Abstract
Pancreatic ductal adenocarcinoma (PDAC) is among the most deadly types of malignancies because of its high ability to metastasize. PDAC is thought to be under hypoxic condition. Therefore, to investigate the mechanism of metastatic processes, chronic-hypoxia-resistant PDAC cells were newly generated under hypoxic condition for 3–6 months and reoxygenation experiments were performed using these chronic-hypoxia-resistant PDAC cells in in vivo-mimicking conditions. Proliferation, invasiveness and tumorigenicity in PDAC cells were significantly increased by reoxygenation. A Hedgehog (Hh) signaling component, Gli1, was significantly increased by reoxygenation. Gli1 knockdown inhibited reoxygenation-induced increases in proliferation and tumorigenicity and decreased invasiveness through suppression of matrix metalloproteinase (MMP) 2 and MMP9. Moreover, inhibition of Sonic Hh and Smoothened abrogated reoxygenation induced increases in proliferation and invasiveness. These results suggest that metastatic processes in PDAC are induced through activation of the Hh signaling pathway. Therefore, the Hh signaling pathway may be a therapeutic target for refractory PDAC in metastatic processes induced by reoxygenation.
Keywords: Hedgehog, invasiveness, pancreatic cancer, proliferation, reoxygenation
Pancreatic ductal adenocarcinoma (PDAC) is one of the most fatal types of cancer.1 Therapy with gemcitabine and tegafur/gimeracil/oteracil potassium (S-1) has demonstrated improvements in prognosis for PDAC in Japan2; however, the effects are still limited. Thus, new effective therapeutic strategies for refractory PDAC are urgently required. The characteristics of invasiveness and high incidence of metastasis are among the causes of the poor prognosis of PDAC.3–5 To complete the metastatic process, cancer cells dissolve the extracellular matrix and basal membrane, progress into adjacent tissue, intrude into lymphatic vessels, and invade blood and metastatic organs.6 Consequently, it is important to understand the tumor microenvironment.
Tumor cells are normally located in hypoxic conditions of <1.3% O2.7 Oxygen tension is 5.3% in mixed venous blood and 3.3–7.9% in well-vascularized organs.8 Therefore, the process by which cancer cells detach from hypoxic tumors and metastasize to secondary sites via the blood stream is thought to be reoxygenation. Some researchers have shown that malignant phenotypic changes induced by reoxygenation, such as increases in proliferation, invasiveness and tumorigenicity, occur through the MEK/ERK and PI3K/Akt signaling pathways.9 However, the other mechanisms of phenotypic changes induced by reoxygenation remain unclear. Previously, we showed that invasiveness of PDAC cells increases under acute hypoxic conditions compared with normoxic conditions.10 However, because cancer cells in vivo are situated in chronic hypoxic conditions,7,11–14 to mimic and analyze in vivo local tumor sites, hypoxia-resistant tumor cells generated under chronic hypoxic conditions are required instead of tumor cells generated under normoxic or acute hypoxic conditions. We have also shown that Hedgehog (Hh) signaling plays a pivotal role in proliferation and invasiveness in PDAC cells under acute hypoxic conditions.10 The Hh signaling pathway is crucial for the growth, patterning and morphogenesis of multiple organs during embryonic development.15–17 Recently, it has been reported that Hh signaling is reactivated in various kinds of cancer, and, therefore, it may be a therapeutic target for cancer therapy.18–23 Of the Hh-related molecules, Gli1 is an activator of target genes and is itself a transcriptional target of the Hh pathway.15,24,25 Therefore, in this study we used Gli1 as a marker of Hh signal activation.
In the present study, to develop a new effective therapeutic strategy for refractory PDAC, especially with regard to proliferation, tumorigenicity and invasion in metastatic processes, we generated hypoxia-resistant PDAC cell lines for reoxygenation experiments focusing on the Hh signaling pathway, and investigated the mechanism of the induction of malignant phenotypes by reoxygenation.
Materials and Methods
Cell lines
PDAC cell lines (AsPC-1 and SUIT-2) were cultured in RPMI1640 (Nacalai Tesque, Kyoto, Japan) supplemented with 10% FBS (Life Technologies, Carlsbad, CA, USA), 100 U/mL penicillin (Meijiseika, Tokyo, Japan) and 100 μg/mL streptomycin (Meijiseika). For normoxic and reoxygenated conditions, cells were cultured in 5% CO2 and 95% air. For hypoxic conditions, cells were cultured in 1% O2, 5% CO2 and 94% N2 using a multi-gas incubator (Sanyo, Tokyo, Japan). To induce hypoxia-resistant AsPC-1 and SUIT-2 cells, these lines were cultured in hypoxic conditions; surviving cells were selected through each cell passage and were cultured under hypoxia for at least 3 months and up to 6 months because these cells we used are functionally stable between 3 and 6 months from initial culture (Fig. S1). A passage culture was performed twice a week. The culture medium used in the experiments and the passage culture was cultured in advance under hypoxia for at least 12 h before use. We named these generated cells chronic hypoxia-resistant PDAC cells (Ch-H-R cells). In proliferation, cell invasion and colony formation assays, Ch-H-R cells were trypsinized for 2 min, were spun down for 5 min after hypoxia-pretreated medium was added and were resuspended with hypoxia-pretreated medium. After cell counting, cells were re-plated to Transwell inserts and culture plates (Becton Deckinson Labware, Flanklin Lakes, NJ, USA). On reoxygenation research, Ch-H-R cells were pre-treated under reoxygenated condition for fewer than 5 days. A invasive activity, one of the major malignant phenotype, kept the same level on these cells (Fig. S2). Cell viability of Ch-H-R cells and reoxygenated Ch-H-R cells were almost over 95% and spindle-like-cell morphology was observed in Ch-H-R cells and reoxygenated Ch-H-R cells. As a control of chronic hypoxia, PDAC cells cultured in hypoxia for fewer than 5 days were named acute hypoxic (Ac-H) cells.
Cell invasion assay
The invasiveness of the cultured cells was assessed by the number of migrating cells through Matrigel-coated Transwell inserts, as described previously.26 The upper surface of a filter (pore size, 8.0 μm; BD Biosciences, Heidelberg, Germany) was coated with basement membrane Matrigel (BD Biosciences). Cells (1.0 × 104) were re-plated to the upper chamber and incubated in a medium for 16 h. The total number of cells that had migrated to the lower side of the filter was counted using a BZ-9000 (Biorevo; Keyence, Osaka, Japan) and Hybrid cell count software (Keyence).
Soft agar colony formation assay
Cultured cells were re-plated at a density of 1.0 × 104 cells/well into six-well plates in a 0.4% agar solution. Three weeks later, the cells were stained with 0.005% crystal violet (Sigma-Aldrich, St. Louis, MO, USA) solution. Colonies were imaged and counted in the same manner as for the cell invasion assay.
RNA interference
SiRNA for Gli1 (ON-TARGETplus SMART pool, L-003896), matrix metalloproteinase (MMP2) (ON-TARGETplus SMART pool, L-005959), MMP9 (ON-TARGETplus SMART pool, L-005970), siRNA for sonic Hh (Shh) (ON-TARGETplus SMART pool, L-006036), smoothened (Smo) (ON-TARGETplus SMART pool, L-005726) and negative control siRNA (ON-TARGETplus si CONTROL non-targeting pool, D-001810) were purchased from Dharmacon (Lafayette, CO, USA) and cells were transfected for 48 h according to the manufacturer's protocol. Cell viability is almost over 95% after gene knockdown experiments.
Isolation of RNA and real-time RT-PCR
Total RNA extraction and real-time RT-PCR were performed as described previously.10 All primer sets amplified fragments of <200-bp long. The primers sequences used were Gli1, forward, 5′-GGTTCAAGAGCCTGGGCTGTGT-3′, and reverse, 5′-GGCAGCATTCTCAGTGATGCTG-3′, MMP2, forward, 5′-TGATCTTGACCAGAATACCATCGA-3′, and reverse, 5′-GGCTTGCGAGGGAAGAAGTT-3′, MMP9, forward, 5′-TGGGCTACGTGACCTATGACAT-3′, and reverse, 5′-GCCCAGCCCACCTCCACTCCTC-3′, Smo, forward, 5′-CAGGTGGATGGGGACTCTGTGAGT-3′, and reverse, 5′-GAGTCATGACTCCTCGGATGAGG-3′, Shh, forward, 5′-GTGTACTACGAGTCCAAGGCAC-3′, and reverse, 5′-AGGAAGTCGCTGTAGAGCAGC-3′, ACTB, forward, 5′-TTGCCGACAGGATGCAGAAGGA-3′, and reverse, 5′-AGGTGGACAGCGAGGCCAGGAT-3′.
In vivo xenograft tumor model
Five-week-old female athymic nude mice (BALB/c nu/nu) were purchased from Charles River Laboratories Japan (Kanagawa, Japan) and acclimatized for 1 week. All animal procedures were approved by the Animal Care and Use Committee at Kyushu University. The cultured cells transfected with Gli1-targeting siRNA or non-targeting control siRNA, and control cells without siRNA were subcutaneously implanted into flank regions (1.0 × 106 cells in RPMI per mouse) of the nude mice. The tumor size was determined once or twice a week and the tumor volume was calculated with the following formula: length × (width)2 × 0.5 (mm3). The mice were killed when their tumor size had reached 2 cm in diameter or if the animals had become moribund during the observation period. The tumors were resected, fixed in 10% formalin and embedded in paraffin for immunohistochemistry.
Immunofluorescent staining
In immunocytochemistry, cultured cells were fixed with 4% paraformaldehyde (Nacalai Tesque) and permeabilized with 0.1% TRITON X-100 (Wako, Osaka, Japan). In immunohistochemistry, retrieval of GLI1 antigen in xenograft tumors was performed by heating tissue sections with 0.01 mol/L sodium citrate buffer (pH 6.0) at 95°C for 10 min. These samples were immersed with Blocking One Hist (Nacalai Tesque) and were incubated with a primary rabbit polyclonal antibody against hypoxia-inducible factor-1α (HIF-1α) (1:100; #3716; Cell Signaling, Danvers, MA, USA), a rabbit polyclonal antibody against MMP2 (1:200; sc-10736; Santa Cruz, Dallas, TX, USA), a rabbit polyclonal antibody against CA9 (1:1000; NB100-417, Novus Biologicals, Lyttelton, CO, USA), a goat polyclonal antibody against GLI1 (1:50; N-16; Santa Cruz) or a goat polyclonal antibody against MMP9 (1:200; sc-6840; Santa Cruz) at room temperature for 1 h, followed by secondary Alexa 488-conjugated goat anti-rabbit IgG, 488-conjugated donkey anti-goat IgG and 594-conjugated rabbit anti-goat IgG (Life Technologies), respectively. The nuclei were counterstained with DAPI (Life Technologies), and the protein expression levels were evaluated by fluorescence intensity under fluorescence microscopy (Carl Zeiss, Tokyo, Japan), as previously described.27 Briefly, CA9, MMP9 and GLI1 were allocated to the green channel, and HIF-1α and MMP2 were allocated to the red channel, while the nucleus was assigned the blue channel to visualize DAPI fluorescence. Red and green channel intensities were plotted as a histogram along a line scan, which revealed the fluorescence intensity. Using the intensity of the nuclear rising edge (stained by DAPI) as a standard, the expression intensity of each protein in single cells was relatively evaluated at the cell's maximum diameter. β-actin was used as a positive control. Staining with secondary antibody alone was used as a negative control.
Statistical analysis
The data are presented as the means ± SD. Student's t-tests were used to compare continuous variables between two groups. P-values of <0.05 was considered statistically significant.
Results
Reoxygenation enhances the malignant potential of pancreatic ductal adenocarcinoma cells
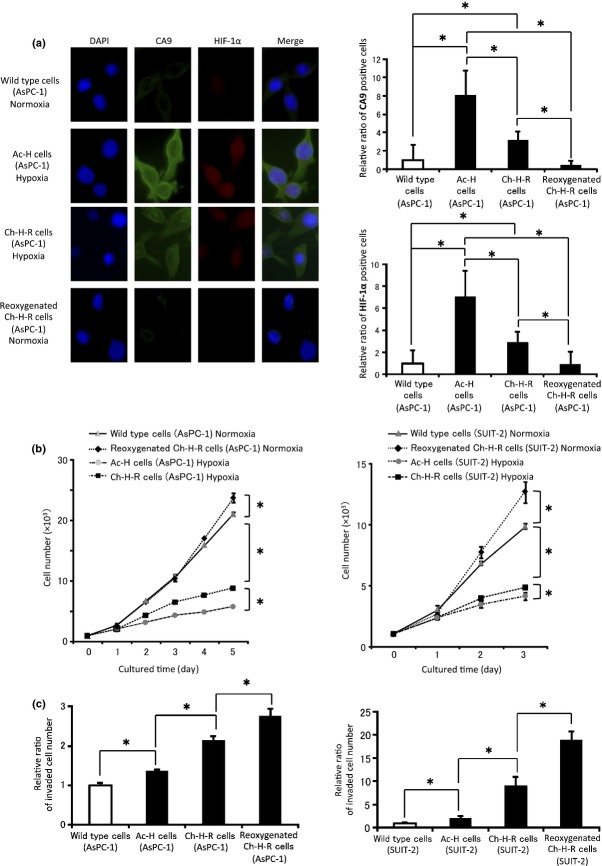
Wild type cells, chronic hypoxia-resistant PDAC cells (Ch-H-R cells), PDAC cells cultured under acute hypoxia (Ac-H cells) and reoxygenated Ch-H-R cells were used to investigate the change of phenotypes by reoxygenation. First, we evaluated HIF-1α, well known as one of the main hypoxic transcriptional factors, and its downstream protein CA9. CA9 positive cells were counted as described in Fig. S3. CA9 and HIF-1α were expressed in Ch-H-R cells and Ac-H cells. However, the amount of CA9 or HIF-1α positive cells in reoxygenated Ch-H-R cells was significantly lower than in Ch-H-R cells and Ac-H cells (Figs1a,S4), and CA9 and HIF-1α protein expression were also confirmed by western blotting analysis (Fig. S5). Next, we examined proliferation and invasiveness, factors of malignant potential. The ability of anchorage-dependent proliferation of reoxygenated Ch-H-R cells was the highest among the four types of cells both in AsPC-1 and SUIT-2 (Fig.1b). Invasiveness in Ch-H-R cells was significantly higher than that in Ac-H cells, and also significantly higher in reoxygenated Ch-H-R cells than in Ch-H-R cells both in AsPC-1 and SUIT-2 (Fig.1c). These results suggest that reoxygenation confers a more aggressive phenotype in PDAC cells.
Figure 1.
Reoxygenation enhances the malignant potential of PDAC cells. (a) Representative images of CA9 and HIF-1α expression by immunofluorescence in wild type cells, Ac-H cells, Ch-H-R cells and reoxygenated Ch-H-R cells derived from AsPC-1 (left panels) (×400). The graph shows the relative ratio of CA9 or HIF-1α positive cells (right panels). (b) Cell number in wild type cells, Ac-H cells, Ch-H-R cells and reoxygenated Ch-H-R cells were evaluated (n = 3). (c) Invaded cell number in wild type cells, Ac-H cells, Ch-H-R cells and reoxygenated Ch-H-R cells were analyzed (n = 3). The graph shows mean ± SD. *P < 0.05.
Reoxygenation enhances Gli1 and matrix metalloproteinase expression
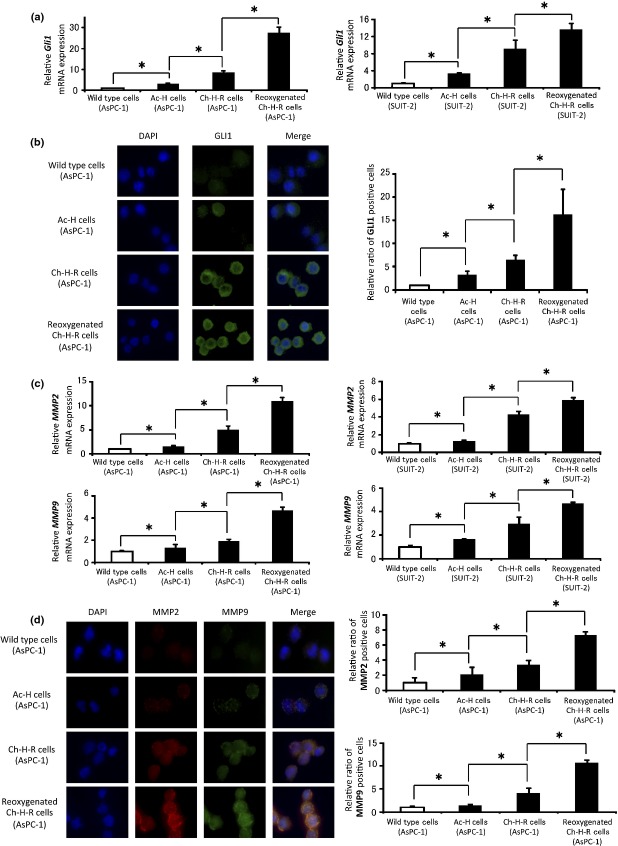
To analyze the mechanism of reoxygenation effects, we investigated the Hh signaling pathway because we previously reported it to be correlated with the augmentation of PDAC cell proliferation and invasiveness under acute hypoxia.10 In this study, Gli1 expression was considered a valuable marker of Hh signal activation. First, we evaluated Gli1 mRNA expression; expression of Gli1 mRNA was significantly higher in Ch-H-R cells than in Ac-H cells, and also significantly higher in reoxygenated Ch-H-R cells than in Ch-H-R cells (Fig.2a). The amount of Gli1 positive cells in reoxygenated Ch-H-R cells was significantly higher than in Ch-H-R cells (Figs2b,S6), and the GLI1 protein expression was also confirmed by western blotting analysis (Fig. S7). Next, we evaluated two matrix metalloproteinases, MMP2 and MMP9, as factors in reoxygenation-enhanced invasion. As expected, MMP2 and MMP9 mRNA expression and the ratios of positive cells were significantly increased after reoxygenation (Figs2c,d,S8), and MMP2 and MMP9 protein expression were also confirmed by western blotting analysis and gelatin zymography (Fig. S9a,b).
Figure 2.
Reoxygenation enhances Gli1 and MMP expression. (a) Gli1 mRNA expression in wild type cells, Ac-H cells, Ch-H-R cells and reoxygenated Ch-H-R cells were analyzed by real-time RT-PCR (n = 3). (b) Representative images of GLI1 expression in wild type cells, Ac-H cells, Ch-H-R cells and reoxygenated Ch-H-R cells derived from AsPC-1 cells by immunofluorescence (left panels) (×400). The graph shows the relative ratio of GLI1 positive cells (right panel). (c) MMP2 (upper panels) and MMP9 (lower panels) mRNA expression in wild type cells, Ac-H cells, Ch-H-R cells and reoxygenated Ch-H-R cells were analyzed by real-time RT-PCR (n = 3). (d) Representative images of MMP2 and MMP9 expression in wild type cells, Ac-H cells, Ch-H-R cells and reoxygenated Ch-H-R cells derived from AsPC-1 cells by immunofluorescence (left panels) (×400). The graph shows the relative ratio of MMP2 or MMP9 positive cells, respectively (right panels). The graph shows mean ± SD. *P < 0.05.
Augmentation of malignant potential by reoxygenation is induced by activation of the Hedgehog signaling pathway
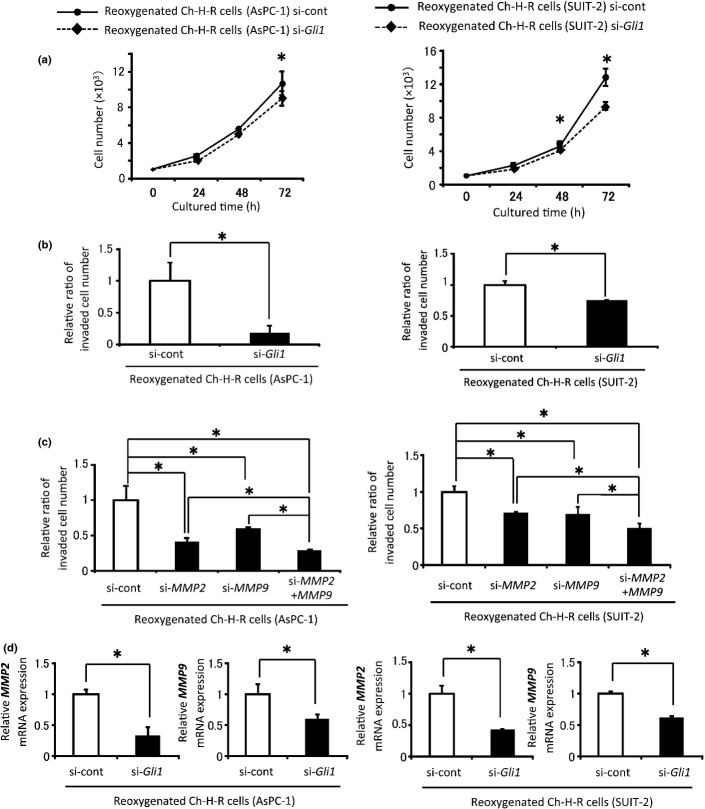
Next, the correlation between activation of Hh signaling and reoxygenation-induced proliferation and invasiveness was investigated. Transfection with Gli1 siRNA in reoxygenated Ch-H-R cells led to an 80–90% decrease in Gli1 mRNA expression (Fig. S10). Gli1 siRNA-transfected reoxygenated cells showed significant decreases in anchorage-dependent proliferation and invasion (Fig.3a,b). To investigate the effect of MMP on invasiveness, MMP siRNA was used. MMP2 and MMP9 knockdown efficiencies were approximately 70–90 and 60–80%, respectively (Fig. S11). The invasiveness of MMP2 and MMP9 siRNA-transfected reoxygenated Ch-H-R cells was significantly lower than that of the control cells (Fig.3c). MMP2 and MMP9 co-knockdown significantly inhibited invasion compared with MMP2 or MMP9 knockdown alone (Fig.3c). In addition, Gli1 knockdown significantly decreased the expression of MMP2 and MMP9 (Fig.3d). Next, to investigate whether the upregulation of Gli1 was through Hh signaling, Shh and Smo knockdown experiments were performed. Shh and Smo knockdown efficiencies were over 90% (Fig. S12). Shh siRNA and Smo siRNA transfected-reoxygenated Ch-H-R cells showed lower anchorage-dependent proliferative (Fig.4a,b) and invasive (Fig.4c,d) abilities than the control in both cell lines. These results suggest that enhanced cell proliferation and invasion by reoxygenation are induced through the activation of Hh signaling.
Figure 3.
Gli1 contributes to the augmentation of malignant potential by reoxygenation. (a) Cell number in control or Gli1 siRNA-transfected-reoxygenated Ch-H-R cells was counted (n = 3). (b) Invaded cell number in control or Gli1 siRNA-transfected-reoxygenated Ch-H-R cells was investigated (n = 3). (c) Reoxygenated Ch-H-R cells were transfected with control, MMP2 or MMP9 siRNA. Then the invaded cell number in these cells was counted (n = 3). (d) MMP2 and MMP9 mRNA expression in Gli1 siRNA transfected-reoxygenated Ch-H-R cells were evaluated by real-time RT-PCR (n = 3). The graph shows mean ± SD. *P < 0.05.
Figure 4.
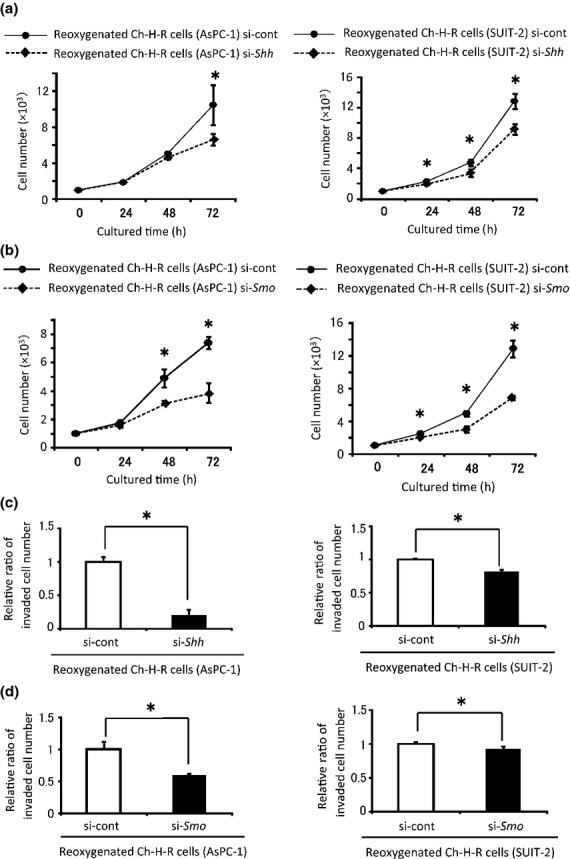
Shh and Smo contribute to the augmentation of malignant potential by reoxygenation. (a, b) Control, Shh (a) or Smo (b) siRNA-transfected-reoxygenated Ch-H-R cells were cultured under normoxia and cell numbers were evaluated (n = 3). (c, d) Invaded cells in control, Shh (c) or Smo (d) siRNA-transfected-reoxygenated Ch-H-R cells were counted (n = 3). The graph shows mean ± SD. *P < 0.05.
Reoxygenation enhances tumorigenicity by Gli1 in vivo
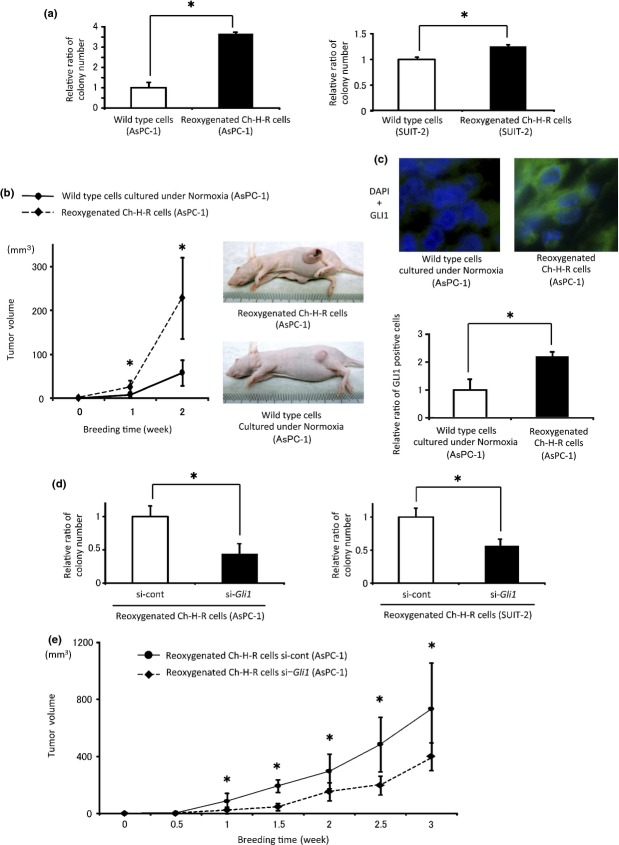
To confirm the observed increase in proliferation by reoxygenation in vitro, we investigated the tumorigenicity of reoxygenated Ch-H-R cells. Normal PDAC cells cultured under normoxia were used as a control in this experiment. Anchorage-independent proliferation in reoxygenated Ch-H-R cells was significantly higher than that in wild type PDAC cells (Fig.5a). Tumors from mice administered with reoxygenated Ch-H-R cells were significantly larger than those from mice injected with wild-type cells (Fig.5b). Gli1 positive cells in tumors from mice injected with reoxygenated Ch-H-R cells were significantly higher than those in the controls (Fig.5c). Next, the effect of Hh signaling in increased tumorigenicity by reoxygenation was examined. Anchorage-independent proliferation in Gli1 siRNA transfected-reoxygenated Ch-H-R cells was significantly lower than that in the control (Fig.5d) in vitro. In vivo, tumors from mice injected with Gli1 siRNA-transfected-reoxygenated Ch-H-R cells were significantly smaller than those from mice injected with control siRNA-transfected cells (Fig.5e). These results suggest that Gli1 plays a pivotal role in tumorigenicity in reoxygenation in vivo.
Figure 5.
Reoxygenation enhances tumorigenicity by Gli1 in vivo. (a) Colony number in wild type cells and reoxygenated Ch-H-R cells was evaluated (n = 3). (b) Wild-type cells cultured under normoxia and reoxygenated Ch-H-R cells derived from AsPC-1 were implanted into flank regions of BALB/c nude mice. Tumor volume was evaluated at the indicated day (left panel) (n = 6). Representative images of tumor-bearing mice (right panels). (c) GLI1 expression in tumors from each mouse was determined by immunofluorescence staining (upper panels) (×630). The graph shows relative ratio of GLI1 positive cells in tumors (lower panel). (d) Colony number in Gli1 siRNA transfected-reoxygenated Ch-H-R cells was estimated (n = 3). (e) Control or Gli1 siRNA-transfected-reoxygenated Ch-H-R cells derived from AsPC-1 were implanted into flank regions of BALB/c nude mice. Tumor volume was evaluated on the indicated day (n = 6). The graph shows mean ± SD. *P < 0.05.
Discussion
Hypoxia and reoxygenation are critical pathophysiological conditions enhanced by blood flow during ischemia-reperfusion injury.28–30 In vivo, local tumor cells are exposed to chronic hypoxia, but are exposed to reoxygenation during the process of invasion and metastasis, in which tumor cells infiltrate the local blood vessels and adhere to the metastatic organs such as the liver and lung. Figure S13 shows the schema of our hypothesis. In the present study, to mimic the tumor microenvironment of reoxygenation, chronic hypoxia-resistant PDAC cell lines were generated by continuous culture for 3–6 months under hypoxia because PDAC cell lines took approximately 1–2 months to adapt (Fig. S1). We confirmed the aggressive phenotype of Ch-H-R cells (Fig.1b,c); therefore, these cells were used in a reoxygenation model that mimicked in vivo conditions in this study. First, we analyzed the expression of HIF-1α, which is an important transcriptional factor for hypoxia,31–34 and its downstream protein, CA9, in wild type cells, Ac-H cells, Ch-H-R cells and reoxygenated Ch-H-R cells. Unexpectedly, the HIF-1α positive ratio in Ch-H-R cells was lower than in Ac-H cells. This result is consistent with a previous report that shows that HIF-1α levels decrease during prolonged chronic hypoxia.35 Because the positive ratio of HIF-1α and CA9 differed between Ac-H cells and Ch-H-R cells, there should be many relationships that can be determined only by analyzing Ch-H-R cells but not Ac-H cells. Consistent with the HIF-1α results, secretion of vascular endothelial growth factor (VEGF), regulated by HIF-1α,36,37 in Ch-H-R cells was significantly lower than in Ac-H cells (Fig. S14). This result may be one reason that the VEGF monoclonal antibody, bevacizumab, is not so effective for PDAC in the clinic. Importantly, although HIF-1α is thought to contribute to tumor aggressiveness,38,39 reoxygenated Ch-H-R cells, which have the lowest ratio of HIF-1α positive cells among Ac-H cells, Ch-H-R cells and reoxygenated Ch-H-R cells, showed the most aggressive phenotype among these three types of cells (Fig.1b,c). In proliferation, because HIF-1α is reported to inhibit the cell cycle under hypoxia,40 low expression of HIF-1α may lead to the high proliferative ability in the reoxygenated Ch-H-R cells. In other functions, the factors other than HIF-1α may contribute to the induction of malignant potential by reoxygenation.
Previously we showed that Hh signaling contributes to the progression of malignant potential for Ac-H cells compared with cells cultured under normoxia independent from HIF-1α expression.10 Therefore, we next investigated the contribution of Hh signaling on phenotypic changes by reoxygenation. Gli1 was used as a marker of Hh signal activation because it is a transcriptional factor and target gene of Hh signaling. Consistent with our previous study,10 Gli1 expression in Ac-H cells was higher than in wild-type cells cultured under normoxia. In this study, Gli1 expression in Ch-H-R cells was higher than in Ac-H cells, and also higher in reoxygenated Ch-H-R cells than in Ch-H-R cells (Figs2a,b, S6, S7). Importantly, the kinetics of these Gli1 expression patterns was almost parallel with the changes of phenotype, such as proliferation and invasiveness. Moreover, inhibition of Shh and Smo abrogated the increased proliferation and invasiveness in reoxygenated Ch-H-R cells (Fig.4). Taken together, these data indicate that reoxygenation-related increases in aggressive phenotypes in PDAC cells may be induced through Hh signaling. However, Gli1 is downstream of several pathways.17,41,42 Koga et al.43 show that estrogen receptor α (ERα) regulates the Hh pathway through Shh induction. Nakashima et al.44 also reveal that nuclear factor (NF)-κB contributes to Hh signaling activation through Shh activation. Other pathways and factors such as ERα and NF-κB may also contribute to the activation of Hh signaling induced by reoxygenation.
Recent studies have demonstrated the important role of MMP in cancer invasion for many solid malignant tumors.45 In particular, type IV collagenase, MMP2 and MMP9 have been well analyzed in the hypoxia-related upregulation of invasiveness in PDAC cells.10,46 We have focused on MMP2 and MMP9 in this study. However, there seems to be a discrepancy between invasive ability (Fig.1) and MMP expression by zymography (Fig. S9B). This discrepancy might be a result of the marginal difference of MMP activity being important and the MMP activation may only occur in the invasive front.
In the present study, we demonstrated for the first time that aggressive reoxygenation-induced phenotypic changes in tumors were induced by upregulation of Gli1 through activation of Hh signaling. Therefore, our results suggest that the Hh signaling pathway may be a therapeutic target for refractory PDAC cells with respect to reoxygenation-induced proliferation, tumorigenicity and invasiveness in metastatic processes, and that an Hh signaling inhibitor may be useful in PDAC treatment.
Acknowledgments
This study was supported by the Japan Society for the Promotion of Science Kakenhi Grant Number 24390303. We thank Ms Kaori Nomiyama for her skillful technical assistance.
Disclosure Statement
The authors have no conflict of interest.
Funding information
Japan Society for the Promotion of Science Kakenhi (24390303).
Supporting Information
Additional supporting information may be found in the online version of this article:
Proliferation of Ch-H-R cells that had cultured under hypoxia until 6 months.
Invasive ability within 5 days of reoxygenation in reoxygenated Ch-H-R cells.
The analysis method of immunofluorescent staining.
Immunocytochemistry of CA9 or hypoxia-inducible factor-1α (HIF-1α) in wild type cells, Ac-H cells, Ch-H-R cells and reoxygenated Ch-H-R cells derived from SUIT-2.
Protein expression of hypoxia-inducible factor-1α (HIF-1α) and CA9 by western blotting analysis.
Immunocytochemistry of GLI1 in wild type cells, Ac-H cells, Ch-H-R cells and reoxygenated Ch-H-R cells derived from SUIT-2.
Protein expression of GLI1 by western blotting analysis.
Immunocytochemistry of MMP2 and MMP9 in wild type cells, Ac-H cells, Ch-H-R cells and reoxygenated Ch-H-R cells derived from SUIT-2.
Protein expression of MMP2 and MMP9 by western blotting analysis and gelatin zymography.
Knockdown efficiency of Gli1 mRNA expression on transfection with Gli1-siRNA in reoxygenated Ch-H-R cells.
Knockdown efficiency of MMP2 and MMP9 mRNA expression on transfection with MMP2 and MMP9-siRNA in reoxygenated Ch-H-R cells respectively.
Knock down efficiency of Shh and Smo siRNA transfected cells.
Schematic figure of hypothesis of Ch-H-R cells and reoxygenated Ch-H-R cells.
Secretion of vascular endothelial growth factor (VEGF) in wild type cells, Ac-H cells, Ch-H-R cells and reoxygenated Ch-H-R cells.
References
- 1.Siegel R, Naishadham D, Jermal A. Cancer statistics. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Egawa S, Toma H, Ohigashi H, et al. Japan Pancreatic Cancer Registry; 30th year anniversary: Japan Pancreas Society. Pancreas. 2012;41:985–92. doi: 10.1097/MPA.0b013e318258055c. [DOI] [PubMed] [Google Scholar]
- 3.Chatterjee D, Rashid A, Wang H, et al. Tumor invasion of muscular vessels predicts poor prognosis in patients with pancreatic ductal adenocarcinoma who received neoadjuvant therapy and pancreaticoduodenectomy. Am J Surg Pathol. 2012;36:552–9. doi: 10.1097/PAS.0b013e318240c1c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shibata K, Matsumoto T, Yada K, et al. Factors predicting recurrence after resection of pancreatic ductal carcinoma. Pancreas. 2005;31:69–73. doi: 10.1097/01.mpa.0000166998.04266.88. [DOI] [PubMed] [Google Scholar]
- 5.Katz MH, Wang H, Fleming JB, et al. Long-term survival after multidisciplinary management of resected pancreatic adenocarcinoma. Ann Surg Oncol. 2009;16:836–47. doi: 10.1245/s10434-008-0295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liotta LA, Stetler-Stevenson WG. Tumor invasion and metastasis: an imbalance of positive and negative regulation. Cancer Res. 1991;51(Suppl):5054s–9s. [PubMed] [Google Scholar]
- 7.Höckel S, Schlenger K, Vaupel P, Höckel M. Association between host tissue vascularity and the prognostically relevant tumor vascularity in human cervical cancer. Int J Oncol. 2001;19:827–32. [PubMed] [Google Scholar]
- 8.Caldwell CC, Kojima H, Lukashev D, et al. Differential effects of physiologically relevant hypoxic conditions on T lymphocyte development and effector functions. J Immunol. 2001;167:6140–9. doi: 10.4049/jimmunol.167.11.6140. [DOI] [PubMed] [Google Scholar]
- 9.Sung SM, Jung DS, Kwon CH, Park JY, Kang SK, Kim YK. Hypoxia/reoxygenation stimulates proliferation through PKC-dependent activation of ERK and Akt in mouse neural progenitor cells. Neurochem Res. 2007;32:1932–9. doi: 10.1007/s11064-007-9390-1. [DOI] [PubMed] [Google Scholar]
- 10.Onishi H, Kai M, Odate S, et al. Hypoxia activates the hedgehog signaling pathway in a ligand-independent manner by upregulation of Smo transcription in pancreatic cancer. Cancer Sci. 2011;102:1144–50. doi: 10.1111/j.1349-7006.2011.01912.x. [DOI] [PubMed] [Google Scholar]
- 11.Höckel M, Schlenger K, Knoop C, Vaupel P. Oxygenation of carcinomas of the uterine cervix: evaluation by computerized O2 tension measurements. Cancer Res. 1991;51:6098–102. [PubMed] [Google Scholar]
- 12.Vaupel P, Schlenger K, Knoop C, Höckel M. Oxygenation of human tumors: evaluation of tissue oxygen distribution in breast cancers by computerized O2 tension measurements. Cancer Res. 1991;51:3316–22. [PubMed] [Google Scholar]
- 13.Höckel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst. 2001;93:266–76. doi: 10.1093/jnci/93.4.266. [DOI] [PubMed] [Google Scholar]
- 14.Koong AC, Mehta VK, Le QT, et al. Pancreatic tumors show high levels of hypoxia. Int J Radiat Oncol Biol Phys. 2000;48:919–22. doi: 10.1016/s0360-3016(00)00803-8. [DOI] [PubMed] [Google Scholar]
- 15.Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059–87. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- 16.Hooper JE, Scott MP. Communicating with Hedgehogs. Nat Rev Mol Cell Biol. 2005;6:306–17. doi: 10.1038/nrm1622. [DOI] [PubMed] [Google Scholar]
- 17.Onishi H, Katano M. Hedgehog signaling pathway as a therapeutic target in various types of cancer. Cancer Sci. 2011;102:1756–60. doi: 10.1111/j.1349-7006.2011.02010.x. [DOI] [PubMed] [Google Scholar]
- 18.Kinzler KW, Bigner SH, Bigner DD, et al. Identification of an amplified, highly expressed gene in a human glioma. Science. 1987;236:70–3. doi: 10.1126/science.3563490. [DOI] [PubMed] [Google Scholar]
- 19.Yanai K, Nagai S, Wada J, et al. Hedgehog signaling pathway is a possible therapeutic target for gastric cancer. J Surg Oncol. 2007;95:55–62. doi: 10.1002/jso.20606. [DOI] [PubMed] [Google Scholar]
- 20.Thayer SP, di Magliano MP, Heiser PW, et al. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature. 2003;425:851–6. doi: 10.1038/nature02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kubo M, Nakamura M, Tasaki A, et al. Hedgehog signaling pathway is a new therapeutic target for patients with breast cancer. Cancer Res. 2004;64:6071–4. doi: 10.1158/0008-5472.CAN-04-0416. [DOI] [PubMed] [Google Scholar]
- 22.Karhadkar SS, Bova GS, Abdallah N, et al. Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature. 2004;431:707–12. doi: 10.1038/nature02962. [DOI] [PubMed] [Google Scholar]
- 23.Nagai S, Nakamura M, Yanai K, et al. Gli1 contributes to the invasiveness of pancreatic cancer through matrix metalloproteinase-9 activation. Cancer Sci. 2008;99:1377–84. doi: 10.1111/j.1349-7006.2008.00822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dahmane N, Lee J, Robins P, Heller P. Ruiz i Altaba A. Activation of the transcription factor Gli1 and the Sonic hedgehog signalling pathway in skin tumours. Nature. 1997;389:876–81. doi: 10.1038/39918. [DOI] [PubMed] [Google Scholar]
- 25.Dai P, Akimaru H, Tanaka Y, Maekawa T, Nakafuku M, Ishii S. Sonic Hedgehog-induced activation of the Gli1 promoter is mediated by GLI3. J Biol Chem. 1999;274:8143–52. doi: 10.1074/jbc.274.12.8143. [DOI] [PubMed] [Google Scholar]
- 26.Zhang H, Morisaki T, Matsunaga H, et al. Protein-bound polysaccharide PSK inhibits tumor invasiveness by down-regulation of TGF-beta1 and MMPs. Clin Exp Metastasis. 2000;18:343–52. doi: 10.1023/a:1010897432244. [DOI] [PubMed] [Google Scholar]
- 27.Kai M, Onishi H, Souzaki M, et al. Semi-quantitative evaluation of CD44(+)/CD24(-) tumor cell distribution in breast cancer tissue using a newly developed fluorescence immunohistochemical staining method. Cancer Sci. 2011;102:2132–8. doi: 10.1111/j.1349-7006.2011.02063.x. [DOI] [PubMed] [Google Scholar]
- 28.Doi K, Horiuchi T, Uchinami M, et al. Hepatic ischemia-reperfusion promotes liver metastasis of colon cancer. J Surg Res. 2002;105:243–7. doi: 10.1006/jsre.2002.6356. [DOI] [PubMed] [Google Scholar]
- 29.Man K, Ng KT, Lo CM, et al. Ischemia-reperfusion of small liver remnant promotes liver tumor growth and metastases–activation of cell invasion and migration pathways. Liver Transpl. 2007;13:1669–77. doi: 10.1002/lt.21193. [DOI] [PubMed] [Google Scholar]
- 30.Nicoud IB, Jones CM, Pierce JM, et al. Warm hepatic ischemia–reperfusion promotes growth of colorectal carcinoma micrometastases in mouse liver via matrix metalloproteinase-9 induction. Cancer Res. 2007;67:2720–8. doi: 10.1158/0008-5472.CAN-06-3923. [DOI] [PubMed] [Google Scholar]
- 31.Semenza GL. Expression of hypoxia-inducible factor 1: mechanisms and consequences. Biochem Pharmacol. 2000;59:47–53. doi: 10.1016/s0006-2952(99)00292-0. [DOI] [PubMed] [Google Scholar]
- 32.Ivan M, Kondo K, Yang H, et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–8. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 33.Jaakkola P, Mole DR, Tian YM, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–72. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 34.Maxwell PH, Wiesener MS, Chang GW, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–5. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 35.Koh MY, Lemos R, Jr, Liu X, Powis G. The hypoxia-associated factor switches cells from HIF-1α- to HIF-2α-dependent signaling promoting stem cell characteristics, aggressive tumor growth and invasion. Cancer Res. 2011;71:4015–27. doi: 10.1158/0008-5472.CAN-10-4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsuzuki Y, Fukumura D, Oosthuyse B, Koike C, Carmeliet P, Jain RK. Vascular endothelial growth factor (VEGF) modulation by targeting hypoxia-inducible factor-1alpha →hypoxia response element →VEGF cascade differentially regulates vascular response and growth rate in tumors. Cancer Res. 2000;60:6248–52. [PubMed] [Google Scholar]
- 37.Iervolino A, Trisciuoglio D, Ribatti D, et al. Bcl-2 overexpression in human melanoma cells increases angiogenesis through VEGF mRNA stabilization and HIF-1-mediated transcriptional activity. FASEB J. 2002;16:1453–5. doi: 10.1096/fj.02-0122fje. [DOI] [PubMed] [Google Scholar]
- 38.Bos R, Zhong H, Hanrahan CF, et al. Levels of hypoxia-inducible factor-1 alpha during breast carcinogenesis. J Natl Cancer Inst. 2001;93:309–14. doi: 10.1093/jnci/93.4.309. [DOI] [PubMed] [Google Scholar]
- 39.Zhong H, De Marzo AM, Laughner E, et al. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999;59:5830–5. [PubMed] [Google Scholar]
- 40.Hackenbeck T, Knaup KX, Schietke R, et al. HIF-1 or HIF-2 induction is sufficient to achieve cell cycle arrest in NIH3T3 mouse fibroblasts independent from hypoxia. Cell Cycle. 2009;8:1386–95. doi: 10.4161/cc.8.9.8306. [DOI] [PubMed] [Google Scholar]
- 41.Mills LD, Zhang Y, Marler RJ, et al. Loss of the transcription factor GLI1 identifies a signaling network in the tumor microenvironment mediating KRAS oncogene-induced transformation. J Biol Chem. 2013;288:11786–94. doi: 10.1074/jbc.M112.438846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y, Ding Q, Yen CJ, et al. The crosstalk of mTOR/S6K1 and Hedgehog pathways. Cancer Cell. 2012;21:374–87. doi: 10.1016/j.ccr.2011.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koga K, Nakamura M, Nakashima H, et al. Novel link between estrogen receptor alpha and hedgehog pathway in breast cancer. Anticancer Res. 2008;28:731–40. [PubMed] [Google Scholar]
- 44.Nakashima H, Nakamura M, Yamaguchi H, et al. Nuclear factor-kappaB contributes to hedgehog signaling pathway activation through sonic hedgehog induction in pancreatic cancer. Cancer Res. 2006;66:7041–9. doi: 10.1158/0008-5472.CAN-05-4588. [DOI] [PubMed] [Google Scholar]
- 45.Nelson AR, Fingleton B, Rothenberg ML, Matrisian LM. Matrix metalloproteinases: biologic activity and clinical implications. J Clin Oncol. 2000;18:1135–49. doi: 10.1200/JCO.2000.18.5.1135. [DOI] [PubMed] [Google Scholar]
- 46.Onishi H, Morisaki T, Nakao F, Odate S, Morisaki T, Katano M. Protein-bound polysaccharide decreases invasiveness and proliferation in pancreatic cancer by inhibition of hedgehog signaling and HIF-1α pathways under hypoxia. Cancer Lett. 2013;28:289–98. doi: 10.1016/j.canlet.2013.02.041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Proliferation of Ch-H-R cells that had cultured under hypoxia until 6 months.
Invasive ability within 5 days of reoxygenation in reoxygenated Ch-H-R cells.
The analysis method of immunofluorescent staining.
Immunocytochemistry of CA9 or hypoxia-inducible factor-1α (HIF-1α) in wild type cells, Ac-H cells, Ch-H-R cells and reoxygenated Ch-H-R cells derived from SUIT-2.
Protein expression of hypoxia-inducible factor-1α (HIF-1α) and CA9 by western blotting analysis.
Immunocytochemistry of GLI1 in wild type cells, Ac-H cells, Ch-H-R cells and reoxygenated Ch-H-R cells derived from SUIT-2.
Protein expression of GLI1 by western blotting analysis.
Immunocytochemistry of MMP2 and MMP9 in wild type cells, Ac-H cells, Ch-H-R cells and reoxygenated Ch-H-R cells derived from SUIT-2.
Protein expression of MMP2 and MMP9 by western blotting analysis and gelatin zymography.
Knockdown efficiency of Gli1 mRNA expression on transfection with Gli1-siRNA in reoxygenated Ch-H-R cells.
Knockdown efficiency of MMP2 and MMP9 mRNA expression on transfection with MMP2 and MMP9-siRNA in reoxygenated Ch-H-R cells respectively.
Knock down efficiency of Shh and Smo siRNA transfected cells.
Schematic figure of hypothesis of Ch-H-R cells and reoxygenated Ch-H-R cells.
Secretion of vascular endothelial growth factor (VEGF) in wild type cells, Ac-H cells, Ch-H-R cells and reoxygenated Ch-H-R cells.