Abstract
The aims of this study were to evaluate the frequency of dose-limiting toxicities and to find the recommended dose of combination chemotherapy with sorafenib and transcatheter arterial infusion (TAI) using cisplatin for patients with advanced hepatocellular carcinoma (HCC), for whom surgical resection, local ablation therapy, or transcatheter arterial chemoembolization were not indicated. Patients received 800 mg sorafenib daily. Cisplatin was given at one of three dosages (level 1, 35 mg/m2/cycle; level 2, 50 mg/m2/cycle; and level 3, 65 mg/m2/cycle) from feeding arteries to the HCC. The treatment was repeated every 4–6 weeks up to a maximum of six cycles, until there were signs of tumor progression or unacceptable toxicity. The dose-limiting toxicities experienced by the 20 enrolled patients were grade 4 increased aspartate aminotransferase at level 1, grade 3 gastrointestinal hemorrhaging at level 1, and grade 3 hypertension at level 3. The common drug-related adverse events that were of severity grade 3 or 4 included the elevation of aspartate aminotransferase (30%), alanine aminotransferase (20%), amylase (30%), and lipase (30%). Partial response was seen in four patients (20%), and 13 patients (65%) had stable disease. The median overall survival and progression-free survival were 9.1 and 3.3 months, respectively. The combination of sorafenib at 800 mg/day with TAI of cisplatin at 65 mg/m2/cycle was determined to be the recommended regimen. A randomized phase II trial of sorafenib alone versus sorafenib plus TAI of cisplatin is currently underway. This study was registered at UMIN as trial number UMIN000001496.
Keywords: Arterial infusion, chemotherapy, cisplatin, hepatocellular carcinoma, sorafenib
Hepatocellular carcinoma is one of the most common types of cancer worldwide.1 Hepatic resection, liver transplantation, and local ablation therapy, including radiofrequency ablation and percutaneous ethanol injection, are considered to be curative treatments for HCC.2–4 Transcatheter arterial chemoembolization has been recognized as an effective but non-curative treatment for patients with large or multifocal, unresectable HCC without vascular invasion or extrahepatic spread.4 However, the majority of patients develop recurrence or metastasis after these treatments, and their HCCs progress to the advanced stages. Two separate phase III trials have reported that sorafenib, an oral multikinase inhibitor, prolongs OS with manageable toxicities.5,6 Thus, sorafenib has been accepted as standard first-line chemotherapy for patients who cannot benefit from resection, transplantation, local ablation therapy, or TACE, and who still have preserved liver function. However, sorafenib treatment has yielded rather unsatisfactory results in terms of OS of patients with advanced HCC.
In Japan, TAI chemotherapy is often given to patients with localized advanced HCC, such as in cases with vascular invasion. Transcatheter arterial infusion likely has better antitumor activity and reduced toxicity compared to systemic chemotherapy, because TAI can increase the local concentration of anticancer drugs while reducing their systemic distribution and accompanying adverse effects.7,8 However, TAI has not been established as a standard treatment for advanced HCC, because the survival benefit has not been evaluated in large-scale prospective randomized trials. Cisplatin alone,9,10 5-FU plus cisplatin,11 and 5-FU plus interferon12 are frequently used chemotherapeutic regimens that have been shown to lead to tumor shrinkage and increased OS. Among these options, TAI of cisplatin does not require an implanted reservoir system, so it is easier to manage its administration. In addition, favorable antitumor efficacy10 has been reported by previous phase II trials. The combination of sorafenib and TAI of cisplatin might be more effective than sorafenib alone for the treatment of advanced HCC. Therefore, we planned a phase I study of the combination chemotherapy of sorafenib and TAI with cisplatin for advanced HCC. The primary endpoint of this trial was to determine the recommended doses of TAI of cisplatin and sorafenib to use for combination therapy, according to the frequency of its DLT. The secondary goal of this study was to evaluate the toxicity and efficacy of this combination in patients with advanced HCC.
Materials and Methods
Patient eligibility
Patients eligible for enrolment in this study had advanced HCC for which surgical resection, local ablation therapy, and TACE were not indicated. Hepatocellular carcinoma was diagnosed by either histologic examination or based on a computed tomographic scan, angiograph, and an increased level of serum AFP or DCP. Eligibility criteria included the following factors: (i) 20–79 years of age; (ii) an Eastern Cooperative Oncology Group performance status score of 0–2; (iii) one or more measurable lesions in the liver; (iv) adequate hematological function (hemoglobin levels of 8.5 g/dL or more, neutrophil counts of 1500 cells/mm3 or more, and platelet counts of 70 000 cells/mm3 or more); (v) adequate hepatic function (serum total bilirubin levels of 2.0 mg/dL or less, serum albumin levels of 2.8 g/dL or more, and serum AST/ALT levels within five times the ULN, Child–Pugh score of seven points or less); (vi) adequate pancreatic function (serum total amylase/lipase levels within two times the ULN); and (vii) adequate renal function (serum creatinine level within normal limits and creatinine clearance of 60 mL/min or more). Previous local therapy for intrahepatic lesions, such as hepatic resection, percutaneous local ablation, or TACE was allowed if it had not been given within the 4 weeks before this treatment. In this study, the eligibility criterion regarding the Child–Pugh classification was set at a score of seven points or less, because sorafenib has been reported to be feasible in patients with Child–Pugh class B.13,14
Patients were excluded from the study if they had a treatment history of sorafenib or cisplatin for HCC, an active infection, uncontrollable hypertension, severe heart disease, refractory pleural effusion or ascites, a severe mental disorder or encephalopathy, an active gastroduodenal ulcer or esophageal bleeding, or active concomitant malignancy. This study also excluded pregnant and lactating women, women of childbearing age unless they were using effective contraception, and patients with other serious medical conditions.
Treatment plan
Sorafenib (Bayer Health Care Pharmaceuticals, West Haven, CT, USA) was given orally at a dose of 800 mg daily. Cisplatin (Nippon Kayaku, Tokyo, Japan) was concurrently administered by a catheter in the proper, right, or left hepatic artery, or another feeding artery, under angiographic guidance with the Seldinger technique on the same day as sorafenib administration at one of three dosages (35 mg/m2/cycle for level 1, 50 mg/m2/cycle for level 2, or 65 mg/m2/cycle for level 3) (Table1). The maximum dose of cisplatin was set according to the dose approved by Japanese insurance for single-use as an intra-arterial therapy.10 The treatment was repeated every 4–6 weeks up to a maximum of six cycles, until there was evidence of tumor progression or unacceptable toxicity. A list of suspension criteria was set, and the treatment of patients receiving sorafenib that met these criteria was interrupted until the toxicities were resolved. When resuming treatment, the dose of sorafenib needed to be reduced to 400 mg daily. If additional dose reduction was required, the dose was reduced to a single 400-mg dose every other day. The suspension criteria for sorafenib were defined as: (i) grade 4 neutropenia or thrombocytopenia; (ii) grade 3 or 4 non-hematological toxicity excluding increased levels of serum AST/ALT/γ-GT, pancreatic enzyme increases, HFSR, hyperglycemia, and constipation; (iii) grade 4 pancreatic enzyme increases with clinical and/or imaging findings of pancreatitis, or a pancreatic adverse event considered to be life threatening; (iv) serum AST/ALT levels of 10 times the ULN; (v) serum creatinine levels of 2.0 mg/dL or more; (vi) grade 2 or 3 HFSR; and (vii) grade 2 or 3 hypertension.
Table 1.
Dosage levels of sorafenib and cisplatin administered to patients with advanced hepatocellular carcinoma
| Level | Sorafenib (mg/day) | Cisplatin TAI (mg/m2/cycle) | Remarks |
|---|---|---|---|
| 1 | 800 | 35 | Starting dose |
| 2 | 800 | 50 | |
| 3 | 800 | 65 | Recommended dose |
The starting criteria for cisplatin TAI were defined as follows: (i) neutrophil counts of 1200/mm3 or more; (ii) thrombocyte counts of 50 000 cells/mm3 or more; (iii) total bilirubin levels of 3.0 mg/dL or less; (iv) AST or ALT levels five times the ULN or less; and (v) creatinine levels of 1.5 mg/dL or less. If these adverse events were outside of the starting criteria, TAI of cisplatin was postponed until the criteria were fulfilled.
Clinical assessments
The trial was an open-label, single-arm phase I study that was carried out at four cancer centers in Japan. The primary endpoints were to evaluate the frequency of DLTs and to determine the recommended doses of sorafenib and cisplatin in a phase II study. Dose escalation followed a standard “3 plus 3” dose escalation design. In other words, at least three patients were enrolled at each of three dosage levels. If one or two DLTs were observed in the initial three patients, three additional patients were entered at the same dosage level. If DLTs were not observed in three of the three patients, or three or more of the six patients treated at that level during the first cycle of treatment, the dose of cisplatin was escalated to the next level. At the highest dosage level, three additional patients were entered and the safety was evaluated carefully during the first three cycles of the nine patients. An additional patient would be included when treatment was terminated for reasons other than DLT before the end of the first course, because it would be impossible to determine the frequency of DLTs. The efficacy and safety evaluation committee determined the recommended dose. Dose-limiting toxicities were defined as follows: (i) febrile neutropenia; (ii) grade 4 leucopenia or grade 4 neutropenia persisting for 7 days or more; (iii) grade 4 thrombocytopenia or thrombocytopenia requiring transfusion; (iv) grade 3 or 4 non-hematological toxicity excluding increased serum AST/ALT/γ-GT levels, increased pancreatic enzyme levels, HFSR, hyperglycemia, or constipation; (v) grade 4 increased pancreatic enzyme levels with clinical and/or imaging findings of pancreatitis, or a pancreatic adverse event considered to be life threatening; (vi) serum AST/ALT levels of 10 times the ULN or more; (vii) serum creatinine levels of 2.0 mg/dL or more; and (viii) any toxicities that necessitated a treatment delay of more than 4 weeks. Toxicities were graded according to the Common Terminology Criteria for Adverse Events, version 3.0. During treatment, a complete blood count with differentials, serum chemistry, and urinalysis was obtained biweekly. Tumor response was evaluated every 6 weeks using RECIST version 1.0. Progression-free survival was defined as the time from enrolment in this trial to the first documentation of progression or death. Overall survival was the time from enrolment in this trial to the date of death or the date of the last follow-up. Both PFS and OS times were calculated using the Kaplan–Meier method.
This protocol was approved for clinical investigation by each institution's review board in accordance with the provisions of the Declaration of Helsinki, Good Clinical Practice guidelines, and local laws and regulations. Written informed consent was obtained from all patients who were considered eligible for participation in this study before enrolment. This study was registered at UMIN as trial number UMIN000001496.
Results
Patient characteristics
A total of 20 patients were enrolled in the trial between December 2008 and August 2010. The patient characteristics are listed in Table2. Seven patients were enrolled at dose level 1, three patients at dose level 2, and 10 patients at dose level 3. This was because we replaced one more patient at dose levels 1 and 3, according to the recommendation of the efficacy and safety evaluation committee. Sorafenib treatment was terminated for one patient at dose level 1 who developed grade 3 erythema multiforme and one patient at dose level 3 who developed hypoglycemia owing to disease progression on the 11th day of the first cycle. Erythema multiforme was a distinctive adverse event of sorafenib and was therefore not considered a DLT in this study. In addition, hypoglycemia was considered unrelated to the combination therapy.
Table 2.
Baseline characteristics of patients with advanced hepatocellular carcinoma enrolled in this study
| Characteristics | Level 1 | Level 2 | Level 3 | Total | |
|---|---|---|---|---|---|
| No. of patients | 7 | 3 | 10 | 20 | |
| Age, years | 30–39 | 0 | 0 | 1 | 1 |
| 40–49 | 0 | 0 | 1 | 1 | |
| 50–59 | 1 | 2 | 1 | 4 | |
| 60–69 | 3 | 0 | 2 | 6 | |
| 70–79 | 3 | 1 | 4 | 8 | |
| PS | 0 | 7 | 3 | 8 | 19 |
| 1 | 0 | 0 | 1 | 1 | |
| Viral marker | HBs Ag (+) | 0 | 1 | 3 | 5 |
| HCV Ab (+) | 3 | 1 | 3 | 7 | |
| Child–Pugh score | 5 | 4 | 2 | 4 | 11 |
| 6 | 1 | 1 | 3 | 5 | |
| 7 | 2 | 0 | 2 | 4 | |
| Portal vein invasion | Vp 3 | 1 | 0 | 3 | 4 |
| Vp 4 | 3 | 0 | 2 | 5 | |
| Distant metastases | Absent | 4 | 2 | 6 | 13 |
| Present | 3 | 1 | 3 | 7 | |
| Stage (UICC v.6) | II | 0 | 1 | 0 | 1 |
| III | 4 | 1 | 6 | 12 | |
| IV | 3 | 1 | 3 | 7 |
HBs Ag, hepatitis B surface antigen; HCV Ab, hepatitis C antibody; PS, performance status; UICC, Union for International Cancer Control; Vp 3, hepatocellular carcinoma invasion of the first-order branch of the portal vein; Vp 4, hepatocellular carcinoma invasion of the main trunk of the portal vein.
The median dose intensity of sorafenib and the median relative dose intensity were 528 mg daily and 66%, respectively (Table3). There was no decrease in the dose of cisplatin. The median number of cycles of cisplatin was 2.8 (range, 1–6 cycles).
Table 3.
Dosage intensity and number of transcatheter arterial infusion (TAI) cycles in patients with advanced hepatocellular carcinoma treated with sorafenib and cisplatin
| Level 1 | Level 2 | Level 3 | Total | |
|---|---|---|---|---|
| No. of enrolled patients | 7 | 3 | 10 | 20 |
| No. of patients with dose reduction of sorafenib (%) | 2 (29) | 1 (33) | 5 (50) | 8 (40) |
| Mean relative dose intensity of sorafenib, % | 91 | 78 | 62 | 66 |
| Mean no. of cisplatin TAI cycles | 3.1 | 1.6 | 3.0 | 2.8 |
Adverse events
The DLTs included grade 4 increased AST, grade 3 gastrointestinal hemorrhage, and grade 3 hypertension. At dose level 1, two of the seven patients experienced DLTs; the first of these patients developed grade 4 increased levels of serum AST on the 13th day of the first cycle, and the second patient experienced grade 3 gastrointestinal bleeding and grade 3 bacteremia on the 13th day of the first cycle. No DLTs occurred in patients receiving dose level 2. At dose level 3, one patient experienced DLT in the form of grade 3 hypertension on the 32nd day of the first cycle, but no other DLTs were seen at this dose level.
The most common grade 3 or 4 drug-related adverse events included increased levels of AST (30%), amylase (30%), lipase (30%), ALP (10%), and γ-GT (10%), anemia (15%), leukopenia (10%), and thrombocytopenia (10%) during the entire periods of the combination therapy (Table4). There were no treatment-related deaths in this trial. Therefore, the combination therapy of TAI of cisplatin at 65 mg/m2 with 800 mg/day sorafenib was considered to be manageable.
Table 4.
Adverse events observed in patients with advanced hepatocellular carcinoma treated with sorafenib and cisplatin by transcatheter arterial infusion (TAI) (n = 20)
| Characteristic | Level 1 |
Level 2 |
Level 3 |
Total |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of pts |
n = 7 |
% | % |
n = 3 |
% | % |
n = 10 |
% | % | % | % | |||||||||
| Grade (CTCAE v.3.0) | 1 | 2 | 3 | 4 | Any | 3/4 | 1 | 2 | 3 | 4 | Any | 3/4 | 1 | 2 | 3 | 4 | Any | 3/4 | Any | 3/4 |
| Leukopenia | 3 | 0 | 1 | 0 | 57 | 14 | 0 | 2 | 0 | 0 | 33 | 0 | 2 | 3 | 1 | 0 | 60 | 10 | 55 | 10 |
| Neutropenia | 2 | 0 | 1 | 0 | 43 | 14 | 1 | 1 | 0 | 0 | 66 | 0 | 3 | 1 | 0 | 0 | 40 | 0 | 45 | 5 |
| Anemia | 1 | 1 | 2 | 0 | 57 | 29 | 1 | 0 | 0 | 0 | 33 | 0 | 2 | 0 | 1 | 0 | 30 | 10 | 35 | 15 |
| Thrombocytopenia | 1 | 2 | 1 | 0 | 57 | 14 | 0 | 2 | 0 | 0 | 66 | 0 | 1 | 6 | 1 | 0 | 80 | 10 | 70 | 10 |
| Hyperbilirubinemia | 4 | 2 | 0 | 0 | 86 | 29 | 3 | 0 | 0 | 0 | 33 | 0 | 3 | 4 | 0 | 0 | 70 | 0 | 80 | 0 |
| AST increased | 0 | 2 | 1 | 1 | 57 | 29 | 0 | 1 | 2 | 0 | 100 | 66 | 2 | 6 | 2 | 0 | 100 | 20 | 85 | 30 |
| ALT increased | 1 | 0 | 2 | 0 | 43 | 29 | 1 | 1 | 1 | 0 | 100 | 33 | 2 | 2 | 1 | 0 | 50 | 10 | 55 | 20 |
| γ-GT increased | 0 | 2 | 0 | 1 | 43 | 14 | 0 | 1 | 0 | 0 | 33 | 33 | 0 | 3 | 1 | 0 | 40 | 10 | 40 | 10 |
| ALP increased | 2 | 0 | 1 | 0 | 43 | 14 | 1 | 1 | 0 | 0 | 66 | 0 | 2 | 1 | 1 | 0 | 40 | 10 | 45 | 10 |
| Amylase increased | 0 | 0 | 0 | 1 | 14 | 14 | 0 | 1 | 0 | 0 | 33 | 0 | 3 | 0 | 5 | 0 | 80 | 50 | 50 | 30 |
| Lipase increased | 0 | 0 | 0 | 1 | 14 | 14 | 1 | 0 | 1 | 0 | 66 | 33 | 2 | 0 | 1 | 3 | 60 | 10 | 45 | 30 |
| Anorexia | 2 | 1 | 0 | 0 | 43 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 1 | 0 | 40 | 10 | 35 | 5 |
| Nausea | 1 | 0 | 0 | 0 | 14 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 30 | 10 | 20 | 5 |
| Hypertension | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 20 | 10 | 10 | 5 |
| Gastrointestinal bleeding | 0 | 0 | 1 | 0 | 14 | 14 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 10 | 10 | 10 | 10 |
| Bacteremia | 0 | 0 | 1 | 0 | 14 | 14 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 5 |
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CTCAE, Common Terminology Criteria for Adverse Events; γ-GT, γ-glutamyl transpeptidase.
Tumor response and survival
No patients had a complete response, five patients (25%) showed partial responses, and 12 patients (60%) showed stable disease. Progressive disease occurred in three patients (15%). During the treatment, the serum AFP level decreased in 12 patients (60%), and the serum DCP level decreased in nine patients (45%). All patients were included in the survival assessment. Of the 20 patients, one is still alive at the time of drafting this manuscript. He survived more than 40 months. He received six courses of combination chemotherapy of sorafenib with TAI of cisplatin. His HCC shrank partially, allowing for surgical resection, and no recurrence was seen. The other 19 patients did not survive. The cause of death was tumor progression in 18 of the patients and myocardial disease in one patient. The median OS and median PFS were 9.1 and 3.3 months, respectively (Fig.1).
Figure 1.
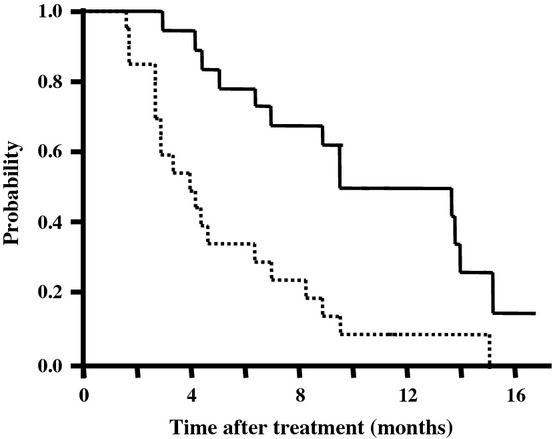
Overall survival curve (solid line) and progression-free survival curve (dashed line) of all patients enrolled in this trial of sorafenib combined with transcatheter arterial infusion of cisplatin for patients with advanced hepatocellular carcinoma.
Discussion
For advanced HCC patients with preserved liver function with a Child–Pugh score of A, sorafenib has been reported to prolong OS compared to placebo with manageable toxicity in two pivotal phase III trials.5,6 However, the OS times of 10.7 months in the SHARP study and 6.5 months in the Asia-Pacific study are still unsatisfactory. Several clinical trials of sorafenib combined with systemic chemotherapy agents or novel molecular targeted agents have been carried out, but few favorable results were reported.15 Combination chemotherapy with TAI may be a promising alternative. Transcatheter arterial infusion can increase the local concentration of anticancer drugs while reducing their systemic distribution and accompanying adverse effects.7,8 Cisplatin is an anticancer agent that has a potency that is directly related to its concentration. The response rate to intra-arterially administered cisplatin has been reported to be 33.8%,10 compared to a response rate of only 9% to systemically administered cisplatin.16 Thus, intra-arterial administration of cisplatin appears to be more effective than systemic administration of cisplatin. Moreover, sorafenib may interact with platinum transporter proteins,17 and exerts a synergistic anticancer effect with cisplatin in preclinical research.18,19 The combined regimen of sorafenib with cisplatin has been tested in clinical trials in patients with pediatric HCC,20 gastric cancer,21–23 lung cancer,24,25 nasopharyngeal carcinoma,26 and solid tumors,27 with favorable outcomes reported. Therefore, the combination of sorafenib with TAI of cisplatin would be expected to have better antitumor efficacy than sorafenib alone in patients with advanced HCC.
In this study, the safety and tolerability of the combination therapy of sorafenib with TAI using cisplatin were investigated in patients with advanced HCC. Although DLTs included grade 4 increased levels of serum AST (level 1), grade 3 gastrointestinal hemorrhage and grade 3 bacteremia (level 1), and grade 3 hypertension (level 1), sorafenib at 800 mg/day combined with cisplatin at 65 mg/m2/cycle (level 3) was well tolerated. The common drug-related adverse events that were of grade 3 or 4 severity included increased levels of AST (30%), ALT (20%), amylase (30%), and lipase (30%). Liver dysfunctions of grade 3 or higher severity were reported in <1.0% of patients in the SHARP study and in no patients in the Asia-Pacific study. The increase of serum transaminase level seemed to be more severe in this combination regimen than with sorafenib alone. This may have been due to TAI of cisplatin, because the increased levels of AST for grades 3 and 4 have been previously reported to be 32–44% in TAI of cisplatin alone.10,28
In this study, administration of sorafenib should have been suspended according to protocol regulations, if grade 2 HFSR was seen. We did not see severe HFSR, but this might lead to a slightly lower dose intensity of sorafenib. Although these severe toxicities were sometimes observed in this study, this regimen was generally manageable, and 800 mg/day sorafenib and 65 mg/m2/cycle cisplatin were acceptable to be the recommended doses. We plan to carry out a randomized phase II study comparing the combination of sorafenib and TAI using cisplatin to sorafenib alone to evaluate the efficacy and safety of the combination at the recommended doses in patients with advanced HCC.
In conclusion, the combination of sorafenib at 800 mg/day combined with cisplatin at 65 mg/m2/cycle was determined to be the recommended regimen for a phase II study in patients with advanced HCC. This regimen was generally manageable, and a randomized phase II trial of sorafenib alone versus the combination of sorafenib with TAI of cisplatin is presently underway.
Acknowledgments
We are grateful to Dr. Junji Furuse, Dr. Keigo Osuga, and Dr. Yasuhiro Matsumura, who served on the efficacy and safety evaluation committee, and to Ms Yoko Yoshimoto, who served on an independent data monitoring committee. We also thank the office administrators (Ms Keiko Kondo and Ms Rubi Mukouyama) for their support. This study was supported by a Grant-in-Aid for Cancer Research from the Ministry of Health, Labour and Welfare, Japan.
Glossary
- AFP
alpha-fetoprotein
- 5-FU
5-fluorouracil
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- DCP
des-γ-carboxy prothrombin
- DLT
dose-limiting toxicity
- γ-GT
γ-glutamyl transpeptidase
- HCC
hepatocellular carcinoma
- HFSR
hand–foot skin reaction
- OS
overall survival
- PFS
progression-free survival
- TACE
transcatheter arterial chemoembolization
- TAI
transcatheter arterial infusion
- ULN
upper limit of normal
Disclosure Statement
Sorafenib (BAY43-9006) was provided without contribution by Bayer Health Care Pharmaceuticals (West Haven, CT, USA), before it was authorized by the Ministry of Health, Labour and Welfare in Japan. Dr. Masafumi Ikeda received lecture fees and research funding from Bayer Yakuhin Ltd, Japan.
Funding information
Ministry of Health, Labour and Welfare, Japan.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362(9399):1907–17. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Di Bisceglie AM, Bruix J, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698–711. doi: 10.1093/jnci/djn134. [DOI] [PubMed] [Google Scholar]
- 4.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–2. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 6.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 7.Ensminger WD, Gyves JW. Regional chemotherapy of neoplastic diseases. Pharmacol Ther. 1983;21:277–93. doi: 10.1016/0163-7258(83)90077-3. [DOI] [PubMed] [Google Scholar]
- 8.Tzoracoleftherakis EE, Spiliotis JD, Kyriakopoulou T, Kakkos SK. Intra-arterial versus systemic chemotherapy for non-operable hepatocellular carcinoma. Hepatogastroenterology. 1999;46:1122–5. [PubMed] [Google Scholar]
- 9.Court WS, Order SE, Siegel JA, et al. Remission and survival following monthly intraarterial cisplatinum in nonresectable hepatoma. Cancer Invest. 2002;20:613–25. doi: 10.1081/cnv-120002486. [DOI] [PubMed] [Google Scholar]
- 10.Yoshikawa M, Ono N, Yodono H, Ichida T, Nakamura H. Phase II study of hepatic arterial infusion of a fine-powder formulation of cisplatin for advanced hepatocellular carcinoma. Hepatol Res. 2008;38:474–83. doi: 10.1111/j.1872-034X.2008.00338.x. [DOI] [PubMed] [Google Scholar]
- 11.Okuda K, Tanaka M, Shibata J, et al. Hepatic arterial infusion chemotherapy with continuous low dose administration of cisplatin and 5-fluorouracil for multiple recurrence of hepatocellular carcinoma after surgical treatment. Oncol Rep. 1999;6:587–91. doi: 10.3892/or.6.3.587. [DOI] [PubMed] [Google Scholar]
- 12.Sakon M, Nagano H, Dono K, et al. Combined intraarterial 5-fluorouracil and subcutaneous interferon-alpha therapy for advanced hepatocellular carcinoma with tumor thrombi in the major portal branches. Cancer. 2002;94:435–42. doi: 10.1002/cncr.10246. [DOI] [PubMed] [Google Scholar]
- 13.Lencioni R, Kudo M, Ye SL, et al. GIDEON (Global Investigation of therapeutic DEcisions in hepatocellular carcinoma and Of its treatment with sorafeNib): second interim analysis. Int J Clin Pract. 2013 doi: 10.1111/ijcp.12352. doi: 10.1111/ijcp.12352. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furuse J, Ishii H, Nakachi K, Suzuki E, Shimizu S, Nakajima K. Phase I study of sorafenib in Japanese patients with hepatocellular carcinoma. Cancer Sci. 2008;99:159–65. doi: 10.1111/j.1349-7006.2007.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abou-Alfa GK, Johnson P, Knox JJ, et al. Doxorubicin plus sorafenib vs doxorubicin alone in patients with advanced hepatocellular carcinoma: a randomized trial. JAMA. 2010;304:2154–60. doi: 10.1001/jama.2010.1672. [DOI] [PubMed] [Google Scholar]
- 16.Okada S, Okazaki N, Nose H, Shimada Y, Yoshimori M, Aoki K. A phase 2 study of cisplatin in patients with hepatocellular carcinoma. Oncology. 1993;50(1):22–6. doi: 10.1159/000227142. [DOI] [PubMed] [Google Scholar]
- 17.Heim M, Scharifi M, Zisowsky J, et al. The Raf kinase inhibitor BAY 43-9006 reduces cellular uptake of platinum compounds and cytotoxicity in human colorectal carcinoma cell lines. Anticancer Drugs. 2005;16:129–36. doi: 10.1097/00001813-200502000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Chen FS, Cui YZ, Luo RC, Wu J, Zhang H. Coadministration of sorafenib and cisplatin inhibits proliferation of hepatocellular carcinoma HepG2 cells in vitro. Nan Fang Yi Ke Da Xue Xue Bao. 2008;28:1684–7. [PubMed] [Google Scholar]
- 19.Wei Y, Shen N, Wang Z, et al. Sorafenib sensitizes hepatocellular carcinoma cell to cisplatin via suppression of Wnt/beta-catenin signaling. Mol Cell Biochem. 2013;381:139–44. doi: 10.1007/s11010-013-1695-6. [DOI] [PubMed] [Google Scholar]
- 20.Schmid I, Haberle B, Albert MH, et al. Sorafenib and cisplatin/doxorubicin (PLADO) in pediatric hepatocellular carcinoma. Pediatr Blood Cancer. 2012;58:539–44. doi: 10.1002/pbc.23295. [DOI] [PubMed] [Google Scholar]
- 21.Sun W, Powell M, O'Dwyer PJ, Catalano P, Ansari RH, Benson AB., 3rd Phase II study of sorafenib in combination with docetaxel and cisplatin in the treatment of metastatic or advanced gastric and gastroesophageal junction adenocarcinoma: ECOG 5203. J Clin Oncol. 2010;28:2947–51. doi: 10.1200/JCO.2009.27.7988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim C, Lee JL, Choi YH, et al. Phase I dose-finding study of sorafenib in combination with capecitabine and cisplatin as a first-line treatment in patients with advanced gastric cancer. Invest New Drugs. 2012;30:306–15. doi: 10.1007/s10637-010-9531-2. [DOI] [PubMed] [Google Scholar]
- 23.Yamada Y, Kiyota N, Fuse N, et al. A phase I study of sorafenib in combination with S-1 plus cisplatin in patients with advanced gastric cancer. Gastric Cancer. 2014;17:161–72. doi: 10.1007/s10120-013-0247-9. [DOI] [PubMed] [Google Scholar]
- 24.Davies JM, Dhruva NS, Walko CM, et al. A phase I trial of sorafenib combined with cisplatin/etoposide or carboplatin/pemetrexed in refractory solid tumor patients. Lung Cancer. 2011;71:151–5. doi: 10.1016/j.lungcan.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paz-Ares LG, Biesma B, Heigener D, et al. Phase III, randomized, double-blind, placebo-controlled trial of gemcitabine/cisplatin alone or with sorafenib for the first-line treatment of advanced, nonsquamous non-small-cell lung cancer. J Clin Oncol. 2012;30:3084–92. doi: 10.1200/JCO.2011.39.7646. [DOI] [PubMed] [Google Scholar]
- 26.Xue C, Huang Y, Huang PY, et al. Phase II study of sorafenib in combination with cisplatin and 5-fluorouracil to treat recurrent or metastatic nasopharyngeal carcinoma. Ann Oncol. 2013;24:1055–61. doi: 10.1093/annonc/mds581. [DOI] [PubMed] [Google Scholar]
- 27.Schultheis B, Kummer G, Zeth M, et al. Phase IB study of sorafenib in combination with gemcitabine and cisplatin in patients with refractory solid tumors. Cancer Chemother Pharmacol. 2012;69:333–9. doi: 10.1007/s00280-011-1685-x. [DOI] [PubMed] [Google Scholar]
- 28.Ikeda M, Okusaka T, Furuse J, et al. A multi-institutional phase II trial of hepatic arterial infusion chemotherapy with cisplatin for advanced hepatocellular carcinoma with portal vein tumor thrombosis. Cancer Chemother Pharmacol. 2013;72:463–70. doi: 10.1007/s00280-013-2222-x. [DOI] [PubMed] [Google Scholar]


