Abstract
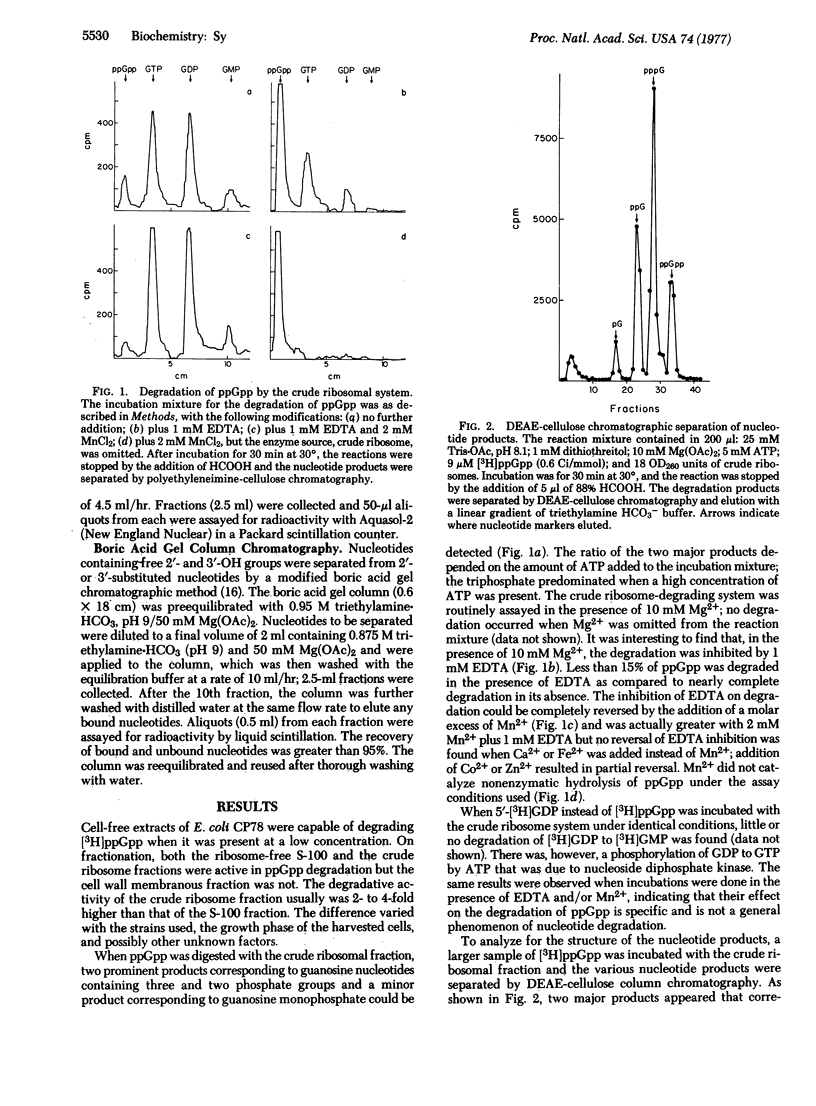
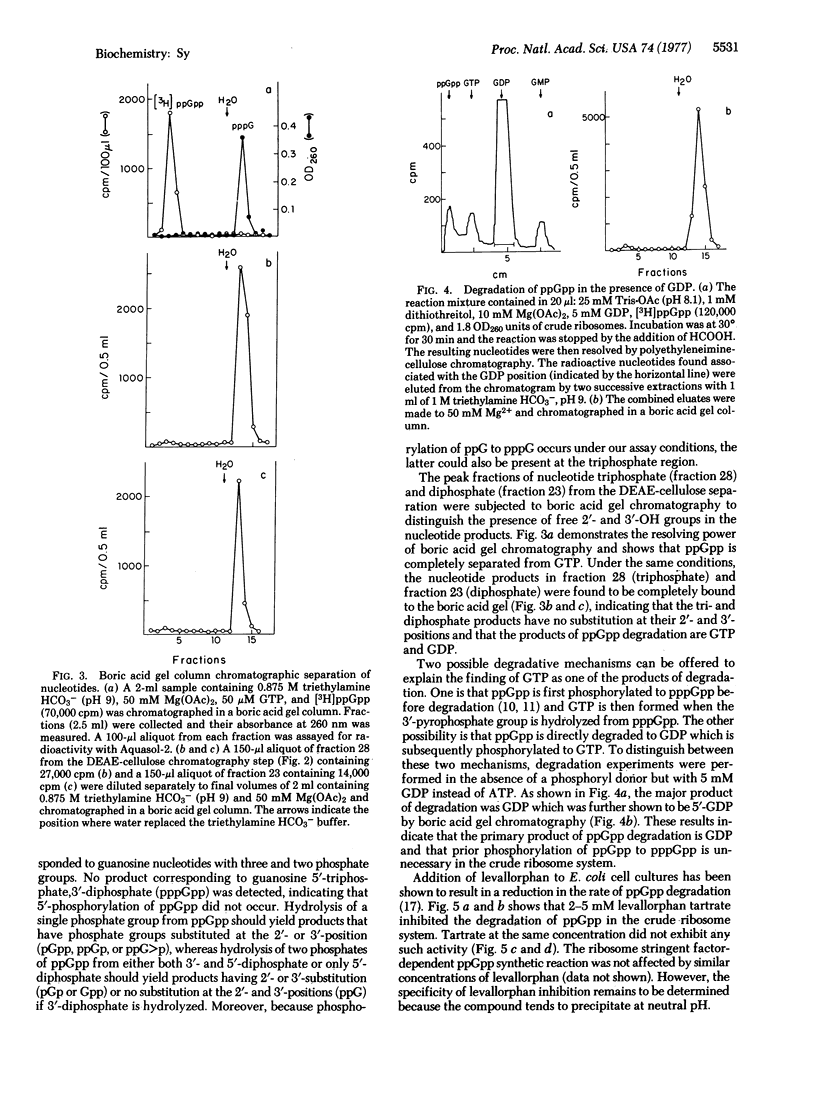
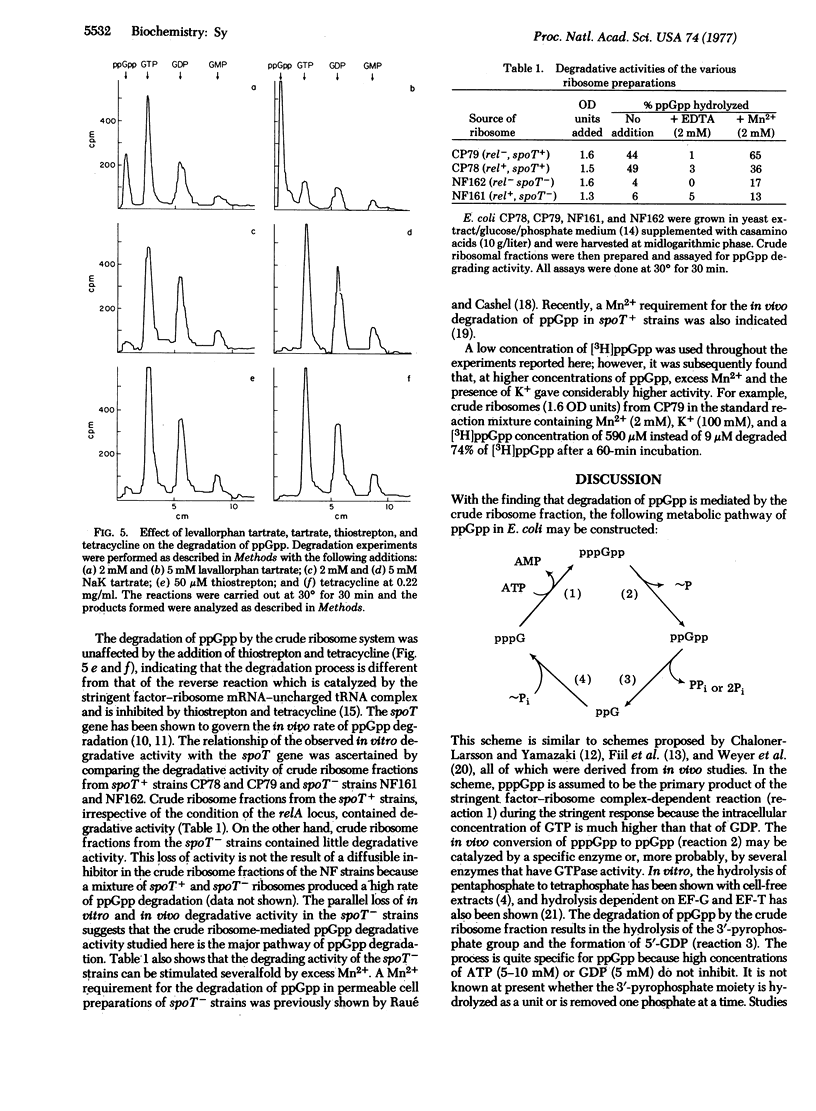
The degradation of guanosine 5'-diphosphate,3'-diphosphate (ppGpp) by the "crude" ribosomal fraction of Escherichia coli CP78 (rel+, spoT+) was demonstrated and characterized. When the 3'-pyrophosphoryl group of ppGpp was hydrolyzed, the primary degradation product was 5'-GDP. Phosphorylation of ppGpp to guanosine 5'-triphosphate,3'-diphosphate (pppGpp) prior to degradation was not necessary. The degradation process required Mn2+ and was inhibited by EDTA. Levallorphan, an inhibitor of in vivo ppGpp degradation, also inhibited ppGpp degradation by the crude ribosome. Thiostrepton and tetracycline did not have any inhibitory effect, indicating that the reaction is not a reversal of pyrophosphorylation catalyzed by the stringent factor/ribosome complex. Crude ribosome fractions from E. coli NF161 and NF162, both spoT-, contained little degrading activity, but similar fractions of E. coli CP79, a relA- and spoT+ strain, contained ppGpp degrading activity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boquet P. L., Devynck M. A., Monnier C., Fromageot P. Inhibition of stable RNA synthesis by levallorphan in Escherichia coli. Implication of compounds MS I and MS II. Eur J Biochem. 1973 Dec 3;40(1):31–42. doi: 10.1111/j.1432-1033.1973.tb03166.x. [DOI] [PubMed] [Google Scholar]
- Brockman R. W., Heppel L. A. On the localization of alkaline phosphatase and cyclic phosphodiesterase in Escherichia coli. Biochemistry. 1968 Jul;7(7):2554–2562. doi: 10.1021/bi00847a016. [DOI] [PubMed] [Google Scholar]
- Cashel M. Regulation of bacterial ppGpp and pppGpp. Annu Rev Microbiol. 1975;29:301–318. doi: 10.1146/annurev.mi.29.100175.001505. [DOI] [PubMed] [Google Scholar]
- Cashel M. The control of ribonucleic acid synthesis in Escherichia coli. IV. Relevance of unusual phosphorylated compounds from amino acid-starved stringent strains. J Biol Chem. 1969 Jun 25;244(12):3133–3141. [PubMed] [Google Scholar]
- Chaloner-Larsson G., Yamazaki H. Synthesis of guanosine 5'-triphosphate,3'-diphosphate in a spo T strain of Escherichia coli. Can J Biochem. 1976 Nov;54(11):935–940. doi: 10.1139/o76-135. [DOI] [PubMed] [Google Scholar]
- De Boer H. A., Bakker A. J., Gruber M. Breakdown of ppGpp in spoT and spoT-cells of Escherichia coli. Manganese and energy requirement and tetracycline inhibition. FEBS Lett. 1977 Jul 1;79(1):19–24. doi: 10.1016/0014-5793(77)80341-4. [DOI] [PubMed] [Google Scholar]
- Fiil N. P., Willumsen B. M., Friesen J. D., von Meyenburg K. Interaction of alleles of the relA, relC and spoT genes in Escherichia coli: analysis of the interconversion of GTP, ppGpp and pppGpp. Mol Gen Genet. 1977 Jan 7;150(1):87–101. doi: 10.1007/BF02425329. [DOI] [PubMed] [Google Scholar]
- Gallant J., Margason G., Finch B. On the turnover of ppGpp in Escherichia coli. J Biol Chem. 1972 Oct 10;247(19):6055–6058. [PubMed] [Google Scholar]
- Hamel E., Cashel M. Role of guanine nucleotides in protein synthesis. Elongation factor G and guanosine 5'-triphosphate,3'-diphosphate. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3250–3254. doi: 10.1073/pnas.70.11.3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund E., Kjeldgaard N. O. Metabolism of guanosine tetraphosphate in Escherichia coli. Eur J Biochem. 1972 Jul 24;28(3):316–326. doi: 10.1111/j.1432-1033.1972.tb01916.x. [DOI] [PubMed] [Google Scholar]
- Raué H. A., Cashel M. Regulation of RNA synthesis in Escherichia coli. III. Degradation of guanosine 5'-diphosphate 3'-diphosphate in cold-shocked cells. Biochim Biophys Acta. 1975 Mar 21;383(3):290–304. [PubMed] [Google Scholar]
- Schott H., Rudloff E., Schmidt P., Roychoudhury R., Kössel H. A dihydroxyboryl-substituted methacrylic polymer for the column chromatographic separation of mononucleotides, oligonucleotides, and transfer ribonucleic acid. Biochemistry. 1973 Feb 27;12(5):932–938. doi: 10.1021/bi00729a022. [DOI] [PubMed] [Google Scholar]
- Stamminger G., Lazzarini R. A. Analysis of the RNA of defective VSV particles. Cell. 1974 Sep;3(1):85–93. doi: 10.1016/0092-8674(74)90044-0. [DOI] [PubMed] [Google Scholar]
- Sy J. A ribosome-independent, soluble stringent factor-like enzyme isolated from a Bacillus brevis. Biochemistry. 1976 Feb 10;15(3):606–609. doi: 10.1021/bi00648a024. [DOI] [PubMed] [Google Scholar]
- Sy J. Reversibility of the pyrophosphoryl transfer from ATP to GTP by Escherichia coli stringent factor. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3470–3473. doi: 10.1073/pnas.71.9.3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyer W. J., de Boer H. A., de Boer J. G., Gruber M. The sequence of ppGpp and pppGpp in the reaction scheme for magic spot synthesis. Biochim Biophys Acta. 1976 Aug 2;442(1):123–127. doi: 10.1016/0005-2787(76)90183-0. [DOI] [PubMed] [Google Scholar]
- Yang H. L., Zubay G., Urm E., Heiness G., Cashel M. Effects of guanosine tetraphosphate, guanosine pentaphosphate, and beta-gamma methylenyl-guanosine pentaphosphate on gene expression of Escherichia coli in vitro. Proc Natl Acad Sci U S A. 1974 Jan;71(1):63–67. doi: 10.1073/pnas.71.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]


