 is typically correlated with higher aggression and a poorer prognostic outlook in breast cancer.(14) Favorable HER-2 function in breast cancer has led to the analysis of HER-2 positivity in other solid tumors. Amplification and/or overexpression of HER-2 has been detected in endometrial,(15) lung,(16) gastric,(17,18) and esophageal cancers.(19) However, the frequency of HER-2 positivity and the role of predicted survival in LARC have not been adequately studied.
In the present study, we determined the status of HER-2 in LARC before nCRT treatment and analyzed whether HER-2 status correlated with clinicopathologic parameters, tumor response, and prognosis to characterize molecular predictive markers.
Materials and Methods
Patients and clinical assessment
The clinical and pathological features were reviewed retrospectively for the purpose of the study. All patients were diagnosed with primary rectal adenocarcinoma and localized within 15 cm above the anal verge, confirmed by rigid rectoscopy. Before treatment, TNM stage was determined by a series of examinations including physical examination, carcinoembryonic antigen serum level, peripheral blood cell count, chest computed tomography (CT), contrast-enhanced CT, and/or MRI of the abdomen and pelvis. The TNM stages were reported according to the American Joint Committee on Cancer (AJCC).(20) A total of 131 patients with clinical stage T3–T4 disease excluding distant metastasis underwent preoperative chemoradiotherapy. Twelve cases were excluded for lack of enough viable tumor cells. The baseline characteristics of the extracted 119 patients were similar to that of the overall population. Informed consent was obtained from all patients and the research protocols were approved by the Ethics Committee of Shandong Cancer Hospital and Institute.
Multimodal treatment
All patients received radiotherapy and chemotherapy followed by surgery. In brief, patients underwent whole pelvis preoperative radiotherapy with a dose of 45 Gy in 25 fractions, and then a boost of 5.4 Gy in three fractions to the primary gross tumor using 3-D conformal irradiation or four-field box technique. Four kinds of chemotherapeutic regimens commonly used in treating colorectal cancer (CRC) were proposed for the patients in the current study. Eligibility criteria for individualized protocol included age, Eastern Cooperative Oncology Group performance status, and economic factors. Patients were grouped according to treatment protocol: 5-fluorouracil group (5-FU), radiotherapy plus continuous infusion of 5-FU (500 mg/m2 days 1–5 and 29–33) followed by surgery and four cycles of continuous infusion of 5-FU (500 mg/m2 days 1–5), repeated every 28 days; capecitabine group, radiotherapy plus capecitabine (850 mg/m2 twice daily, days 1–14 and 22–35) followed by surgery and four cycles of capecitabine (1000–1250 mg/m2 twice daily, days 1–14), repeated every 21 days; 5-FU and oxaliplatin group, radiotherapy plus 5-FU (500 mg/m2 days 1–5 and 29–33) and oxaliplatin (85 mg/m2 days 1 and 29) followed by surgery and eight cycles 5-FU (2400 mg/m2 days 1–2) and oxaliplatin (100 mg/m2 day 1), repeated every 14 days; and capecitabine and oxaliplatin group, radiotherapy plus capecitabine (850 mg/m2 b.i.d., days 1–14 and 22–35) and oxaliplatin (85 mg/m2 days 1 and 22) followed by surgery and four cycles of capecitabine (850–1000 mg/m2 twice daily, days 1–14) and oxaliplatin (130 mg/m2 day 1), repeated every 21 days.
Pathologic assessment
Histopathological examination was carried out by two pathologists who were blinded to all clinical data. Pathologic TNM staging was carried out on the surgical specimens to assess for tumor downstaging analysis.
Tumor response was also evaluated using the tumor regression grade (TRG) system proposed by Dworak et al.(21) Details as follows: grade 0, no regression; grade 1, minor regression, dominant tumor mass with obvious fibrosis in 25% or less of the tumor mass; grade 2, moderate regression, dominantly fibrotic changes with tumor cell fibrosis in 26–50% of the tumor mass; grade 3, good regression, dominant fibrosis outgrowing the tumor mass, more than 50% of tumor regression; and grade 4, total regression, no viable tumor cells, only fibrotic mass. In the present study, TRG 3 and 4 were defined as “good response”, whereas TRG 0–2 were defined as “poor response.”
Immunohistochemical analysis of HER-2 status
Tumor samples were collected from pretreatment tumor biopsies. The HER-2 status was assessed using paraffin-embedded tissue samples that were cut into 5-μm slices. The process of staining was carried out according to the standard immunohistochemistry (IHC) protocol. Briefly, all sections were deparaffinized in xylene and rehydrated with distilled water through a graded series of ethanol solutions. Antigen retrieval was carried out under high pressure for 2 min. Non-specific binding was blocked by the application of normal rabbit serum (Beijing Zhongshan Golden Bridge Biotechnology, Beijing, China). The sections were stained with primary monoclonal rabbit anti-human HER-2 antibody (Abcam, Cambridge, UK) in humidified chamber at 37°C for 60 min, with a diluted ratio of 1:200. Secondary goat anti-rabbit antibody was incubated at 37°C for 15 min (Beijing Zhongshan Golden Bridge Biotechnology). HER-2 expression was visualized using 3,3′-diaminobenzidine and subsequently counterstained in hematoxylin. At last, all sections were dehydrated through a graded series of alcohol and xylene before being mounted under a coverslip.
Because of the inherent biological differences between breast cancer and cancers of the gastrointestinal tract, the similarity of rectal cancer and gastric cancer, the intensity of staining, and the percentage of cancer cells with staining intensity were used to determine the IHC score according to the ToGA trial:(18) absent (IHC 0); low (IHC1+), faint intensity in 10% or more; moderate (IHC2+), weak–moderate intensity in 10% or more; and high (IHC3+), strong intensity in 10% or more of cancer cells.
Amplification of HER-2 gene
HER-2 gene amplification was assessed in all specimens by the FISH using the PathVysion HER-2 DNA Probe Kit/centromere (CEP) 17 probes (Abbott Molecular, Des Plaines, IL, USA). Briefly, 5-μm tissue slides were deparaffinized and the specimens fixed prior to assay with the PathVysion Kit. After denaturation of DNA, 10 μL probe mixture was applied to the target area of the slide and immediately a coverslip was placed on the probe. Then slides were placed in the prewarmed humidified hybridization chamber and incubated at 37°C overnight. After washing and counterstaining, the complete tissue section was scanned to detect any subpopulation of amplified cells. A total of 60 representative nuclei from the invasive tumor were scored. A specimen with an HER-2/CEP17 ratio of 2.0 or more in invasive cells was classified as HER-2 amplification consistent with ToGA guidelines.(18)
Follow-up
Patients underwent a standardized post-treatment follow-up including physical examinations, carcinoembryonic antigen serum level, peripheral blood cell count, and chest X-ray every 3 months for the first 2 years and every 6 months thereafter. Patients also underwent abdominal and pelvic CT or MRI every 6 months. Colonoscopy was carried out within 1 year after treatment and then once every 2–3 years. Disease-free survival (DFS) was defined as the interval between the initiation date of treatment and any evidence of local or systemic cancer recurrence. Overall survival (OS) was defined as the interval between the initiation date of treatment and death from any causes or censored date of last contact for surviving patients with a median follow-up of 56 months.
Statistical analysis
For all the statistical analyses, spss 17.0 (SPSS Inc., Chicago, IL, USA) was used. The association of HER-2 status with other clinicopathologic parameters (age, sex, cT, cN, differentiation, and distance from anal verge) was assessed using the χ2-test. The association of TRG or downstaging with clinicopathologic parameters including HER-2 status was also assessed using the χ2-test. The impact of the HER-2 status on OS was determined using the Kaplan–Meier method. P-values <0.05 or 95% were considered statistically significant differences.
Results
Patient characteristics
The clinicopathological parameters of the 119 patients are detailed in Table 1. In brief, there were 70 (58.8%) male and 49 (41.2%) female with a median age of 62.1 years. Forty-two (35.3%) tumors were localized in the lower 6 cm from anal verge with 77 (64.7%) tumors localized in more than 6 cm above the anal verge. Among 119 patients with T3–4 tumors, there were 68 cases assessed as T3 and 51 cases as T4. Thirty-nine patients were clinically assessed as stage II, and 80 patients were clinically assessed as stage III. Regarding chemotherapy regimens: 24 patients received 5-FU and oxaliplatin, 32 patients received continuous infusion of 5-FU, 36 patients received capecitabine only and 27 patients received capecitabine and oxaliplatin.
Table 1.
Correlations between human epidermal growth factor receptor-2 (HER-2) expression and clinicopathological parameters in patients with locally advanced rectal cancer
| Clinicopathological parameters | Cases | HER-2 status | P-value | |
|---|---|---|---|---|
| Positiven (%) | Negativen (%) | |||
| Age, years | ||||
| <62 | 50 | 10 (45) | 40 (41) | 0.717 |
| ≥62 | 69 | 12 (55) | 57 (59) | |
| Sex | ||||
| Male | 74 | 13 (59) | 61 (63) | 0.740 |
| Female | 45 | 9 (41) | 36 (37) | |
| Histology | ||||
| Differentiated | 66 | 10 (45) | 56 (62) | 0.209 |
| Undifferentiated | 53 | 12 (55) | 41 (38) | |
| Distance from anal verge, cm | ||||
| <6 | 42 | 6 (27) | 36 (37) | 0.383 |
| ≥6 | 77 | 16 (73) | 61 (63) | |
| cT | ||||
| 3 | 68 | 11 (50) | 57 (59) | 0.453 |
| 4 | 51 | 11 (50) | 40 (41) | |
| cN | ||||
| Negative | 39 | 8 (36) | 31 (32) | 0.691 |
| Positive | 80 | 14 (64) | 66 (68) | |
| Chemotherapy regimens | ||||
| 5-FU/capecitabine | 68 | 14 (64) | 54 (56) | 0.495 |
| Capecitabine/5-FU and oxaliplatin | 51 | 8 (36) | 43 (44) | |
5-FU, 5-fluorouracil.
A comparison between pretreatment staging results and histopathological diagnosis after surgery (Table 2) revealed AJCC downstaging in 63 (52.9%) patients. According to the histopathological TRG system, there were 52 (43.7%) patients with good response (TRG 3–4) and 67 (56.3%) patients with poor response (TRG 0–2).
Table 2.
Tumor and nodal locoregional staging before treatment and after surgery in patients with locally advanced rectal cancer
| Tumor | cT3n (%) | cT4n (%) |
|---|---|---|
| pT0 | 11 (16) | 4 (8) |
| pT1 | 12 (18) | 5 (10) |
| pT2 | 15 (22) | 9 (18) |
| pT3 | 29 (43) | 10 (20) |
| pT4 | 1 (1) | 23 (44) |
| Nodal | cN0 | cN+ |
| pN0 | 39 (100) | 43 (54) |
| pN+ | 0 (0) | 37 (46) |
| Downstaging | 63 (53) | |
| Non-downstaging | 56 (47) | |
| Tumor regression grading | ||
| TRG 0 | 4 (3) | |
| TRG 1 | 18 (15) | |
| TRG 2 | 45 (38) | |
| TRG 3 | 37 (31) | |
| TRG 4 | 15 (13) | |
HER-2 status and correlation with clinicopathologic parameters
There were 31 (26.0%) cases of the 119 investigated rectal tumors classified as IHC score 0, 29 (24.4%) cases classified as IHC score 1+, 42 (35.3%) cases classified as IHC score 2+, and 17 (14.3%) cases classified as IHC score 3+ (Fig. 1). Amplification of the HER-2 gene was detected in 5% (3/60) of IHC 0–1, 12% (5/42) of IHC 2+, and 94% (16/17) of IHC 3+ samples (Fig. 2).
Figure 1.
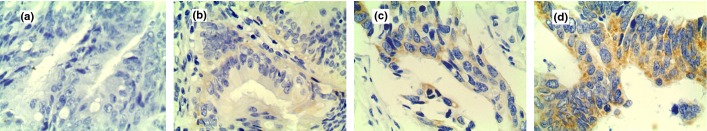
Immunohistochemical staining for human epidermal growth factor receptor-2 (HER-2) in rectal cancer cells. (a) No staining observed (0). (b) Faint staining detected in >10% of tumor cells (1+). (c) Moderate staining observed in >10% of tumor cells (2+). (d) Strong staining observed in >10% of tumor cells (3+). Magnification, ×400.
Figure 2.
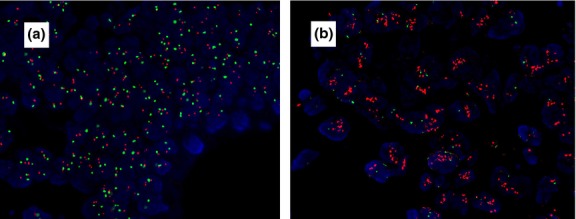
Fluorescence in situ hybridization in rectal cancer cells. (a) Tumors showed no HER-2 amplification. (b) Tumors showed HER-2 amplification with a HER-2/CEP17 ratio >2.0. Magnification, ×400.
The HER-2 status was defined as positive if tissue samples were scored as IHC3+ or IHC 2+ plus gene-amplified, which is the group that derived greatest benefit from trastuzumab in ToGA(18) and the standard approved in Europe for trastuzumab eligibility.(22) The remaining cases were considered HER-2 negative if they were scored as IHC 0–1 or IHC 2+ without gene-amplification. In all, 17 cases scored as IHC3+ and 5 cases scored as IHC 2+ plus gene-amplified were determined as HER-2 positive. Correlation analyses showed a lack of significant association of HER-2 positivity with any pretreatment clinicopathologic parameters such as sex, age, differentiation, cT, cN, or AJCC stage.
Correlations between clinicopathological parameters and tumor response
To determine the ability of clinicopathological parameters to predict downstaging and good histopathological tumor response to nCRT, the χ2-test was used (Table 3). Lack of node metastasis showed significant correlation with downstaging (P = 0.036) and good tumor regression (P = 0.019). Tumors graded as T3 were more likely to achieve good response than those graded as T4 (P = 0.048). Based on AJCC staging analysis, 9 cases in 22 HER-2-positive patients achieved downstaging, and 54 cases in 97 HER-2-negative patients (P = 0.210). Base on TRG analysis, 6 cases in 22 HER-2-positive patients achieved good response, and 46 cases in 97 HER-2-negative patients (P = 0.085). It seemed that HER-2-negative tumors might be more sensitive to nCRT. However, the associations both in downstaging and TRG response were not statistically significant (P = 0.210 and P = 0.085).
Table 3.
Correlations between clinicopathological parameters and tumor response in patients with locally advanced rectal cancer
| Clinicopathological parameters | Cases | TNM | P-value | TRG | P-value | ||
|---|---|---|---|---|---|---|---|
| Downstaging n (%) | Non-downstaging n (%) | Good response n (%) | Poor response n (%) | ||||
| Age, years | |||||||
| <62 | 50 | 26 (41) | 24 (43) | 0.861 | 25 (48) | 25 (37) | 0.238 |
| ≥62 | 69 | 37 (59) | 32 (57) | 27 (52) | 42 (63) | ||
| Sex | |||||||
| Male | 74 | 44 (70) | 30 (54) | 0.303 | 35 (67) | 39 (58) | 0.310 |
| Female | 45 | 19 (30) | 26 (46) | 17 (33) | 28 (42) | ||
| Distance from anal verge, cm | |||||||
| <6 | 42 | 24 (38) | 18 (32) | 0.498 | 19 (36) | 23 (34) | 0.802 |
| ≥6 | 77 | 39 (62) | 38 (68) | 33 (63) | 44 (66) | ||
| Histology | |||||||
| Differentiated | 66 | 32 (51) | 34 (61) | 0.277 | 25 (48) | 41 (61) | 0.153 |
| Undifferentiated | 53 | 31 (49) | 22 (39) | 27 (52) | 26 (39) | ||
| cT stage | |||||||
| 3 | 68 | 37 (59) | 31 (55) | 0.528 | 35 (67) | 33 (49) | 0.048 |
| 4 | 51 | 26 (41) | 25 (45) | 17 (33) | 34 (51) | ||
| cN stage | |||||||
| Negative | 39 | 26 (41) | 13 (23) | 0.036 | 23 (44) | 16 (24) | 0.019 |
| Positive | 80 | 37 (59) | 43 (77) | 29 (56) | 51 (76) | ||
| AJCC stage | |||||||
| II | 39 | 22 (35) | 17 (30) | 0.597 | 20 (38) | 19 (28) | 0.244 |
| III | 80 | 41 (65) | 39 (69) | 32 (61) | 48 (72) | ||
| Chemotherapy regimens | |||||||
| 5-FU/capecitabine | 68 | 32 (51) | 36 (64) | 0.138 | 27 (52) | 41 (61) | 0.239 |
| Capecitabine/5-FU and oxaliplatin | 51 | 31 (49) | 20 (36) | 25 (48) | 26 (39) | ||
| HER-2 | |||||||
| Positive | 22 | 9 (14) | 13 (23) | 0.210 | 6 (12) | 16 (24) | 0.085 |
| Negative | 97 | 54 (86) | 43 (77) | 46 (88) | 51 (76) | ||
5-FU, 5-fluorouracil; AJCC, American Joint Committee on Cancer; HER-2, human epidermal growth factor receptor-2; TRG, tumor regression grade.
Survival analysis
Node status (N), HER-2 status, and depth of invasion (T) were found to be significantly correlated with 5-year DFS and OS, as listed in Table 4. The 5-year DFS of 22 patients with HER-2 positive status was significantly shorter than that of 97 patients with HER-2 negative status (P = 0.015) (Fig. 3a). In the total study population, HER-2-negative patients had significantly better OS as compared with HER-2-positive patients (P = 0.026) (Fig. 3b). Compared with the 5-FU/capecitabine group, the patients in the 5-FU/capecitabine and oxaliplatin group had longer DFS and OS, but the difference was not statistically significant (P = 0.213 and P = 0.346).
Table 4.
Correlations between clinicopathological parameters and survival in locally advanced rectal cancer
| Parameters | Cases | 5-year DFS, % | P-value | 5-year OS, % | P-value |
|---|---|---|---|---|---|
| Age, years | |||||
| <62 | 50 | 66.3 | 0.801 | 80.1 | 0.391 |
| ≥62 | 69 | 62.3 | 68.5 | ||
| Sex | |||||
| Male | 74 | 65.6 | 0.629 | 74.1 | 0.924 |
| Female | 45 | 61.3 | 71.1 | ||
| Distance from anal verge, cm | |||||
| <6 | 42 | 61.2 | 0.379 | 72.5 | 0.731 |
| ≥6 | 77 | 65.3 | 73.0 | ||
| Histology | |||||
| Differentiated | 66 | 66.0 | 0.893 | 77.9 | 0.338 |
| Undifferentiated | 53 | 61.8 | 67.5 | ||
| cT stage | |||||
| 3 | 68 | 70.3 | 0.037 | 78.7 | 0.041 |
| 4 | 51 | 55.3 | 63.1 | ||
| cN stage | |||||
| Negative | 39 | 77.1 | 0.021 | 84.2 | 0.027 |
| Positive | 80 | 57.2 | 65.9 | ||
| Chemotherapy regimens | |||||
| 5-FU/capecitabine | 68 | 60.7 | 0.213 | 72.6 | 0.346 |
| Capecitabine/5-FU and oxaliplatin | 51 | 68.4 | 75.0 | ||
| HER-2 | |||||
| Positive | 22 | 50.8 | 0.015 | 55.6 | 0.026 |
| Negative | 97 | 67.2 | 76.8 | ||
5-FU, 5-fluorouracil; DFS, disease-free survival; HER-2, human epidermal growth factor receptor-2; OS, overall survival.
Figure 3.
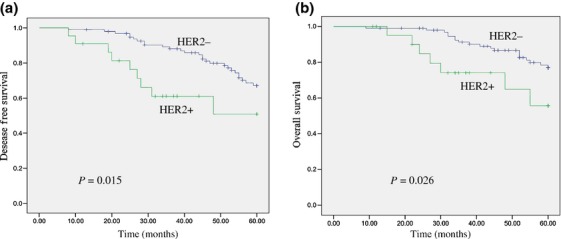
Kaplan–Meier estimates of disease-free survival (DFS) and overall survival (OS) rates in relation to human epidermal growth factor receptor-2 (HER-2) status. (a) HER-2 positivity in rectal cancers correlate with a shorter DFS curves (P = 0.015). (b) HER-2 positivity in rectal cancers correlate with a shorter OS curves (P = 0.026).
Discussion
In this study, we determined the HER-2 frequency in LARC by FISH and IHC. In the pretreatment biopsies, 17 cases with IHC3+ and 5 cases with IHC 2+ plus gene-amplification were determined as HER-2 positive. The positivity of 18.5% was not consistent with other previously published reports. Park et al.(23) detected positive immunostaining (2+ or 3+) for the HER-2 protein in 65 (47.4%) of the 137 colorectal carcinomas. Drebber et al.(24) found HER-2 staining in 27% of 54 rectal cancers biopsies. In a quite recent study, HER-2 expression analysis of CRC tissues revealed HER-2 moderate overexpression in 13 (14%) patients and strong overexpression in 10 (11%) patients.(25) This discrepancy between our results and those of the previous studies can be explained by following factors. First, the standard for HER-2 positivity is varied, even in Park's study both IHC 2+ and IHC 3+ considered as HER-2 positive. Second, as indicated in the previous report,(26) there were differences of HER-2 positivity between biopsies and resected regimens. More importantly, most studies just determined the HER-2 status by IHC. We used both IHC and FISH to assess the status of HER-2, which may have influenced results. In line with previous studies,(24,26) we did not find any correlation between HER-2 positivity and clinicopathologic parameters.
Human epidermal growth factor receptor-2 is a therapeutic target in a variety of malignant tumors. It has been used to forecast patients' therapeutic response to adjuvant chemotherapy and endocrine therapy in breast cancer.(27) In metastatic colorectal cancer patients, HER-2 gene copy number status was indicated to correlate with the clinical response to anti-EGFR-targeted therapy (P = 0.0006).(28) In this study, we determined the association between HER-2 status and the pathologic response to nCRT in LARC based on the tumor regression grade system and downstaging system.
The TRG system proposed by Dworak in resected specimens is a method to assess treatment response to neoadjuvant therapy(21) and could be used for assessing tumor response and for early determination of treatment efficacy.(29,30) Based on TRG analysis, 6 cases in 22 HER-2-positive patients achieved good response, as did 46 (47%) cases in 97 HER-2-negative patients (P = 0.085). Tumor–node–metastasis downstaging has also been widely used to assess tumor response in LARC. After comparing the pretreatment staging results with histopathological diagnosis after surgery, we found 9 (41%) cases in 22 HER-2-positive patients achieved downstaging, as did 54 (56%) cases in 97 HER-2-negative patients (P = 0.210). The associations both in downstaging and TRG response were not statistically significant. The results from our current study are in accordance with the previous study.(26) But our study, for the first time, provides us with a trend that HER-2-positive tumors may be resistant to nCRT.
Overexpression of HER-2 has been found to serve as an independent predictor of shorter OS and DFS in many solid tumors.(17–19,31) However, data with respect to the putative role of HER-2 as an independent prognostic parameter in rectal cancer have so far been conflicting. A study from Japan reported OS for Dukes stage B patients was significantly lower in the group with cytoplasmic HER-2 overexpression compared to the group without expression.(32) Lim et al.(23) detected HER-2 overexpression in 23 patients (25%) and found the OS and DFS of patients negative for HER-2 expression was significantly better than that of patients positive for HER-2 expression (P = 0.018 and P = 0.021). The results from our current study are in accordance with those of the above-mentioned study. Both DFS and OS of patients with HER-2 positivity were indicated to be significantly shorter than those of patients with HER-2 negativity (P = 0.015 and P = 0.026, respectively). However, our study does not support the associations observed in other studies between HER-2 status and prognosis. Based on analysis of pretherapeutic biopsies, Drebber et al.(21) found patients with a higher HER-2 expression showed a significant survival benefit compared with patients having low protein levels. With a median follow-up of 46.5 months, patients with HER-2 positivity showed in trend a better DFS (P = 0.1) and a significant benefit in cancer-specific survival (P = 0.03).(24) We speculate that several reasons can explain the conflict between studies. First, different detection methods were used. Second, the discrepancies between data from Asia and data from Europe and North America may, at least partly, influence the results. Finally, the inherent limitation of previous studies was that the number of patients was small. Only 22 tumors in our study were determined to be HER-2-positive, which may contribute to discrepancies and errors in the results. Large consistent cohort studies and multicenter trials are needed to further validate the role of HER-2 status in rectal cancer.
However, some inherent limitations of this study might lead to biased results. First, the current study was retrospective and the number of patients was limited. Second, the chemotherapeutic regimens were various, which might influence patient response to CRT and prognosis. In this study, patients in the 5-FU/capecitabine and oxaliplatin group had better a response rate and a little longer DFS and OS than patients in the 5-FU/capecitabine group, but the difference was not statistically significant. The results are in accordance with a previous study.(33–35) It was hypothesized that radiotherapy with optimal radiosensitization by 5-FU/capecitabine already maximizes tumor responses and improvement in prognosis with little room for further improvement with the incorporation of oxaliplatin.(35)
In summary, 22 cases in 119 patients classified as IHC 3+ or IHC 2+ plus gene-amplified were determined as HER-2 positive. HER-2 positivity was not associated with tumor response to nCRT in LARC, but it provided us with a trend that HER-2-positive tumors may be resistant to nCRT. HER-2 positivity before nCRT was significantly associated with poor 5-year DFS (P = 0.015) and 5-year OS (P = 0.026). To further validate the role of HER-2 status in rectal cancer, large consistent cohort studies are needed.
Acknowledgments
The work was supported by National Natural Science Foundation of China (No. 81301868) and by the Shandong Province Science and Technology Development Program (Nos. 2012YD18086 and 2012YD18053).
Disclosure Statement
There authors have no conflict of interest.
References
- 1.Jemal A, Bray F, Center MM, et al. Forman D: global cancer statistics. CA Cancer J Clin. 2011;61:69–70. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.van Gijn W, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-Year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12:575–82. doi: 10.1016/S1470-2045(11)70097-3. [DOI] [PubMed] [Google Scholar]
- 3.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–40. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 4.Roh MS, Colangelo LH, O'Connell MJ, et al. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol. 2009;27:5124–30. doi: 10.1200/JCO.2009.22.0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rödel C, Liersch T, Becker H, et al. Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: initial results of the German CAO(ARO/AIO-04 randomised phase3 trial. Lancet Oncol. 2012;13:679–87. doi: 10.1016/S1470-2045(12)70187-0. [DOI] [PubMed] [Google Scholar]
- 6.Eich HT, Stepien A, Zimmermann C, et al. Neoadjuvant radiochemotherapy and surgery for advanced rectal cancer: prognostic significance of tumor regression. Strahlenther Onkol. 2001;187:225–30. doi: 10.1007/s00066-011-2113-1. [DOI] [PubMed] [Google Scholar]
- 7.Schechter AL, Stern DF, Vaidyanathan L. The neu oncogene: an erb-B-related gene encoding a 185 000-M(r) tumour antigen. Nature. 1984;312:513–6. doi: 10.1038/312513a0. [DOI] [PubMed] [Google Scholar]
- 8.Casalini P, Iorio MV, Galmozzi E, et al. Role of HER receptors family in development and differentiation. J Cell Physiol. 2004;200:343–50. doi: 10.1002/jcp.20007. [DOI] [PubMed] [Google Scholar]
- 9.Coussens L, Yang-Feng TL, Liao YC, et al. Tyrosine kinase receptor with extensive homology to EGF receptor shares chromosomal location with neu oncogene. Science. 1985;230:1132–9. doi: 10.1126/science.2999974. [DOI] [PubMed] [Google Scholar]
- 10.Eriksen JG, Steiniche T, Overgaard J, et al. The influence of epidermal growth factor receptor and tumor differentiation on the response to accelerated radiotherapy of squamous cell carcinomas of the head and neck in the randomized DAHANCA 6 and 7 Study. Radiother Oncol. 2005;74:93–100. doi: 10.1016/j.radonc.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 11.Giralt J, Eraso A, Armengol M, et al. Epidermal growth factor receptor is a predictor of tumor response in locally advanced rectal cancer patients treated with preoperative radiotherapy. Int J Radiat Oncol Biol Phys. 2002;54:1460–5. doi: 10.1016/s0360-3016(02)03752-5. [DOI] [PubMed] [Google Scholar]
- 12.Kim JS, Kim JM, Li S, et al. Epidermal growth factor receptor as a predictor of tumor downstaging in locally advanced rectal cancer patients treated with preoperative chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2006;66:195–200. doi: 10.1016/j.ijrobp.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 13.Kaptain S, Tan LK, Chen B. Her-2/neu and breast cancer. Diagn Mol Pathol. 2001;10:139–52. doi: 10.1097/00019606-200109000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Rubin I, Yarden Y. The basic biology of HER2. Ann Oncol. 2001;12:S3–8. doi: 10.1093/annonc/12.suppl_1.s3. [DOI] [PubMed] [Google Scholar]
- 15.Tangjitgamol S, Tanvanich S, Srijaipracharoen S, et al. Expression of estrogen receptor, progesterone receptor, and Her-2/neu in primary and extra-corporeal endometrial cancer. Histol Histopathol. 2013;28:787–94. doi: 10.14670/HH-28.787. [DOI] [PubMed] [Google Scholar]
- 16.Hirashima N, Takahashi W, Yoshii S, et al. Protein overexpression and gene amplification of c-erb B-2 in pulmonary carcinomas: a comparative immunohistochemical and fluorescence in situ hybridization study. Mod Pathol. 2001;14:556–62. doi: 10.1038/modpathol.3880350. [DOI] [PubMed] [Google Scholar]
- 17.Rüschoff J, Hanna W, Bilous M, et al. HER2 testing in gastric cancer: a practical approach. Mod Pathol. 2012;25:637–50. doi: 10.1038/modpathol.2011.198. [DOI] [PubMed] [Google Scholar]
- 18.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–97. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 19.Yoon HH, Shi Q, Sukov WR, et al. Association of HER2/ErbB2 expression and gene amplification with pathologic features and prognosis in esophageal adenocarcinomas. Clin Cancer Res. 2012;15:546–54. doi: 10.1158/1078-0432.CCR-11-2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sobin LH, Compton CC. TNM seventh edition: what's new, what's changed: communication from the International Union Against Cancer and the American Joint Committee on Cancer. Cancer. 2010;116:5336–9. doi: 10.1002/cncr.25537. [DOI] [PubMed] [Google Scholar]
- 21.Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis. 1997;12:19–23. doi: 10.1007/s003840050072. [DOI] [PubMed] [Google Scholar]
- 22.Albarello L, Pecciarini L, Doglioni C. HER2 testing in gastric cancer. Adv Anat Pathol. 2011;18:53–9. doi: 10.1097/PAP.0b013e3182026d72. [DOI] [PubMed] [Google Scholar]
- 23.Park DI, Kang MS, Oh SJ, et al. HER-2/neu overexpression is an independent prognostic factor in colorectal cancer. Int J Colorectal Dis. 2007;22:491–7. doi: 10.1007/s00384-006-0192-8. [DOI] [PubMed] [Google Scholar]
- 24.Drebber U, Madeja M, Odenthal M, et al. β-catenin and Her2/neu expression in rectal cancer: association with histomorphological response to neoadjuvant therapy and prognosis. Int J Colorectal Dis. 2011;26:1127–34. doi: 10.1007/s00384-011-1213-9. [DOI] [PubMed] [Google Scholar]
- 25.Lim SW, Kim HR, Kim HY, et al. Over-expression of Her-2 in colorectal cancer tissue, but not in serum, constitutes an independent worse prognostic factor. Cell Oncol (Dordr) 2013;36:311–21. doi: 10.1007/s13402-013-0136-6. [DOI] [PubMed] [Google Scholar]
- 26.Conradi LC, Styczen H, Sprenger T, et al. Frequency of HER-2 Positivity in Rectal Cancer and Prognosis. Am J Surg Pathol. 2013;37:522–31. doi: 10.1097/PAS.0b013e318272ff4d. [DOI] [PubMed] [Google Scholar]
- 27.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 28.Martin V, Landi L, Molinari F, et al. HER2 gene copy number status may influence clinical efficacy to anti-EGFR monoclonal antibodies in metastatic colorectal cancer patients. Br J Cancer. 2013;108:668–75. doi: 10.1038/bjc.2013.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rödel C, Martus P, Papadoupolos T, et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol. 2005;23:8688–96. doi: 10.1200/JCO.2005.02.1329. [DOI] [PubMed] [Google Scholar]
- 30.Rosenberg R, Nekarda H, Zimmermann F, et al. Histopathological response after preoperative radiochemotherapy in rectal carcinoma is associated with improved overall survival. J Surg Oncol. 2008;97:8–13. doi: 10.1002/jso.20844. [DOI] [PubMed] [Google Scholar]
- 31.Harada S, Mick R, Roses RE, et al. The significance of HER-2/neu receptor positivity and immunophenotype in ductal carcinoma in situ with early invasive disease. J Surg Oncol. 2011;104:458–65. doi: 10.1002/jso.21973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Osako T, Miyahara M, Uchino S, et al. Immunohistochemical study of c-erbB-2 protein in colorectal cancer and the correlation with patient survival. Oncology. 1998;55:548–55. doi: 10.1159/000011911. [DOI] [PubMed] [Google Scholar]
- 33.Gérard JP, Azria D, Gourgou-Bourgade S, et al. Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: results of thephase III trial ACCORD 12/0405-Prodige 2. J Clin Oncol. 2010;28:1638–44. doi: 10.1200/JCO.2009.25.8376. [DOI] [PubMed] [Google Scholar]
- 34.Gérard JP, Azria D, Gourgou-Bourgade S, et al. 2012;30:389. Clinical results at 3 years of the accord 12 randomized trial in rectal cancer [abstract]. ASCO Meeting Abstracts. [Google Scholar]
- 35.Aschele C, Cionini L, Lonardi S, et al. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer:pathologic results of the STAR-01 randomized phase III trial. J Clin Oncol. 2011;29:2773–80. doi: 10.1200/JCO.2010.34.4911. [DOI] [PubMed] [Google Scholar]


