Abstract
The aim of the current study is to evaluate the prognostic value of anemia, an easily estimable parameter in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP) immunochemotherapy. A total of 157 patients with newly diagnosed diffuse large B-cell lymphoma treated with ≥1 cycle of R-CHOP were included. Hemoglobin level without red cell transfusion within 7 days of initiation of treatment was chosen as a parameter of baseline cancer-induced anemia. To investigate the clinical significance of chemotherapy-induced anemia and its recovery after completion of treatment, 87 patients in complete remission for ≥6 months from the time of the last cycle of R-CHOP were grouped and analyzed separately. Patients with a cancer-induced anemia of hemoglobin <10 g/dL showed inferior event-free and disease-free survival compared to those with hemoglobin ≥10 g/dL. This finding was observed irrespective of the status of pre-treatment bone marrow involvement. In multivariate analysis, hemoglobin <10 g/dL was found to be an international prognostic index-independent prognostic factor. Risk of relapse was significantly higher for patients who were still anemic at 6 months after R-CHOP, compared to those who achieved complete recovery from chemotherapy-induced anemia within 6 months.
Keywords: Anemia, biomarkers, diffuse large B-cell lymphoma, non-Hodgkin's lymphoma, prognosis
Anemia is a common finding in cancer patients, with prevalence ranging from 30% to 90%.(1) Anemia observed in cancer patients can be broadly divided into two categories, CIA and TIA. The former denotes anemia that is influenced or exacerbated by malignancy itself. The etiology of CIA seems to be multifactorial.(2) For example, IDA associated with tumor-site blood loss, suppressed erythropoiesis through BM infiltration of tumor, and suppression of iron utilization affected by cytokines released from tumor cells. Poor nutritional status of cancer patients may also contribute to the incidence of CIA. Therapy-induced anemia is caused by anticancer treatment, usually chemotherapy that suppresses hematopoiesis of BM and/or induces kidney toxicity.(3) Radiotherapy to the skeleton is also a well-known cause of TIA.(4).
As with other types of malignancy, CIA is commonly observed in patients with NHL at the time of diagnosis.(5,6) Among patients with follicular lymphoma, a representative indolent lymphoma, Hb < 12.0 g/dL was found to be an independent prognostic factor and has become one of the factors of the FLIPI(7) and FLIPI 2.(8) In a retrospective evaluation of 591 patients with intermediate-grade NHL, Morrow et al.(9) reported that baseline CIA (Hb < 12.0 g/dL) was associated with poor patient characteristics and a predictor of non-responsiveness to chemotherapy. In a previous French study among 2210 patients with high-grade NHL treated with intensive induction chemotherapeutic regimen ACVB (doxorubicin, cyclophosphamide, vincristine, bleomycin, and prednisone), the baseline Hb level <8.5 g/dL was an independent predictive factor for early death.(10).
Although those studies(5,7–10) support the prognostic value of CIA in patients with NHL, they mainly included patients with heterogeneous subtypes of NHLs in the pre-rituximab era. In addition, the clinical significance of TIA, particularly CTIA, has not been studied in patients with NHL. Therefore, we evaluated the prognostic value of CIA assessed by baseline Hb and the relation of CTIA to treatment outcomes, limited to patients with DLBCL, the most common subtype of NHL,(11) treated with R-CHOP immunochemotherapy.
Materials and Methods
Patients
Patients were included if they were: (i) diagnosed with DLBCL according to the 2008 World Health Organization criteria; (ii) ≥20 years old; (iii) treated with ≥1 cycle of conventional three-weekly R-CHOP regimen as described previously(12) at Gachon University Gil Medical Center (Incheon, Korea); and (iv) had ≥1 report of CBC testing and bilateral BM study within 7 days before initiation of the first cycle of R-CHOP immunochemotherapy. Patients with DLBCL accompanied by a central nervous system lesion, transformed from indolent B-cell lymphoma, or associated with HIV infection, were excluded. Patients with IDA either demonstrated by laboratory tests for ferrokinetics or suggested according to low mean cellular volume of RBC in a CBC report or microcytic and hypochromic RBC morphology from peripheral blood smear, were included only if the IDA appeared to be caused by lymphoma. For example, a male patient with IDA and bulky small bowel DLBCL with a history of tarry stool was included, whereas a young female patient with single cervical lymph node-involved DLBCL of Ann Arbor stage IA, along with IDA caused by menorrhagia, was excluded. Patients with autoimmune or non-autoimmune hemolytic anemia, megaloblastic anemia, or anemia affected by BM insufficiency from hematologic disease other than DLBCL were ineligible for inclusion in the current study. Administration of erythropoiesis-stimulating agents was not allowed by the institutional policy.
This study was reviewed and approved by the IRB of the Gachon University Gil Medical Center and the informed consent was waived by the IRB considering the retrospective nature of the study with minimal risk (approval number: GBIRB2014-176).
Assessment of anemia
The institutional LLN of Hb in CBC was set as 13.0 g/dL for males and 12.5 g/dL for female patients. Severity of anemia was graded by Hb level according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0, as follows: G1, <LLN − 10.0 g/dL; G2, <10.0 to ≤8.0 g/dL; G3, <8.0 g/dL or transfusion indicated; and G4, life threatening.
Baseline Hb was chosen as a marker of CIA and defined as the lowest Hb level within 7 days before initiation of R-CHOP without RBC transfusion. To evaluate the clinical significance of CTIA and its recovery after completion of R-CHOP, patients who satisfied the following criteria were separated as a subgroup, named Group CR6, and further evaluated: (i) patients who had CR confirmed by 18flurorodeoxyglucose PET/computed tomography after six to eight cycles of R-CHOP with or without subsequent radiotherapy to a non-skeletal area; (ii) patients who maintained CR for ≥6 months from the time of the last cycle of R-CHOP; and (iii) patients who did not receive radiotherapy to the skeletal area nor consolidative autologous stem cell transplantation. For those patients, review and analysis of nadir Hb level during R-CHOP therapy and the lower Hb levels at three (90 ± 15 days) and six (180 ± 30 days) months after the date of the last cycle of R-CHOP with their treatment outcomes was carried out.
Statistical analysis
Event-free survival denotes the time from initiation of treatment to any treatment failure, including disease progression, relapse, or discontinuation of treatment for any reason. Overall survival was defined as the time from initiation of treatment until death from any cause. Disease-free survival, the time from initiation of therapy to disease progression or relapse, was also estimated in order to exclude the impact of early TRM or non-lymphoma-related death. The Kaplan–Meier method was used for analysis of survival and the log–rank test was used for comparison. Multivariate analysis was carried out by entering into a backward Cox regression model, with variables of P < 0.1 in univariate analysis. Either Fisher's exact test or the Chi-square test was used as appropriate for dichotomizing and analysis of continuous variables. Each value was two-sided, and statistical significance was accepted at P < 0.05.
Results
Patient characteristics
For the period from March 2006 to August 2013, 181 adult patients were diagnosed with DLBCL. Among those, 157 patients (median age, 58 years) satisfied the inclusion criteria and 24 patients were excluded. The reasons of exclusion were: received supportive care only because of poor performance status (n = 6), central nervous system involvement of DLBCL (n = 6), lack of information on bilateral BM biopsy results (n = 5), transfer to another hospital after diagnosis (n = 2), received immunochemotherapy other than R-CHOP (n = 1; R-CHOP plus etoposide), and had anemia considered as non-lymphomatous cause (n = 4; two pre-menopausal female patients had IDA with definitive history of menorrhagia, one patient had preceding pancytopenia which was thought to be originated from her underlying rheumatologic disorder, and one patient with Ann Arbor stage II DLBCL had both anemia and thrombocytopenia with a BM cellularity of <25%, suggestive of non-severe aplastic anemia). One male and one female patient with IDA were included because their IDA were thought to be related to lymphoma: a 66-year-old male patient who had a lymphomatous involvement of small bowel with overt hematochezia, and a 70-year-old female also with small bowel lesion with a positive result for stool Hb test.
Of the 157 included patients, 87 (55.4%; median age, 55 years) were classified as Group CR6. A detailed summary of characteristics of all patients and Group CR6 is shown in Table 1.
Table 1.
Patient characteristics
| Parameters | Entire patients (n = 157) | Group CR6 (n = 87) |
|---|---|---|
| Gender | ||
| Male | 68 (43.3%) | 37 (42.5%) |
| Age | ||
| Median | 58 | 55 |
| Range | 20–89 | 20–80 |
| >60 years | 73 (46.5%) | 35 (40.2%) |
| ECOG† performance status | ||
| ≥2 | 46 (29.3%) | 13 (14.9%) |
| Serum lactate dehydrogenase | ||
| Elevated | 76 (48.4%) | 35 (40.2%) |
| Ann Arbor stage | ||
| I | 26 (16.6%) | 15 (17.2%) |
| II | 55 (35.0%) | 34 (39.1%) |
| III | 34 (21.7%) | 23 (26.4%) |
| IV | 42 (26.8%) | 15 (17.2%) |
| Extranodal sites | ||
| ≥2 sites | 45 (28.7%) | 18 (20.7%) |
| Standard IPI‡ risk | ||
| Low | 76 (48.4%) | 53 (60.9%) |
| Low-intermediate | 26 (16.6%) | 14 (16.1%) |
| High-intermediate | 19 (12.1%) | 8 (9.2%) |
| High | 36 (23.0%) | 12 (13.8%) |
| B symptom | ||
| Present | 44 (28.0%) | 21 (24.1%) |
| Bulky lesion | ||
| Present | 33 (21.0%) | 18 (20.7%) |
| Bone marrow involvement | ||
| Present | 25 (15.9%) | 8 (9.2%) |
| Hans classification | ||
| GCB§ subtype | 44 (28.0%) | 27 (31.0%) |
| Non-GCB subtype | 69 (43.9%) | 41 (47.1%) |
| Not classified | 44 (28.0%) | 19 (21.8%) |
| Serum albumin | ||
| <3.5 g/dL | 42 (26.8%) | 17 (19.5%) |
| Treatment | ||
| 6 to 8 cycles of R-CHOP¶ with or without radiotherapy | 97 | 87 |
| 6 to 8 cycles of R-CHOP followed by ASCT†† | 11 | – |
| 3 to 4 cycles of R-CHOP followed by radiotherapy | 20 | – |
| Not be completed due to treatment-related mortality | 10 | – |
| Not be completed due to patient's refusal or lost follow-up, etc | 6 | – |
Group CR6 was made up of a subgroup of patients who: (i) had complete remission confirmed by 18flurorodeoxyglucose PET /computed tomography after six to eight cycles of R-CHOP with or without subsequent radiotherapy to a non-skeletal area; (ii) maintained complete remission for ≥6 months from the time of the last cycle of R-CHOP; and (iii) did not receive radiotherapy to the skeletal area nor consolidative autologous stem cell transplantation. ASCT, autologous 6 stem cell transplantation; ECOG, Eastern Cooperative Oncology Group; GCB, germinal center B cell-like; IPI, International Prognostic Index; RCHOP, rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone.
Overall treatment outcomes
A total of 129 patients (82.2%) achieved CR at the final response evaluation. Six patients (3.8%) showed a partial response and six (3.8%) had progressive disease during R-CHOP treatment. Sixteen patients (10.2%) were not evaluable for the final response evaluation because their planned R-CHOP could not be completed.
During the median follow-up period of 38.4 months, 40 events (24.8%; 10 patients for TRM, 6 patients for progressive disease during R-CHOP, 1 patient for non-lymphoma-related death by rupture of an ascending aortic aneurysm, and 23 patients for relapsed DLBCL after CR) and 38 deaths (24.2%) were reported. The estimated EFS rate of all patients at 2 and 3 years was 75.4% and 73.6%, respectively. The OS rate at 2 and 3 years was 78.0% and 76.0%, respectively.
Among Group CR6, there were 11 events (12.6%; all events were disease relapse) and 9 deaths (10.3%; all deaths were lymphoma-related), with a median follow-up period of 37.1 months. Disease-free survival was the same as EFS because all events that occurred in Group CR6 were disease relapse. The DFS rate at 2 and 3 years for those patients was 88.7% and 88.7%, respectively, and the OS rate at 2 and 3 years was 94.8% and 92.8%, respectively.
Analysis of cancer-induced anemia
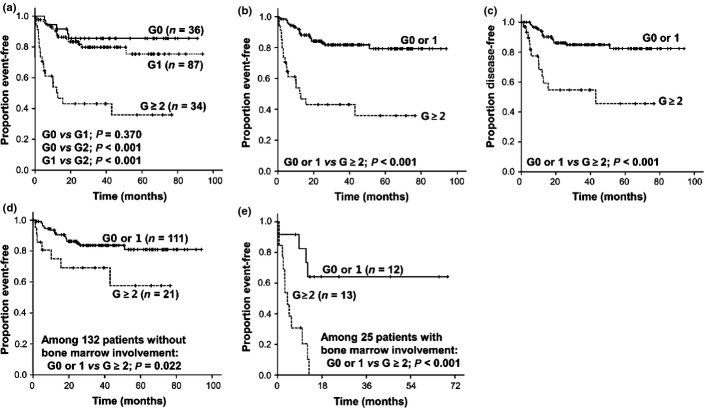
The mean baseline Hb was 11.9 ± SD 1.8 g/dL in male patients and 10.9 ± SD 1.6 g/dL in female patients (P = 0.001). One hundred and twenty-one patients (77.1%) had G ≥ 1 and 34 patients (21.7%) had G ≥ 2 baseline anemia. Patients with a CIA of G ≥ 2 anemia showed inferior EFS compared to those with no (G0) or G1 anemia (Fig. 1a,b). Among 10 patients who had TRM, 7 had G ≥ 2 baseline anemia (P = 0.001). However, such a significant difference of survival between patients with G ≥ 2 anemia and those with G0 or 1 anemia was well maintained in analysis of DFS instead of EFS (P < 0.001; Fig. 1c).
Fig. 1.
Analyses of the impact of anemia on treatment outcomes in patients with diffuse large B-cell lymphoma treated with R-CHOP immunochemotherapy (n = 157). (a, b) Kaplan–Meier plots for event-free survival according to the grade of baseline anemia. (c) Kaplan–Meier plot for disease-free survival according to the grade of baseline anemia. (d, e) Kaplan–Meier graphs for event-free survival according to bone marrow status and baseline anemia.
A significantly lower mean baseline Hb was observed in patients with BM involvement compared to those without BM involvement (10.1 ± SD 1.7 g/dL vs 11.5 ± SD 1.7 g/dL, P < 0.001). Most IPI factors, BM involvement, and serum albumin level <3.5 g/dL was significantly associated with the incidence of G ≥ 2 anemia (Table 2). Inferior survival of patients with G ≥ 2 anemia was observed irrespective of BM status (Fig. 1d,e).
Table 2.
Relations of patient characteristics to cancer-induced anemia and chemotherapy-induced anemia in patients with diffuse large B-cell lymphoma treated with R-CHOP immunochemotherapy (n = 157)
| Cancer-induced anemia (n = 157) | Chemotherapy-induced anemia (n = 87) | |||
|---|---|---|---|---|
| G0 or 1 (n = 123) | G ≥ 2 (n = 34) | Normalized (n = 74) | Still anemic (n = 13) | |
| Gender, male | 56 | 12 | 30 | 7 |
| versus female | 67 | 22 | 44 | 6 |
| P | 0.286 | 0.371 | ||
| Age ≥60 years | 52 | 21 | 25 | 10 |
| versus <60 years | 71 | 13 | 49 | 3 |
| P | 0.044 | 0.003 | ||
| ECOG performance status ≥2 | 27 | 15 | 8 | 5 |
| versus 0 or 1 | 96 | 19 | 66 | 8 |
| P | <0.001 | 0.010 | ||
| Lactose dehydrogenase elevated | 55 | 21 | 29 | 6 |
| versus not elevated | 68 | 13 | 45 | 7 |
| P | <0.078 | 0.637 | ||
| Ann Arbor stage III or IV | 51 | 25 | 30 | 8 |
| versus I or II | 72 | 9 | 44 | 5 |
| P | 0.001 | 0.159 | ||
| ≥2 Extranodal site(s) | 25 | 20 | 12 | 6 |
| versus none or single site | 98 | 14 | 62 | 7 |
| P | <0.001 | 0.014 | ||
| Standard IPI high or high-intermediate | 32 | 23 | 13 | 7 |
| versus low or low-intermediate | 91 | 11 | 61 | 6 |
| P | <0.001 | 0.004 | ||
| B symptoms present | 23 | 21 | 15 | 6 |
| versus absent | 100 | 13 | 59 | 7 |
| P | <0.001 | 0.044 | ||
| Bulky disease present | 24 | 9 | 16 | 2 |
| versus absent | 99 | 25 | 58 | 11 |
| P | 0.378 | 0.609 | ||
| Bone marrow involvement present | 12 | 13 | 7 | 1 |
| versus absent | 111 | 21 | 67 | 12 |
| P | <0.001 | 0.839 | ||
| Hans classification GCB subtype | 39 | 5 | 24 | 3 |
| versus non-GCB subtype | 54 | 15 | 34 | 7 |
| P | 0.159 | 0.497 | ||
| Serum albumin <3.5 g/dL | 22 | 20 | 13 | 4 |
| versus ≥3.5 g/dL | 101 | 14 | 61 | 9 |
| P | <0.001 | 0.268 | ||
ECOG, Eastern Cooperative Oncology Group; GCB, germinal center B cell-like; IPI, International Prognostic Index.
In univariate analysis, each IPI factor, BM involvement, presence of B symptoms, and G ≥ 2 baseline anemia showed an association with EFS. In multivariate analysis, baseline G ≥ 2 anemia was found to be an IPI-independent prognostic factor (Table 3). Actually patients who had both G ≥ 2 anemia and BM involvement (n = 13) showed extremely poor prognosis (Fig. 1e).
Table 3.
Univariate and multivariate analysis for event-free survival and disease-free survival in all patients with diffuse large B-cell lymphoma treated with R-CHOP immunochemotherapy (n = 157)
| Parameters | For event-free survival | For disease-free survival | ||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Univariate analysis | ||||
| IPI score ≥3 | 5.6 (2.9–10.8) | <0.001 | 5.5 (2.6–11.9) | <0.001 |
| Age >60 years | 2.3 (1.2–4.4) | 0.011 | 1.3 (0.6–2.7) | 0.460 |
| Elevated lactose dehydrogenase | 3.8 (1.9–7.9) | <0.001 | 4.2 (1.8–9.8) | <0.001 |
| Ann Arbor stage III or IV | 3.2 (1.6–6.5) | 0.001 | 4.0 (1.7–9.4) | <0.001 |
| ECOG performance status ≥2 | 11.1 (5.5–22.5) | <0.001 | 10.4 (4.7–23.1) | <0.001 |
| ≥2 extranodal sites | 3.5 (1.9–6.5) | <0.001 | 3.5 (1.7–7.4) | 0.001 |
| Presence of B symptoms | 2.9 (1.6–5.5) | 0.001 | 2.8 (1.3–5.8) | 0.008 |
| Presence of bulky lesion | 0.8 (0.3–1.8) | 0.538 | 0.6 (0.2–1.7) | 0.306 |
| Bone marrow involvement | 6.3 (3.3–12.0) | <0.001 | 5.7 (2.6–12.4) | <0.001 |
| Non-GCB by Hans classification (vs GCB) | 1.8 (0.8–4.1) | 0.168 | 1.7 (0.7–4.4) | 0.257 |
| Serum albumin <3.5 g/dL | 2.0 (1.1–3.8) | 0.031 | 1.1 (0.5–2.6) | 0.785 |
| Grade ≥2 anemia | 5.1 (2.7–9.5) | <0.001 | 4.4 (2.1–9.3) | <0.001 |
| Multivariate analysis; IPI as separate 5 marker | ||||
| ECOG performance status ≥2 | 7.3 (3.3–15.8) | <0.001 | 8.9 (4.0–20.1) | <0.001 |
| Bone marrow involvement | 2.0 (1.0–4.2) | 0.051 | – | – |
| Grade ≥2 anemia | 3.0 (1.5–5.8) | 0.001 | 2.9 (1.4–6.3) | 0.006 |
| Multivariate analysis; IPI as a single marker | ||||
| IPI score ≥3 | 2.8 (1.3–6.2) | 0.009 | 3.2 (1.3–7.7) | 0.010 |
| Bone marrow involvement | 2.9 (1.4–6.2) | 0.005 | 2.7 (1.1–6.7) | 0.025 |
| Grade ≥2 anemia | 3.3 (1.7–6.4) | <0.001 | 3.0 (1.4–6.7) | 0.004 |
CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; GCB, germinal center B cell-like; HR, hazard ratio; IPI, International Prognostic Index.
Among patients in Group CR6, baseline anemia G ≥ 2 did not showed an association with the difference of DFS (P = 0.356; Fig. 2a).
Fig. 2.
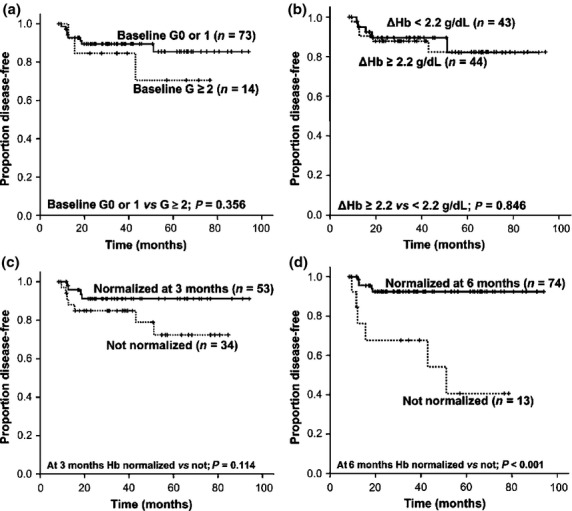
Analyses of the impact of anemia on treatment outcomes in a subgroup of patients (group CR6) (n = 87). Kaplan–Meier curves of disease-free survival according to the grade of baseline anemia (a), the mean gap (Δ) between baseline and nadir hemoglobin (Hb) during immunochemotherapy (b), and the normalization of Hb 3 months (c) and 6 months (d) after completion of the planned therapy.
Analysis of chemotherapy-induced anemia
All 87 patients in Group CR6 experienced CTIA: the mean nadir Hb was 9.6 ± SD 1.7 g/dL in male patients and 9.1 ± SD 1.1 g/dL in female patients (P = 0.139). Sixty patients (69.0%) had a G ≥ 2 nadir Hb and 19 patients (21.8%) had a G ≥ 3 nadir Hb during R-CHOP. Mean gap between baseline Hb and nadir Hb during immunochemotherapy (ΔHb) of each patient was 2.2 ± SD 1.4 g/dL. Severity of nadir Hb did not show a significant association with inferior DFS (P = 0.711 for G ≥ 2 anemia and P = 0.061 for G ≥ 3 anemia). No difference of DFS was observed between patients with a ΔHb ≥ 2.2 g/dL and those with a ΔHb < 2.2 g/dL (P = 0.846; Fig. 2b).
Of the 87 patients, 53 (60.9%) and 74 (85.1%) patients reported a normalized Hb in CBC at 3 and 6 months after completion of R-CHOP, respectively. Patients who were still anemic at 6 months after R-CHOP had a significantly higher rate of relapse (six relapses out of 13 patients vs five relapses out of 74 patients; P = 0.001) with notably inferior DFS, compared to those who achieved a complete recovery from CTIA within 6 months (Fig. 2d). Failure to normalization of Hb at 6 months after immunochemotherapy was an independent factor predicting disease relapse, along with IPI factors (Table 4).
Table 4.
Univariate and multivariate analysis for disease-free survival in group CR6 of patients with diffuse large B-cell lymphoma treated with R-CHOP immunochemotherapy (n = 87)
| Parameters | Hazard ratio (95% CI) | P-value |
|---|---|---|
| Univariate analysis | ||
| IPI score ≥3 | 4.2 (1.3–13.9) | 0.017 |
| Age >60 years | 1.6 (0.5–5.4) | 0.414 |
| Elevated lactose dehydrogenase | 7.0 (1.5–32.3) | 0.013 |
| Ann Arbor stage III or IV | 3.0 (0.8–11.5) | 0.100 |
| ECOG performance status ≥2 | 7.8 (2.4–25.7) | 0.001 |
| ≥2 extranodal sites | 3.3 (1.0–10.7) | 0.051 |
| Presence of B symptoms | 1.6 (0.5–5.6) | 0.439 |
| Presence of bulky lesion | 0.3 (0.1–2.6) | 0.293 |
| Bone marrow involvement | 5.6 (1.5–21.4) | 0.011 |
| Non-GCB by Hans classification (vs GCB) | 1.4 (0.4–6.0) | 0.637 |
| Serum albumin <3.5 g/dL | 1.2 (0.3–5.4) | 0.850 |
| Anemia not recovered at 6 months | 8.0 (2.4–26.2) | 0.001 |
| Multivariate analysis; IPI as separate 5 marker | ||
| Elevated lactose dehydrogenase | 5.5 (1.1–26.6) | 0.034 |
| Bone marrow involvement | 7.2 (1.5–34.6) | 0.013 |
| Anemia not recovered at 6 months | 11.5 (3.1–43.3) | <0.001 |
| Multivariate analysis; IPI as a single marker | ||
| Bone marrow involvement | 11.6 (2.5–53.7) | 0.002 |
| Anemia not recovered at 6 months | 12.5 (3.3–47.5) | <0.001 |
Group CR6 was made up of a subgroup of patients who: (i) had complete remission confirmed by 18flurorodeoxyglucose PET/computed tomography after six to eight cycles of R-CHOP with or without subsequent radiotherapy to a non-skeletal area; (ii) maintained complete remission for ≥6 months from the time of the last cycle of R-CHOP; and (iii) did not receive radiotherapy to the skeletal area nor consolidative autologous stem cell transplantation. CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; GCB, germinal center B cell-like; IPI, International Prognostic Index.
Discussion
In the current study, baseline G ≥ 2 anemia was an independent poor prognostic factor in the analyzed patients. Patients who failed to recover from CTIA within 6 months after completion of six to eight cycles of R-CHOP were more likely to experience relapse.
Unlike follicular lymphoma, in which Hb < 12.0 g/dL was known to be prognostic,(7,8) the current study showed that no difference of EFS between patients with G0 and G1 anemia and only G ≥ 2 anemia showed an association with inferior EFS. In a previous Taiwanese study evaluating 100 patients with DLBCL treated with rituximab-containing immunochemotherapy, Hb < 12.0 g/dL did not show a significant association with inferior progression-free survival or OS,(13) consistent with our result. It is not certain whether mild-degree G1 anemia does not have any impact on outcomes at all or might have shown a significant relation to poor survival if it were analyzed in a larger patient population. Nevertheless, we can cautiously suggest that inferior survival is prominent when baseline anemia is G ≥ 2, at least in patients with DLBCL, which includes a large majority of high grade NHLs.
In the current study, G ≥ 2 CIA showed an association with early TRM during R-CHOP and this result is in line with the result of a previous GELA study,(10) in which Hb < 8.5 g/dL was identified as a predictor of death within 100 days of treatment initiation. However, the prognostic value was preserved when we censored the episodes of TRMs by analysis of DFS instead of EFS. Considering the maintenance of the prognostic power in multivariate analysis on DFS, baseline anemia seems not to be a byproduct caused by malnutrition and/or impaired performance status. Rather, it seems to be a biomarker reflecting both the risk of early death due to toxicity of treatment and rapid progression of the disease or later relapse. Confirmation of these findings in a larger cohort can clearly define more detailed meaning and the role of baseline CIA in patients with aggressive NHL including DLBCL.
Some may argue that baseline CIA may not be an independent factor but a consequence of BM involvement, an important prognostic factor of DLBCL. For example, when the prognostic role of platelet count was evaluated, thrombocytopenia showed an association with inferior survival but limited to patients with BM involvement.(6,14) Indeed, in our study the incidence of G ≥ 2 baseline anemia showed strong correlation with the incidence of BM involvement (P < 0.001). However, the prognostic value of G ≥ 2 CIA was independent from the baseline BM status of patients (Fig. 1d,e, Table 3). Bone marrow involvement may actually be just a part of various factors causing CIA. In a recent study by Tisi et al.(15) evaluating the characteristics of CIA in 53 patients with DLBCL, influence of lymphomatous BM involvement had no impact on the occurrence of anemia: no difference of Hb level was observed according to the BM status (median, 11.8 g/dL for patients without BM infiltration vs 10.9 g/dL for those with BM infiltration, P = 0.27). By contrast, an elevated level of interleukin-6, a pro-inflammatory cytokine, was the dominant factor affecting anemia, which has the typical signs of anemia of chronic disease. They also concluded that reduced erythropoietin production plays a minor but significant role in development of anemia.(15) These results suggest that not only BM suppression by tumor but various factors, including poor utilization of stored iron, excessive production of pro-inflammatory cytokines, and blunted erythropoietin synthesis may result in CIA that has a prognostic value. Another possibility is that patients with G ≥ 2 anemia may have an occult BM involvement, which can only be detected by molecular clonal assays.(16) Further evaluation of clonality of BM samples with molecular testing in patients with NHL who have moderate to severe CIA would be of interest.
We selected Group CR6 (n = 87) in order to identify any anemia-related parameters that would be predictive of later disease relapse after CR. In those patients, G ≥ 2 baseline anemia was not predictive of relapse, along with the severity (ΔHb) of CTIA. By comparison, recovery from CTIA within 6 months was an identified factor predicting an inferior survival. Because CTIA has a distinct cause, it has been assumed(17) and sometimes evaluated(18,19) separately from CIA in previous studies of erythropoiesis-stimulating agents in malignancies. Due to the limited sample size, we cannot be conclusive that recovery from CTIA within 6 months is a biomarker predictive of relapse. For example, although patients up to 89 years of age were included, all events after 6 months are disease-related; this could be explained only by the small patient number. However, further evaluation in larger patient cohorts may reveal the actual value of successful recovery from CTIA. In DLBCL, other post-treatment CBC changes have been studied: late onset neutropenia after rituximab was found to be a relatively common phenomenon and no association of overall prognosis was reported.(20,21) Lymphopenia after completion of rituximab-containing immunochemotherapy appears to be a risk factor for disease relapse.(22,23) Future extension of the prognostic implication of CTIA to T-cell or natural killer cell lymphoma is also warranted because RBC is not a target of rituximab, a mAb to CD20 surface antigen of B-lymphocyte.
In conclusion, CIA assessed by pre-treatment Hb < 10.0 g/dL was overall prognostic. Failure to complete recovery from CTIA within 6 months of completion of R-CHOP was predictive of DLBCL relapse. The current study raises an interest and necessity of further investigation into CIA and CTIA, parameters that are very easy to analyze with almost no additional cost.
Glossary
Abbreviations
- BM
bone marrow
- CBC
complete blood cell count
- CIA
cancer-induced anemia
- CR
complete remission
- CTIA
chemotherapy-induced anemia
- DFS
disease-free survival
- DLBCL
diffuse large B-cell lymphoma
- EFS
event-free survival
- FLIPI
Follicular Lymphoma International Prognostic Index
- G
grade
- Hb
hemoglobin
- IDA
iron deficiency anemia
- IPI
International Prognostic Index
- IRB
Institutional Review Board
- LLN
lower limit of normal
- NHL
non-Hodgkin's lymphoma
- OS
overall survival
- RBC
red blood cells
- R-CHOP
rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone
- TIA
therapy-induced anemia
- TRM
treatment-related mortality
Disclosure Statement
The authors have no conflict of interest.
References
- 1.Knight K, Wade S, Balducci L. Prevalence and outcomes of anemia in cancer: a systematic review of the literature. Am J Med. 2004;116(Suppl 7A):11S–26S. doi: 10.1016/j.amjmed.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 2.Spivak JL. The anaemia of cancer: death by a thousand cuts. Nat Rev Cancer. 2005;5:543–55. doi: 10.1038/nrc1648. [DOI] [PubMed] [Google Scholar]
- 3.Groopman JE, Itri LM. Chemotherapy-induced anemia in adults: incidence and treatment. J Natl Cancer Inst. 1999;91:1616–34. doi: 10.1093/jnci/91.19.1616. [DOI] [PubMed] [Google Scholar]
- 4.Jefferies S, Rajan B, Ashley S, Traish D, Brada M. Haematological toxicity of cranio-spinal irradiation. Radiother Oncol. 1998;48:23–7. doi: 10.1016/s0167-8140(98)00024-3. [DOI] [PubMed] [Google Scholar]
- 5.Moullet I, Salles G, Ketterer N, et al. Frequency and significance of anemia in non-Hodgkin's lymphoma patients. Ann Oncol. 1998;9:1109–15. doi: 10.1023/a:1008498705032. [DOI] [PubMed] [Google Scholar]
- 6.Conlan MG, Armitage JO, Bast M, Weisenburger DD. Clinical significance of hematologic parameters in non-Hodgkin's lymphoma at diagnosis. Cancer. 1991;67:1389–95. doi: 10.1002/1097-0142(19910301)67:5<1389::aid-cncr2820670519>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 7.Solal-Celigny P, Roy P, Colombat P, et al. Follicular lymphoma international prognostic index. Blood. 2004;104:1258–65. doi: 10.1182/blood-2003-12-4434. [DOI] [PubMed] [Google Scholar]
- 8.Federico M, Bellei M, Marcheselli L, et al. Follicular lymphoma international prognostic index 2: a new prognostic index for follicular lymphoma developed by the international follicular lymphoma prognostic factor project. J Clin Oncol. 2009;27:4555–62. doi: 10.1200/JCO.2008.21.3991. [DOI] [PubMed] [Google Scholar]
- 9.Morrow TJ, Volpe S, Gupta S, Tannous RE, Fridman M. Anemia of cancer in intermediate-grade non-Hodgkin's lymphoma. South Med J. 2002;95:889–96. [PubMed] [Google Scholar]
- 10.Dumontet C, Mounier N, Munck JN, et al. Factors predictive of early death in patients receiving high-dose CHOP (ACVB regimen) for aggressive non-Hodgkin's lymphoma: a GELA study. Br J Haematol. 2002;118:210–7. doi: 10.1046/j.1365-2141.2002.03565.x. [DOI] [PubMed] [Google Scholar]
- 11.Huh J. Epidemiologic overview of malignant lymphoma. Korean J Hematol. 2012;47:92–104. doi: 10.5045/kjh.2012.47.2.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hong J, Park S, Park J, et al. Evaluation of prognostic values of clinical and histopathologic characteristics in diffuse large B-cell lymphoma treated with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone therapy. Leuk Lymphoma. 2011;52:1904–12. doi: 10.3109/10428194.2011.588761. [DOI] [PubMed] [Google Scholar]
- 13.Chen LP, Lin SJ, Yu MS. Prognostic value of platelet count in diffuse large B-cell lymphoma. Clin Lymphoma Myeloma Leuk. 2012;12:32–7. doi: 10.1016/j.clml.2011.09.215. [DOI] [PubMed] [Google Scholar]
- 14.Bloomfield CD, McKenna RW, Brunning RD. Significance of haematological parameters in the non-Hodgkin's malignant lymphomas. Br J Haematol. 1976;32:41–6. doi: 10.1111/j.1365-2141.1976.tb01873.x. [DOI] [PubMed] [Google Scholar]
- 15.Tisi MC, Bozzoli V, Giachelia M, et al. Anemia in diffuse large B-cell non-Hodgkin lymphoma: the role of interleukin-6, hepcidin and erythropoietin. Leuk Lymphoma. 2014;55:270–5. doi: 10.3109/10428194.2013.802314. [DOI] [PubMed] [Google Scholar]
- 16.Mitterbauer-Hohendanner G, Mannhalter C, Winkler K, et al. Prognostic significance of molecular staging by PCR-amplification of immunoglobulin gene rearrangements in diffuse large B-cell lymphoma (DLBCL) Leukemia. 2004;18:1102–7. doi: 10.1038/sj.leu.2403376. [DOI] [PubMed] [Google Scholar]
- 17.Steensma DP. Is anemia of cancer different from chemotherapy-induced anemia? J Clin Oncol. 2008;26:1022–4. doi: 10.1200/JCO.2007.15.3874. [DOI] [PubMed] [Google Scholar]
- 18.Leyland-Jones B, Semiglazov V, Pawlicki M, et al. Maintaining normal hemoglobin levels with epoetin alfa in mainly nonanemic patients with metastatic breast cancer receiving first-line chemotherapy: a survival study. J Clin Oncol. 2005;23:5960–72. doi: 10.1200/JCO.2005.06.150. [DOI] [PubMed] [Google Scholar]
- 19.Smith RE, Jr, Aapro MS, Ludwig H, et al. Darbepoetin alpha for the treatment of anemia in patients with active cancer not receiving chemotherapy or radiotherapy: results of a phase III, multicenter, randomized, double-blind, placebo-controlled study. J Clin Oncol. 2008;26:1040–50. doi: 10.1200/JCO.2007.14.2885. [DOI] [PubMed] [Google Scholar]
- 20.Dunleavy K, Tay K, Wilson WH. Rituximab-associated neutropenia. Semin Hematol. 2010;47:180–6. doi: 10.1053/j.seminhematol.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rozman S, Sonc M, Novakovic BJ. Late-onset neutropenia following primary treatment of diffuse large B-cell lymphoma with rituximab-containing therapy. Leuk Lymphoma. 2012;53:1945–8. doi: 10.3109/10428194.2012.679266. [DOI] [PubMed] [Google Scholar]
- 22.Aoki T, Nishiyama T, Imahashi N, Kitamura K. Lymphopenia following the completion of first-line therapy predicts early relapse in patients with diffuse large B cell lymphoma. Ann Hematol. 2012;91:375–82. doi: 10.1007/s00277-011-1305-1. [DOI] [PubMed] [Google Scholar]
- 23.Porrata LF, Inwards DJ, Ansell SM, et al. New-onset lymphopenia assessed during routine follow-up is a risk factor for relapse postautologous peripheral blood hematopoietic stem cell transplantation in patients with diffuse large B-cell lymphoma. Biol Blood Marrow Transplant. 2010;16:376–83. doi: 10.1016/j.bbmt.2009.10.029. [DOI] [PubMed] [Google Scholar]