Abstract
Narrow-band imaging (NBI) has been reported to be useful for detecting superficial-type esophageal or head and neck squamous cell carcinoma (SCC), and in the present study we have used NBI to detect non-carcinomatous lesions, such as basal cell hyperplasia (BCH) accompanied by microvascular irregularities; these non-carcinomatous lesions were pathologically discriminated from squamous cell carcinoma of the pharynx. The aim of the present study was to clarify the endoscopic characteristics of BCH that contribute to the discrimination of superficial-type head and neck SCC (HNSCC). We examined the key endoscopic findings capable of distinguishing BCH from SCC using 26 BCH and 37 superficial-type SCC of the pharynx that had been pathologically diagnosed at our institution between January 2008 and July 2012. The clinicopathological factors were also compared. The size of the BCH lesions was significantly smaller (P < 0.001), and their intervascular transparency was more clearly observed (P < 0.001). Intra-epithelial papillary capillary loop (IPCL) shapes were less variable and monotonous (P < 0.001), and the distribution of the IPCL was more regular with an interval comparable to that of SCC (P < 0.001), although no significant differences in the sharpness of the lesion border, dilatation of IPCL and tortuosity of the IPCL were seen between the BCH and SCC lesions. This study revealed that BCH was an independent entity in terms of not only pathological findings, but also endoscopic findings observed using NBI, such as the regular distribution of IPCL and the preserved intervascular transparency.
Keywords: Basal cell hyperplasia, head and neck cancer, intra-epithelial papillary capillary loop, narrow-band imaging, pharynx
New innovations in endoscopic modalities have enabled more lesions, such as small or flat-type lesions in the mucosa of the gastrointestinal tract and the head and neck region, to be detected at an early phase. One of these revolutionary modalities is narrow-band imaging (NBI), which makes it possible to visualize microvascular irregularities, also known as intra-epithelial papillary capillary loop (IPCL) abnormalities, of the squamous epithelium. Superficial-type squamous epithelial lesions have been identified using NBI in combination with magnifying endoscopy (NBI-ME).(1–5) The strategy behind this method is to recognize the characteristics of IPCL in neoplastic lesions, such as dilatation, tortuosity, caliber changes in one IPCL, variations in multiple IPCL and color changes in the background mucosa, which are completely different from those seen in normal squamous epithelium.(1) The sensitivity and specificity of NBI endoscopy for the detection of esophageal squamous cell carcinoma (SCC) and high-grade intraepithelial neoplasia are reported to be 90.9 and 95.4%, respectively, suggesting that NBI may be a useful and reliable method of screening for early neoplastic lesions in the esophagus.(4) Muto et al. (2004) first reported that it was possible to identify superficial HNSCC during an early phase as a “brownish area” using NBI-ME, although, up to then, it had been possible to detect only advanced SCC using the conventional modality in the head and neck region.(6)
Thus, early-phase superficial-type SCC, which cannot be detected by an ordinary endoscopy examination, can be detected using NBI-ME. Patients with superficial-type SCC can benefit from early detection through the preservation of important organs, such as the pharynx and larynx, associated with critical and physiological functions including swallowing and vocalization, because since such lesions can be completely resected endoscopically. However, superficial and flat lesions are sometimes recognized in the pharynx as brownish areas using NBI but are not pathologically diagnosed as SCC or dysplasia when a biopsy specimen is examined pathologically. Most of these lesions are pathologically diagnosed as basal cell hyperplasia (BCH) with IPCL atypia.(7) BCH accompanied by microvascular abnormalities was recently reported.(7) BCH exhibit IPCL abnormalities, such as upward extension, dilatation and branching, but do not fulfill the criteria for dysplasia with regard to structural and cellular atypia, which are clearly observed in neoplastic lesions arising in squamous epithelium.(7,8) As described above, the squamous epithelial lesions that are recognized as brownish areas using NBI-ME can include BCH as well as neoplastic lesions. Therefore, the aim of the current study was to determine the endoscopic features of BCH in the pharynx using NBI and to clarify how neoplastic lesions, such as SCC, can be discriminated from other lesions that are recognized as brownish areas so as to enable endoscopic diagnoses. The construction of new diagnostic criteria based on the NBI-ME findings in the present study may contribute to appropriate decisions regarding whether superficial squamous epithelial lesions should be treated or followed up carefully.
Materials and Methods
Patients
Patients who were pathologically diagnosed as having BCH based on endoscopic biopsy samples of the pharynx obtained between January 2008 and July 2012 at our institute were included in the present study. Endoscopic images of superficial-type HNSCC that had been diagnosed pathologically were also used to compare the endoscopic findings for BCH and SCC of the pharynx. For all cases, clear images were obtained using NBI with or without magnification using a GIF H260Z (Olympus, Tokyo, Japan) or in some cases using a GIF Y0002 (Olympus) for a much higher-power magnification (maximum of 380-fold magnification). Cases without NBI-ME images or with only unsuitable images (blurry or unfocused) were excluded from this study. All the biopsy samples were diagnosed pathologically based on the World Health Organization classification of tumors (head and neck tumors)(8); BCH with IPCL atypia was diagnosed based on the criteria reported by our group.(7) Finally, 26 cases of BCH and 37 cases of HNSCC were analyzed in the current study.
Methods
The endoscopic images for each case were examined in detail with respect to location, macroscopic type, size, sharpness of the margin, intervascular transparency, and IPCL-findings including dilatation, tortuosity, shapes and distribution. Intervascular transparency and intervascular color changes in which the transparency was preserved or slightly impaired were also examined except in elevated lesions, which were hard to evaluate and compare.
The sizes of the BCH were estimated using forceps with a width of 6 mm when open (Radial Jaw [Boston Scientific, MA, USA]); the sizes of the SCC were measured pathologically.
The margin of each lesion was categorized into two groups: clear or ambiguous. We classified intervascular transparency into three categories: (i) preserved; (ii) slightly impaired (not completely transparent, but easily recognizable subepithelial vessels); and (iii) impaired (difficult or impossible to recognize subepithelial vessels). In cases where the lesion exhibited both preserved and (slightly) impaired intervascular transparency, the type of transparency was re-evaluated as “(slightly) impaired”. We classified the intervascular color changes into “positive” for apparently brownish areas and “negative” for areas that were not apparently brownish.
The caliber changes in the IPCL, which are a major characteristic of IPCL observed in SCC, were not examined in the present study because the GIF H260Z did not enable a magnification sufficient for measuring and comparing the calibers of the capillaries of the IPCL. Therefore, only IPCL dilatation was examined. The shapes of the IPCL were classified into three categories as follows: monotonous (most IPCL were composed of two to four loop-shaped capillaries), variable (IPCL were composed of various shapes including one-loop, two-to-four loop or non-loop capillaries) and intermediate (the shapes of the IPCL were regular).
In cases where the IPCL were distributed regularly between avascular or loosely vascular areas, we defined the appearance as a regular distribution of IPCL.
In cases where follow-up endoscopies were performed, the changes in the endoscopic findings were evaluated.
Statistical analysis
The lesion size and the endoscopic findings were statistically analyzed to clarify the features of BCH. Cases in which the sizes were not available were excluded from this analysis. The categorical data regarding endoscopic findings were compared using the Fisher exact test or the χ2-test, as appropriate. spss version 11 (SPSS Inc., Chicago, IL, USA) was used for all the statistical analyses. All the statistical tests were two-tailed, and the statistical significance was defined as P < 0.05.
This study was approved by the institutional review board of our hospital on 29 January 2013 (approved clinical study number 2012-298) and was undertaken in conformity with the provisions of the Declaration of Helsinki in 1995 (as revised in Tokyo in 2004).
Results
The characteristics of the BCH patients and those of the BCH and SCC lesions are shown in Tables 1 and 2, respectively. Three BCH cases had tiny satellite lesions adjacent to the main lesions. Each of these cases was considered as one lesion that included the satellite lesions. In three SCC cases, accurate sizes were unknown because of a piecemeal resection. The sizes of the BCH were significantly smaller (P < 0.001) than those of the SCC.
Table 1.
Patient characteristics
| n = 26 | |
| Median age (range) in years | 70 (53–83) |
| Sex: Male/female | 24/2 |
| Past or current history of SCC | |
| ESCC | 22 |
| HNSCC | 13 |
| Without SCC | 3 |
ESCC, esophageal squamous cell carcinoma; HNSCC, head and neck squamous cell carcinoma; SCC, squamous cell carcinoma.
Table 2.
Lesion characteristics
| BCH (n = 26) | SCC (n = 37) | |
|---|---|---|
| Number (%) | Number (%) | |
| Location | ||
| Oropharynx | 20 (73) | 14 (38) |
| Posterior wall | 15 (58) | 7 (19) |
| Lateral wall | 3 (8) | 3 (8) |
| Uvula | 1 (4) | 0 (0) |
| Vallecula | 1 (4) | 4 (11) |
| Hypopharynx | 8 (27) | 23 (62) |
| Posterior wall | 5 (15) | 2 (5) |
| Piriform sinus | 3 (12) | 18 (49) |
| Postcricoid area | 0 (0) | 3 (8) |
| Macroscopic type | ||
| Depressed | 0 (0) | 3 (8) |
| Elevated | 7 (19) | 11 (30) |
| Flat | 19 (73) | 23 (62) |
| Mixed with flat and elevated | 2 (8) | 0 (0) |
| Size | ||
| 1–5 mm | 23 (88) | 3 (8) |
| 6–10 mm | 1 (4) | 13 (35) |
| >10 mm | 2 (8) | 18 (49) |
| NA | 3 (8) | |
BCH, basal cell hyperplasia; NA, not available; SCC, squamous cell carcinoma.
Typical BCH and HNSCC cases are shown in Fig. 1. Both lesions were recognized as brownish areas on the NBI images; however, intervascular transparency and a regular distribution were only observed in the BCH lesion. The endoscopic findings for the BCH and SCC lesions are shown in Table 3. The typical findings for BCH were defined as a regular distribution of IPCL composed of 2–4 loop-shaped (“lasso-like”) capillaries, with all the cases except for one exhibiting this typical finding (Fig. 1). As a result, the BCH were significantly smaller (P < 0.001), had a more preserved intervascular transparency (flat type) (P < 0.001), had fewer variations in IPCL shapes (P < 0.001) and had a more regular distribution of IPCL (P < 0.001) than the SCC. In contrast, no significant difference in the sharpness of the margin was seen between the BCH and SCC lesions (P = 0.17), and the IPCL of both of them were dilated and tortuous. Among the 21 cases of non-elevated (flat or mixed with flat and elevated) BCH, the intervascular transparency was not impaired in any of the cases (i.e. preserved or slightly impaired) and the intervascular color changes were positive in two cases (10%, 2/21). Among the 26 cases of non-elevated (flat or depressed) SCC, 20 cases (77%) had an impaired intervascular transparency, and among the remaining 6 cases, the intervascular color change was positive in 3 cases (50%, 3/6). The features of BCH and SCC are summarized in Fig. 2.
Figure 1.
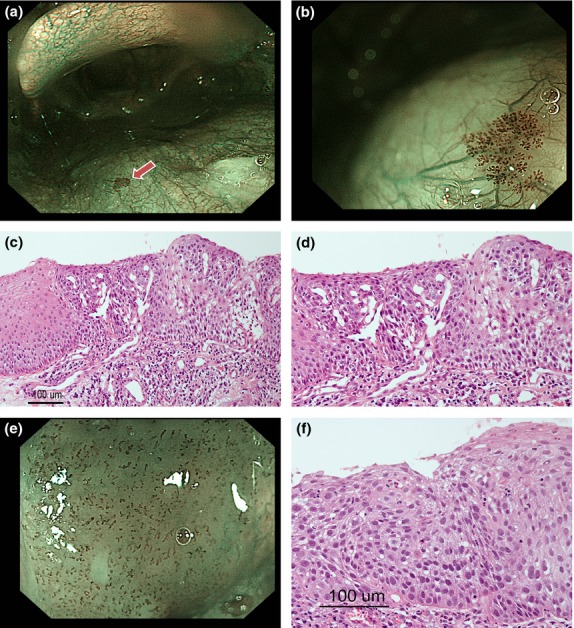
Narrow-band imaging (NBI) view and pathological findings of basal cell hyperplasia (BCH) and head and neck squamous cell carcinoma (HNSCC). (a) NBI view of BCH (arrowhead) without magnification. (b) NBI view of BCH with magnification. (c,d) (c) Pathological findings of BCH. Basal cell hyperplasia is visible within the area around the dilated IPCL, and the maturation of superficial cells is preserved. (d) The area around IPCL in figure (c) was enlarged. (e) NBI view of HNSCC with magnification. (f) Pathological findings of HNSCC, H.E. The whole epithelial layer is occupied by atypical cells.
Table 3.
Endoscopic findings
| BCH (n = 26) | SCC (n = 37) | P-value | |
|---|---|---|---|
| Number (%) | Number (%) | ||
| Border of the margin | |||
| Clear | 24 (92) | 37 (100) | 0.17 |
| Ambiguous | 2 (8) | 0 (0) | |
| Intervascular transparency | (n = 21) | (n = 26) | |
| Preserved | 15 (71) | 3 (12) | <0.001 |
| Slightly impaired | 6 (29) | 3 (12) | |
| Impaired | 0 (0) | 20 (77) | |
| IPCL findings | |||
| Dilatation | |||
| Yes | 26 (100) | 37 (100) | NA |
| No | 0 (0) | 0 (0) | |
| Tortuosity | |||
| Yes | 26 (100) | 37 (100) | NA |
| No | 0 (0) | 0 (0) | |
| Shapes | |||
| Monotonous | 25 (96) | 1 (3) | <0.001 |
| Intermediate | 1 (4) | 4 (11) | |
| Variable | 0 (0) | 32 (86) | |
| Distribution | |||
| Regular | 26 (100) | 4 (11) | <0.001 |
| Irregular | 0 (0) | 33 (89) | |
BCH, basal cell hyperplasia; IPCL, intra-epithelial papillary capillary loop; NA, not available; SCC, squamous cell carcinoma.
Figure 2.
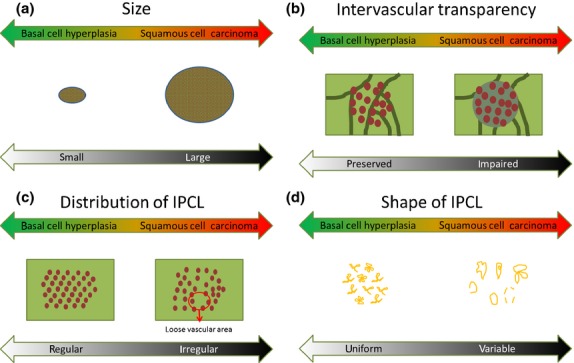
Schema of the four parameters (size, a; intervascular transparency, b; distribution of intra-epithelial papillary capillary loop (IPCL), c; shape of IPCL, d) for the differential diagnosis of basal cell hyperplasia (BCH) and squamous cell carcinoma (SCC). BCH tend to have the features on the left side of the schema, whereas SCC tend to have those on the right side.
Follow-up endoscopies were performed for 13 cases. The median follow-up period was 958 days (range: 182–2263 days). Follow-up biopsies were performed in five cases, and the diagnoses remained unchanged. Among them, the lesions in two cases changed in size, with 1 case showing a slight enlargement and the other showing a slight shrinkage. The other cases did not show any endoscopic changes.
Discussion
Basal cell hyperplasia in the pharynx was shown to exhibit characteristic NBI findings. The fact that some lesions that are clearly recognized as brownish areas in the pharynx turn out to not be carcinomas but BCH is clinically important, as accurate diagnosis can reduce unnecessary endoscopies or treatments. Muto et al. report 148 lesions resected using peroral endoscopic laryngopharyngeal mucosal resection.(9) All of these patients were treated under general anesthesia, and 17 patients (16%) required a temporary tracheostomy. This treatment is minimally invasive but does place physical and economic burden on the patient. Consequently, an accurate diagnosis is important to reduce unnecessary treatment. Lesions diagnosed as BCH are thought to have a low potential to change into cancer. Therefore, BCH is categorized as a non-cancerous entity.
For accurate diagnosis, we were able to obtain more information regarding brownish areas using NBI-ME than using NBI without magnification. In this study, we found that the color of the epithelium (in this study we used the term “intervascular color change”) in HNSCC observed using NBI varied from light green to brown, and color changes were difficult to evaluate objectively; instead, it was somewhat easier to evaluate intervascular transparency objectively. We analyzed intervascular transparency as one of the important features, while intervascular color changes were analyzed when the transparency was not impaired because the impaired transparency might strongly affect the color change.
Based on our data, we defined the typical findings of BCH as a regular distribution of IPCL composed of 2–4 loop-shaped (“lasso-like”) capillaries; all the cases in this series except for one had these typical findings. However, some minor variations existed with regard to intervascular transparency, intervascular color changes and macroscopic type. Consequently, the lesions with typical BCH findings are likely to have various genetic backgrounds. Based on the statistical analyses, the key points for differentiating BCH and SCC are shown in Fig. 2. Most of the BCH were small but were relatively easily recognizable, probably because they had a clear margin and a dense and regular distribution of dilated IPCL; however, we could not evaluate the IPCL density. In addition to these features, the IPCL of BCH were dilated and tortuous, causing the BCH to be misdiagnosed endoscopically as SCC in our previous endoscopic studies. No superficial HNSCC with both preserved transparency and the typical findings of BCH were observed. In contrast, 11 BCH cases exhibited both of these features without an intervascular color change, which was a finding of early ESCC. Thus, this type of BCH is unlikely to require intensive follow up. The long-term changes remain uncertain, however, so some follow up may be needed.
There are several limitations in the present study, including its retrospective design and the fact that it is a single-center study. We need to validate the outcome of this study in a prospective study.
For further understanding and clinical use, we have presented the images of a case with stepwise magnification in a schema shown in Fig. 3. An endocytoscope is not indispensable, but is helpful for understanding the features of BCH and SCC. In daily clinical situations, it may be difficult to magnify the pharynx adequately because of the gag reflex or the lack of magnifying endoscopes at some institutes. Even if it is impossible or difficult to magnify the images, the evaluation of intervascular transparency alone should be helpful, to some extent, in making a differential diagnosis.
Figure 3.
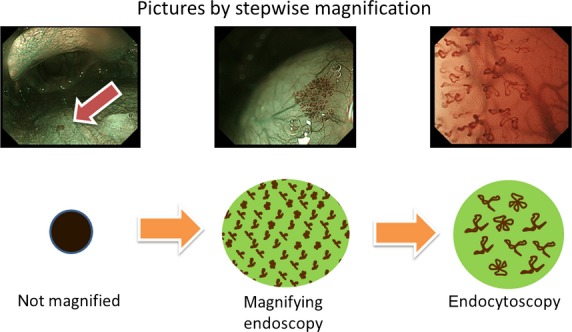
Narrow-band imaging view of basal cell hyperplasia with stepwise magnification and its schema. The left image is without magnification, the middle image is with magnification, and the right image is with magnification using an endocytoscope.
Finally, several points require further clarification. Whether BCH has malignant potential or not remains unclear, and whether BCH consists of a sole entity or includes several entities also remains uncertain. Long-term follow up or a gene analysis may resolve these questions.
In summary, we have presented the features of BCH in the pharynx as observed using NBI-ME; these findings are expected to be useful in the differential diagnosis of brownish areas in the pharynx as either BCH or SCC.
Acknowledgments
This work was supported by the National Cancer Center Research and Development Fund (23-A-15).
Disclosure Statement
The authors have no conflict of interest.
References
- 1.Yoshida T, Inoue H, Usui S, Satodate H, Fukami N, Kudo SE. Narrow-band imaging system with magnifying endoscopy for superficial esophageal lesions. Gastrointest Endosc. 2004;59:288–95. doi: 10.1016/s0016-5107(03)02532-x. [DOI] [PubMed] [Google Scholar]
- 2.Ishihara R, Inoue T, Uedo N, et al. Significance of each narrow-band imaging finding in diagnosing squamous mucosal high-grade neoplasia of the esophagus. J Gastroenterol Hepatol. 2010;25:1410–5. doi: 10.1111/j.1440-1746.2010.06378.x. [DOI] [PubMed] [Google Scholar]
- 3.Muto M, Minashi K, Yano T, et al. Early detection of superficial squamous cell carcinoma in the head and neck region and esophagus by narrow band imaging: a multicenter randomized controlled trial. J Clin Oncol. 2010;28:1566–72. doi: 10.1200/JCO.2009.25.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takenaka R, Kawahara Y, Okada H, et al. Narrow-band imaging provides reliable screening for esophageal malignancy in patients with head and neck cancers. Am J Gastroenterol. 2009;104:2942–8. doi: 10.1038/ajg.2009.426. [DOI] [PubMed] [Google Scholar]
- 5.Lee CT, Chang CY, Lee YC, et al. Narrow-band imaging with magnifying endoscopy for the screening of esophageal cancer in patients with primary head and neck cancers. Endoscopy. 2010;42:613–9. doi: 10.1055/s-0030-1255514. [DOI] [PubMed] [Google Scholar]
- 6.Muto M, Nakane M, Katada C, et al. Squamous cell carcinoma in situ at oropharyngeal and hypopharyngeal mucosal sites. Cancer. 2004;101:1375–81. doi: 10.1002/cncr.20482. [DOI] [PubMed] [Google Scholar]
- 7.Fujii S, Yamazaki M, Muto M, Ochiai A. Microvascular irregularities are associated with composition of squamous epithelial lesions and correlate with subepithelial invasion of superficial-type pharyngeal squamous cell carcinoma. Histopathology. 2010;56:510–22. doi: 10.1111/j.1365-2559.2010.03512.x. [DOI] [PubMed] [Google Scholar]
- 8.Cardesa AN, Gale N, Nadal A, Zidar N. Hypopharynx, larynx and trachea. In: Barnes L, Eveson JW, Reichar P, Sidransky D, editors. World Health Organization Classification of Tumours, Pathology and Genetics of Head and Neck Tumors. Lyon, France: IARC Press; 2005. pp. 118–21. [Google Scholar]
- 9.Muto M, Satake H, Yano T, et al. Long-term outcome of transoral organ-preserving pharyngeal endoscopic resection for superficial pharyngeal cancer. Gastrointest Endosc. 2011;74:477–84. doi: 10.1016/j.gie.2011.04.027. [DOI] [PubMed] [Google Scholar]


