Abstract
Interstitial lung disease (ILD) occurrence and risk factors were investigated in the Japanese non-small-cell lung cancer, post-marketing, large-scale surveillance study, POLARSTAR. All patients with unresectable, recurrent/advanced non-small-cell lung cancer who were treated with erlotinib in Japan between December 2007 and October 2009 were enrolled. Primary endpoints were patterns of ILD and risk factors for onset of ILD and ILD-related death. Overall survival, progression-free survival, and occurrence of adverse drug reactions were secondary endpoints. Interstitial lung disease was confirmed in 429 (4.3%) patients. Concurrent/previous ILD (hazard ratio, 3.19), emphysema or chronic obstructive pulmonary disease (hazard ratio, 1.86), lung infection (hazard ratio, 1.55), smoking history (hazard ratio, 2.23), and period from initial cancer diagnosis to the start of treatment (<360 days; hazard ratio, 0.58) were identified as significant risk factors for developing ILD by Cox multivariate analysis. Logistic regression analysis identified Eastern Cooperative Oncology Group performance status 2–4 (odds ratio, 2.45 [95% confidence interval, 1.41–4.27]; P = 0.0016), ≤50% remaining normal lung area (odds ratio, 3.12 [1.48–6.58]; P = 0.0029), and concomitant honeycombing with interstitial pneumonia (odds ratio, 6.67 [1.35–32.94]; P = 0.02) as poor prognostic factors for ILD death. Median overall survival was 277 days; median progression-free survival was 67 days. These data confirm the well-characterized safety profile of erlotinib. Interstitial lung disease is still an adverse drug reaction of interest in this population, and these results, including ILD risk factors, give helpful information for treatment selection and monitoring. Erlotinib efficacy was additionally confirmed in this population. (POLARSTAR trial ML21590.)
Keywords: Erlotinib, interstitial lung disease, Japanese, non-small-cell lung cancer, surveillance
Erlotinib is an orally administered EGFR TKI that has demonstrated survival benefits over placebo (median OS 6.7 vs 4.7 months, respectively; P = 0.002) with acceptable tolerability in previously treated patients with NSCLC.(1) Promising survival data were also reported in two Japanese phase 2 trials of erlotinib in patients with advanced NSCLC (median OS 13.5–14.7 months).(2,3) This led to the approval of erlotinib in Japan for the treatment of patients with recurrent/advanced NSCLC after failure on at least one prior chemotherapy regimen.
Interstitial lung disease has been reported as an AE of special interest in erlotinib-treated Japanese patients with NSCLC in 4.9% (6/123) of patients with a mortality rate of 2.4% (3/123 patients).(2–4) Similar incidences of ILD have been reported in Japanese patients with NSCLC treated with the EGFR TKI gefitinib, suggesting this may be a class-related AE.(5,6)
Risk factors for developing ILD have been previously reported primarily in gefitinib-treated patients. Kudoh et al.(6) reported old age, smoking history, pre-existing ILD, poor ECOG PS, short duration since NSCLC diagnosis, and ≤50% normal lung area as ILD risk factors, with all of the factors, except ECOG PS and short duration since NSCLC diagnosis, also being associated with poor ILD prognosis (fatal ILD). Hotta et al.(7) reported existing pulmonary fibrosis, poor ECOG PS, and prior irradiation as risk factors for ILD. Pre-existing pulmonary fibrosis and poor ECOG PS have also been shown to be associated risk factors for ILD in patients treated with either gefitinib or erlotinib.(8)
POLARSTAR was a large-scale surveillance study including all Japanese patients with NSCLC treated with erlotinib,(9) undertaken as a post-approval commitment in Japan to monitor safety and efficacy. The objectives were to obtain decisive information on the incidence of ILD, risk factors for developing ILD, and the efficacy of erlotinib. Here, we report the final analysis of the POLARSTAR surveillance study investigating the safety and efficacy of erlotinib treatment in Japanese patients with NSCLC.
Methods
Study design
All patients with unresectable, recurrent/advanced NSCLC who were treated with erlotinib in Japan between December 2007 and October 2009 were enrolled. Eligible patients receiving erlotinib (150 mg orally, once daily), from the 1027 institutions that could prescribe erlotinib, were monitored until erlotinib therapy termination or completion of 12 months of treatment. The study was approved by the relevant ethics committees and patients gave informed consent to participate in the analysis.
Assessments
Demographic and baseline data were collected for each patient, including age, gender, body mass index, tumor histology, ECOG PS, smoking history, and medical history (including hepatic dysfunction, renal dysfunction, cardiovascular disease, and lung disorders). Safety data were collected at 1, 6, and 12 months after the start of erlotinib therapy. All AE reports were collected and graded using the National Cancer Institute Common Terminology Criteria for AEs version 3.0 and coded using the Medical Dictionary for Regulatory Activities version 14.1 thesaurus terms.
Outcome measures
Primary endpoints were patterns of occurrence of ILD and risk factors for onset of ILD. Overall survival and PFS were secondary endpoints and were assessed according to the treating physician's standard clinical practice. The pattern of ADRs, excluding ILD, was an additional secondary endpoint.
Statistical analyses
The sample size determination is previously described.(9) Briefly, 3000 patients were to be enrolled to detect an AE in one case out of 3000 patients with at least a power of 95%; however, during enrolment, target accrual was increased to 10 000 patients by the Japanese Health Authority to further evaluate the safety and efficacy of erlotinib. The increased patient number allows high sensitivity regarding low-frequency ADRs. The safety population comprised all patients who received erlotinib and had case report form data available. The efficacy population comprised all patients included in the safety population, except those where erlotinib therapy was prescribed off-label (i.e. in the first-line setting) at the time of this study, or where a patient's therapeutic history was unknown.
Median PFS and OS were estimated using Kaplan–Meier methodology. Patients without data for the duration of the observation period or from the time of treatment initiation were excluded from the PFS analyses.
Statistical analyses were carried out using Statistical Analysis Software version 9.1 and 9.2 (SAS Institute, Cary, NC). Multivariate Cox regression analysis using a stepwise model was carried out to determine risk factors for ILD; occurrence of ILD was used as the dependent variable. Exploratory variables with P > 0.05 were not included in the final model. In the final step, additional multivariate analyses were carried out to investigate two-factor interactions; statistical significance was set at P < 0.05. This method is described in more detail in the interim analysis publication.(9)
To examine factors affecting poor prognosis in ILD, a stepwise, 5% significance level, multivariate logistic regression analysis was carried out with an analysis set of 310 patients in whom an ILD diagnosis was confirmed by the ILD Review Committee. The target variable was fatal ILD; exploratory variables included gender, age, primary lesion, histological type, smoking history, ECOG PS, honeycomb lung, non-metastatic lesions, and remaining normal lung. The exploratory variables were chosen by the results of a univariate analysis using ILD death as the target variable, with baseline characteristics and characteristics previously reported to affect poor ILD prognosis as the univariate exploratory variables.
Results
A total of 10 708 patients were enrolled in this study. Of these, 9909 patients were evaluated for the final safety analysis and 9663 patients were evaluated for the final efficacy analysis (Fig. 1). Baseline characteristics are shown in Table 1. Of note, more males than females were enrolled; the majority of patients had adenocarcinoma histology (80.9%) and most had ECOG PS 0–1 (74.0%).
Fig. 1.
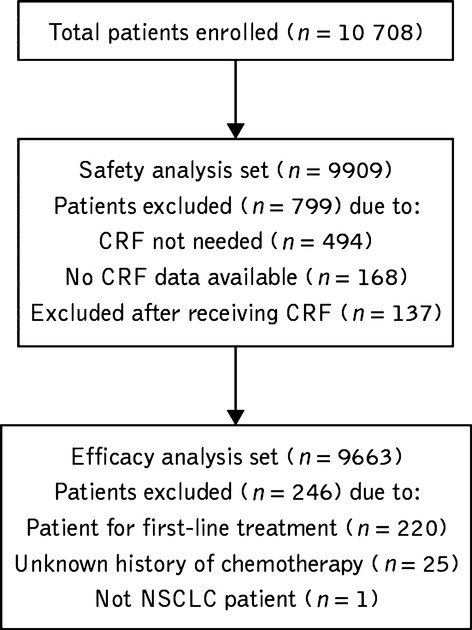
Disposition of patients with unresectable, recurrent/advanced non-small-cell lung cancer who were treated with erlotinib in Japan between December 2007 and October 2009 and who were included in the final analysis. CRF, case report form; NSCLC, non-small-cell lung cancer.
Table 1.
Baseline characteristics of patients with unresectable, recurrent/advanced non-small-cell lung cancer who were treated with erlotinib in Japan between December 2007 and October 2009
| Characteristic | Patients, n (%) (n = 9909) |
|---|---|
| Gender | |
| Male | 5300 (53.5) |
| Female | 4609 (46.5) |
| Age | |
| <65 years | 4466 (45.1) |
| 65–74 years | 3382 (34.1) |
| ≥75 years | 2059 (20.8) |
| Histology | |
| Adenocarcinoma | 7950 (80.9) |
| Squamous cell | 1285 (13.1) |
| Large cell | 155 (1.6) |
| Other | 438 (4.5) |
| ECOG PS | |
| 0–1 | 7315 (74.0) |
| 2–4 | 2576 (26.0) |
| Smoking history | |
| No | 4366 (44.9) |
| Yes | 5367 (55.1) |
| Number of previous treatment lines | |
| 0 | 220 (2.2) |
| 1 | 2481 (25.1) |
| 2 | 2646 (26.8) |
| 3 | 1993 (20.2) |
| 4 | 1546 (15.6) |
| ≥5 | 998 (10.1) |
| Previous gefitinib treatment | |
| Yes | 4396 (44.7) |
| No | 5446 (55.3) |
ECOG PS, Eastern Cooperative Oncology Group performance status.
Safety analysis
Adverse drug reactions were reported in 79.1% (7835/9909) of patients, the most common being skin disorders (67.4%), including rash (60.9%), diarrhea (21.5%), hepatitis, hepatic failure and hepatic function disorder (9.8%), eye disorders (3.3%) and hemorrhage (1.6%; Table 2). Median time to onset of ADRs was 9 days for rash, 8 days for diarrhea, 13 days for hepatitis, hepatic failure, and hepatic function disorder, 15 days for eye disorders, and 16 days for hemorrhage.
Table 2.
Incidence of the most common adverse drug reactions (ADRs) in patients with unresectable, recurrent/advanced non-small-cell lung cancer who were treated with erlotinib in Japan between December 2007 and October 2009
| ADR | All grades | Grade ≥3 | ||
|---|---|---|---|---|
| Patients | Patients | |||
| n | % | n | % | |
| ILD | 429 | 4.3 | 257 | 2.6 |
| Skin disorder | ||||
| Rash | 6032 | 60.9 | 673 | 6.8 |
| Dry skin | 738 | 7.4 | 30 | 0.3 |
| Pruritus | 351 | 3.5 | 13 | 0.1 |
| Paronychia | 654 | 6.6 | 77 | 0.8 |
| Hepatitis, hepatic failure, hepatic function disorder | 976 | 9.8 | 183 | 1.8 |
| Diarrhea | 2133 | 21.5 | 137 | 1.4 |
| Eye disorders | 331 | 3.3 | 19 | 0.2 |
| Corneal disorders | 186 | 1.9 | 11 | 0.1 |
| Hemorrhage | 158 | 1.6 | 46 | 0.5 |
| Gastrointestinal hemorrhage | 39 | 0.4 | 20 | 0.2 |
ILD, interstitial lung disease.
Interstitial lung disease
Incidence
Of the patients analyzed, 491 patients had 497 ILD-like events, of which 62 events were deemed non-ILD by the independent ILD Review Committee. In total, 429 (4.3%) patients were classified as having ILD (310 confirmed and reported by the ILD Review Committee, 119 patients not confirmed by the ILD Review Committee due to not having an evaluated image [n = 93], too difficult to distinguish from tumor progression [n = 4], and too difficult to distinguish from pneumonia due to insufficient evaluable images or clinical findings [n = 22] were still classified as ILD), with an overall mortality rate of 1.5% and a mortality rate of 35.7% in patients with ILD.
The majority of ILD cases (58.5%) were reported within 4 weeks of receiving erlotinib. The incidence of ILD (per 100 patient-weeks) was 0.63–0.81 within 4 weeks of the start of erlotinib treatment and 0.09–0.27 from 6 weeks after the start of erlotinib treatment (Fig. 2). Univariate analysis identified patients who were female, patients with non-adenocarcinoma histology, those with a period of treatment from initial NSCLC diagnosis to the start of treatment <360 days, concomitant or previous emphysema or COPD, concomitant or previous ILD, concomitant or previous lung infections, concomitant hepatic disorders, concomitant renal disorders, history of allergies, smoking history, ECOG PS 2–4, prior chest radiotherapy, pre-treatment lactate dehydrogenase, and no previous treatment with gefitinib as risk factors for ILD development (Table 3). Age at start of treatment, body mass index, concurrent cardiovascular disorders, number of chemotherapy regimens and previous treatment with gemcitabine were variables that were not identified as risk factors from the univariate analysis. Multivariate analysis showed that concurrent/previous ILD (HR, 3.19), concurrent/previous emphysema or COPD (HR, 1.86), concurrent/previous lung infection (HR, 1.55), smoking history (HR, 2.25), and period from initial NSCLC diagnosis to the start of treatment (<360 days; HR, 0.58) were identified as significant risk factors for developing ILD by multivariate analysis (Table 3).
Fig. 2.
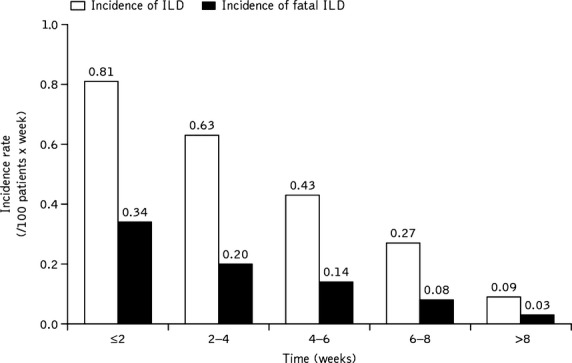
Incidence rate of interstitial lung disease (ILD) stratified by time from start of erlotinib treatment to onset of ILD. The 34 patients without data for either the duration of observation or the time from the start of erlotinib treatment to the onset of ILD were excluded from the analysis. Value determined by dividing the number of patients developing ILD during the specified duration of observation by the patient-days during the observation period (total duration [number of days] of observation of all patients receiving erlotinib during the specified duration of observation).
Table 3.
Cox regression univariate and multivariate analysis of factors affecting the incidence of interstitial lung disease (ILD) in patients with unresectable, recurrent/advanced non-small-cell lung cancer (NSCLC) who were treated with erlotinib in Japan between December 2007 and October 2009
| Variables | Criterion variable | Evaluation variable | Χ2 value | P-value | HR | 95% CI |
|---|---|---|---|---|---|---|
| Univariate analysis | ||||||
| Gender | Male | Female | 76.3424 | <0.0001 | 0.390 | 0.315–0.481 |
| Age (years) | <55 | ≥55 | 2.257 | 0.133 | 1.256 | 0.933–1.692 |
| Body mass index (kg/m2) | <25 | ≥25 | 2.4468 | 0.1178 | 0.788 | 0.585–1.062 |
| Histology | Adenocarcinoma | Non-adenocarcinoma | 32.0958 | <0.0001 | 1.847 | 1.494–2.283 |
| Period from initial NSCLC diagnosis to the start of treatment | <360 days | ≥360 days | 20.1885 | <0.0001 | 0.638 | 0.525–0.776 |
| Concurrent/previous emphysema or COPD | No | Yes | 85.1118 | <0.0001 | 3.071 | 2.420–3.898 |
| Concurrent/previous ILD | No | Yes | 88.7072 | <0.0001 | 3.862 | 2.915–5.116 |
| Concurrent/previous lung infection | No | Yes | 18.7152 | <0.0001 | 1.979 | 1.453–2.697 |
| Concurrent hepatic disorder | No | Yes | 4.9716 | 0.0258 | 1.426 | 1.044–1.949 |
| Concurrent renal disorder | No | Yes | 9.1417 | 0.0025 | 1.611 | 1.183–2.195 |
| Concurrent cardiovascular disorder | No | Yes | 2.8576 | 0.0909 | 1.191 | 0.973–1.459 |
| History of allergies | No | Yes | 5.2846 | 0.0215 | 1.358 | 1.046–1.764 |
| Smoking history | No | Yes | 87.4412 | <0.0001 | 2.896 | 2.318–3.620 |
| ECOG PS | 0–1 | 2–4 | 20.0203 | <0.0001 | 1.620 | 1.311–2.001 |
| Prior chest radiation therapy | No | Yes | 11.9016 | 0.0006 | 1.431 | 1.167–1.753 |
| Baseline lactate dehydrogenase† | – | –† | 7.0077 | 0.0081 | 1 | 1–1 |
| Number of chemotheraphy regimens for the primary diseases | – | –† | 1.2809 | 0.2577 | 1.033 | 0.977–1.092 |
| History of gemcitabine treatment | No | Yes | 0.1141 | 0.7355 | 0.967 | 0.797–1.174 |
| History of gefitinib treatment | No | Yes | 38.7111 | <0.0001 | 0.517 | 0.420–0.636 |
| Multivariate analysis | ||||||
| Concurrent/previous ILD | No | Yes | 55.3796 | <0.0001 | 3.187 | 2.349–4.325 |
| Smoking history | No | Yes | 34.1327 | <0.0001 | 2.246 | 1.712–2.946 |
| Concurrent/previous emphysema or COPD | No | Yes | 20.704 | <0.0001 | 1.860 | 1.424–2.431 |
| Period from initial NSCLC diagnosis to the start of treatment | <360 days | ≥360 days | 19.3818 | <0.0001 | 0.581 | 0.456–0.740 |
| Concurrent/previous lung infection | No | Yes | 6.5905 | 0.0103 | 1.550 | 1.109–2.165 |
| ECOG PS | 0–1 | 2–4 | 8.9467 | 0.0028 | 1.431 | 1.131–1.809 |
| History of gefitinib treatment | No | Yes | 5.3133 | 0.0212 | 0.729 | 0.557–0.954 |
| Number of chemotherapy regimens† | – | –† | 10.4136 | 0.0013 | 1.121 | 1.046–1.201 |
Objective variable: occurrence or non-occurrence of ILD. Explanatory variables: gender, age, body mass index, histological type, concurrent/previous emphysema or chronic obstructive pulmonary disease (COPD), concurrent/previous ILD, concurrent/previous lung infection, concomitant hepatic disorder, concomitant renal disorder, period from initial NSCLC diagnosis to the start of treatment, concomitant cardiovascular disease, history of allergies, smoking history, Eastern Cooperative Oncology Group performance status (ECOG PS), radiotherapy (chest), pretreatment lactate dehydrogenase, number of chemotherapy regimens for the primary disease, history of gemcitabine treatment, history of gefitinib treatment.
Analyzed as a continuous quantity. NSCLC, non-small-cell lung cancer; ILD, interstitial lung disease.; CI, confidence interval; HR, hazard ratio.
Outcomes of ILD
Of the confirmed cases of ILD, 75 (17.5%) patients fully recovered, 154 (35.9%) patients improved their condition, 32 (7.5%) patients did not recover, five (1.2%) patients had sequelae, 153 (35.7%) patients died, and 10 (2.3%) patients had unknown outcomes.
The outcome of ILD by CT image pattern was investigated in 283 patients out of the 310 patients deemed as having confirmed ILD by the independent ILD Review Committee. Diffuse alveolar damage-like pattern on CT was defined as abnormalities that showed non-segmental ground-glass attenuation or airspace consolidation with traction bronchiectasis and loss of volume. In the 63 patients with CT-DAD-like pattern, six (9.5%) patients recovered, 12 (19.1%) patients improved, three (4.8%) patients did not recover, one (1.6%) patient had residual ILD sequelae, and 41 (65.1%) patients died. In the 220 patients with a CT-non-DAD-like pattern, 37 (16.8%) patients recovered, 95 (43.2%) patients improved, 13 (5.9%) patients did not recover, one (0.5%) patient had residual ILD sequelae, 71 (32.3%) patients died, and three (1.4%) patients had unknown outcomes.
Fatal outcome of ILD
The multivariate logistic analysis identified ECOG PS 2–4 (OR, 2.45), ≤50% remaining normal lung area (OR, 3.12), and concomitant honeycombing with interstitial pneumonia (OR, 6.67) as poor prognostic factors for ILD death (Table 4).
Table 4.
Interstitial lung disease (ILD) poor prognosis risk factors from the final analysis results for Post-Launch All-patient-Registration Surveillance in Tarceva®-treated non-small-cell lung cancer patients (POLARSTAR)
| Risk factors for ILD-related death | Criterion variable | Evaluation variable | Χ2 value | P-value | OR | 95% CI |
|---|---|---|---|---|---|---|
| ECOG PS 2–4 | 0–1 | 2–4 | 9.974 | 0.0016 | 2.45 | 1.41–4.27 |
| ≤50% normal lung area | >50 | ≤50 | 8.896 | 0.0029 | 3.12 | 1.48–6.58 |
| Concomitant honeycombing | No | Yes | 5.414 | 0.02 | 6.67 | 1.35–32.94 |
CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; OR, odds ratio.
A total of 12 patients reported concomitant honeycombing and interstitial pneumonia; of these patients, nine patients died of ILD, two patients improved their condition, and one patient did not recover. Of those who died, eight were determined as having CT-non-DAD-like pattern on CT scan and the remaining patient was determined as having CT-DAD-like pattern.
Efficacy
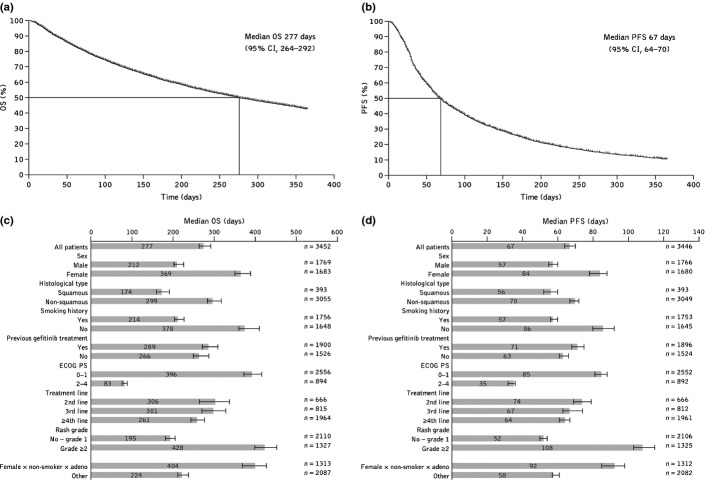
Median OS was 277 days (95% CI, 264–292), with a 6-month survival rate of 62.6% and a 12-month survival rate of 42.8% (Fig. 3a). Median PFS was 67 days (95% CI, 64–70), with a 6-month progression-free rate of 25.8% and a 12-month progression-free rate of 10.6% (Fig. 3b). Compared with the overall population, median OS and PFS appeared to be longer in female patients, non-smokers, patients with ECOG PS 0–1, and patients with grade ≥2 rash (Fig. 3c,d).
Fig. 3.
(a) Overall survival (OS) and (b) progression-free survival (PFS) assessed by Kaplan–Meier methodology in the overall population of patients with unresectable, recurrent/advanced non-small-cell lung cancer who were treated with erlotinib in Japan between December 2007 and October 2009; (c) median OS and (d) PFS in patient subpopulations. CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status.
Discussion
The development of drug-induced acute pulmonary disorders or interstitial pneumonia caused by EGFR TKIs is a common problem; this has particular importance in Japan, because a variety of evidence has suggested that Japanese populations are more vulnerable to these disorders. This large-scale POLARSTAR study provides further decisive information on this issue. Final data from the POLARSTAR study confirm that erlotinib has a well-characterized safety profile with proven efficacy in Japanese patients in routine clinical practice.
In the final analysis from POLARSTAR, the rates of ILD development and mortality in patients with ILD (4.3% and 35.7%, respectively) were comparable with the ILD-associated incidence rates of 3–5% and mortality rates of 27.9–50.0% previously reported among Japanese patients with NSCLC and ILD treated with gefitinib or erlotinib.(2,3,5,6,9) In the POLARSTAR analysis, it was shown that ILD onset was typically soon after initiation of erlotinib, with the highest incidence occurring during the first 4 weeks. Physicians should therefore monitor patients for the symptoms of ILD, which usually occur within 8 weeks of treatment initiation. These findings are further supported by those reported in Japanese NSCLC studies with gefitinib.(5,6)
The risk factors identified as significant primary risk factors (HR, ≥1.5) for ILD occurrence or exacerbation using a Cox proportional hazards multivariate analysis were concurrent/previous ILD, concurrent/previous lung infection, concurrent/previous emphysema or COPD, and smoking history. Cox proportional hazards multivariate analysis was selected for this assessment as the authors considered that a time-dependent analysis was needed, as there was no information regarding the ILD development point in the initial analysis. Concurrent/previous emphysema or COPD was newly identified as a significant primary risk factor for ILD occurrence when analyzed in 9909 patients compared with the result of the interim analysis of 3488 patients (Table 5).(9,10) As ILD is a collective term for a variety of different lung conditions, it is important to be careful not to misdiagnose conditions as ILD, as this will affect the risk factor analysis.
Table 5.
Comparison of the interstitial lung disease (ILD) analysis from the interim and final analysis results for Post-Launch All-patient-Registration Surveillance in Tarceva®-treated non-small-cell lung cancer patients (POLARSTAR)
| Endpoint | Interim analysis (safety, n = 3488) (efficacy, n = 3453) | Final analysis (safety, n = 9909) (efficacy, n = 9663) |
|---|---|---|
| ILD analysis | ||
| Patients with confirmed ILD, n (%) | 158 (4.5) | 429 (4.3) |
| ILD-related mortality rate, % | 1.6 | 1.5 |
| ILD-related mortality rate in ILD patients | 34.8 | 35.7 |
| Risk factors for ILD development, HR | ||
| Previous/concurrent ILD | 4.1 | 3.2 |
| Previous/concurrent Emphysema or COPD | – | 1.9 |
| Previous/concurrent lung infection | 2.0 | 1.6 |
| Smoking history | 3.0 | 2.2 |
| ECOG PS 2–4 | 1.6 | 1.4 |
| <360 days from diagnosis to treatment | – | 0.58 |
COPD, chronic obstructive pulmonary disease; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio.
The period from initial NSCLC diagnosis to the start of treatment (<360 days) was not considered as a risk factor for ILD that needed to be highlighted at this time (HR, 0.58), as the clinical grounds for this factor were not clear. Stage of progression of primary disease or bias of observational period from initial NSCLC diagnosis to termination of treatment were speculated to be the reason; however, details of these reasons are uncertain. In contrast to this analysis, risk factors for ILD associated with gefitinib have been reported as ECOG PS ≥2, smoking history, concomitant interstitial pneumonia, and prior chemotherapy.(5,7,8)
The multivariate analysis identified ECOG PS 2–4, ≤50% remaining normal lung area and concomitant honeycombing with interstitial pneumonia as poor prognostic factors for ILD death in POLARSTAR. Many patients with idiopathic interstitial pneumonias have idiopathic pulmonary fibrosis or non-specific interstitial pneumonia, which have a heterogeneous natural progression, with some patients remaining stable for extended periods, while others show steady worsening of the condition.(11) Some patients with chronic idiopathic interstitial pneumonias, such as idiopathic pulmonary fibrosis and non-specific interstitial pneumonia, experience acute exacerbations characterized by suddenly progressive and severe respiratory failure, with new lung opacities and pathological lesions of DAD.(12) It should be noted that there are racial differences between Mongolians (including the Japanese) and Caucasians in the frequency of acute exacerbations.(13) In the POLARSTAR study, the outcome of ILD by CT image pattern was investigated in 283 patients out of the 310 patients deemed as having confirmed ILD by the independent ILD Review Committee. The mortality rate for ILD among patients who were deemed to have CT-DAD-like pattern was higher than that seen among patients who were deemed as having CT-non-DAD-like pattern (65.1% vs 32.2%, respectively). Those patients with honeycombing and interstitial pneumonia (n = 12) had a high risk of poor prognosis, regardless of their CT pattern. Therefore, physicians should be actively aware of the symptoms of ILD and it is suggested to carefully monitor for these symptoms by CT image or X-ray throughout the disease course. Once physicians recognize ILD, they should immediately discontinue the EGFR TKI and should take the necessary steps to manage the ILD.
The final efficacy results from POLARSTAR are in line with the results of our interim analysis of the study (Table 6).(9) The final efficacy results (median OS, 277 days; median PFS, 67 days) were also comparable with efficacy reported in previous clinical trials of erlotinib treatment. The BR.21 study reported median PFS of 2.2 months (67 days) versus 1.8 months (55 days) and OS of 6.7 months (203 days) versus 4.7 months (143 days) for erlotinib and placebo, respectively, in the second- or third-line setting.(1) Kubota et al. investigated second-line erlotinib in Japanese patients, resulting in a median PFS of 77 days and OS of 14.7 months (447 days).(2) In a second phase 2 study in Japanese patients with NSCLC, second-line erlotinib treatment resulted in median OS of 13.5 months (410 days).(3)
Table 6.
Comparison of the efficacy endpoints from the interim and final analysis results for Post-Launch All-patient-Registration Surveillance in Tarceva®-treated non-small-cell lung cancer patients (POLARSTAR)
| Endpoint | Interim analysis (safety, n = 3488) (efficacy, n = 3453) | Final analysis (safety, n = 9909) (efficacy, n = 9663) |
|---|---|---|
| Efficacy endpoints | ||
| Median OS, days | 260 | 277 |
| 6-month OS rate, % | 62.2 | 62.6 |
| 12-month OS rate, % | 40.9 | 42.8 |
| Median PFS, days | 64 | 67 |
| 6-month PFS rate, % | 23.7 | 25.8 |
| 12-month PFS rate, % | 9.6 | 10.6 |
OS, overall survival; PFS, progression-free survival.
We acknowledge that there are several limitations of this study, including the fact that this was a single-arm observational study with no control group, and the lack of a strict observation period, unlike a clinical trial. The lack of information on EGFR mutation status is also considered a limitation as this is known to strongly affect the efficacy of erlotinib. The lack of patient selection criteria may also be seen as a limitation; however, this may mean that our study population was more representative of the actual Japanese population than would be the case in a clinical trial, especially because of the large patient population in this study. The information on EGFR TKI-associated ILD in this study is thought to be decisive; it provides valuable information for treatment considerations and monitoring in Japanese patients with EGFR mutant or wild-type lung cancer.
Healthcare providers should carefully observe patients during treatment with erlotinib to ascertain whether the patient has any of the risk factors detailed in this analysis. After suspicion of the onset of ILD and diagnosis by CT, it is important to follow the patient's status continuously and carefully monitor their risk level. The final safety and efficacy data from the large-scale POLARSTAR surveillance study confirm that erlotinib has a well-characterized safety profile with proven efficacy in Japanese patients; however, the risk of ILD should still be monitored.
Acknowledgments
The authors would like to thank all patients who participated in the study and clinical personnel involved in data collection, investigators, and site staff. The authors also thank Joanna Musgrove at Gardiner-Caldwell Communications for third-party editorial assistance, which was funded by Chugai Pharmaceutical Co. Ltd.
Glossary
- ADR
adverse drug reaction
- AE
adverse event
- CI
confidence interval
- COPD
chronic obstructive pulmonary disease
- CT
computed tomography
- DAD
diffuse alveolar damage
- ECOG
PS Eastern Cooperative Oncology Group performance status
- EGFR
epidermal growth factor receptor
- HR
hazard ratio
- ILD
interstitial lung disease
- NSCLC
non-small-cell lung cancer
- OR
odds ratio
- OS
overall survival
- PFS
progression-free survival
- POLARSTAR
Post-Launch All-patient-Registration Surveillance in Tarceva®-treated NSCLC patients
- TKI
tyrosine-kinase inhibitor
Disclosure Statement
KN, SK, YO, TJ, MA, NY, and MF have all participated as independent advisory board members for erlotinib, reimbursed by Chugai Pharmaceutical Co. Ltd. YO also has an immediate family member who is an employee of Chugai Pharmaceutical Co. Ltd. HA, YI, ME, TJ, MK, KK, FS, HT, AG, and YF have all participated as independent ILD Review Committee members for erlotinib, reimbursed by Chugai Pharmaceutical Co. Ltd. AS and TI are full-time employees of Chugai Pharmaceutical Co. Ltd. This trial was designed, funded, and monitored by Chugai Pharmaceutical Co. Ltd. Data were gathered, analyzed, and interpreted by Chugai with input from all authors. The corresponding author had full access to the relevant data and took full responsibility for the final decision to submit the report for publication. Although technically classed as a clinical trial, the POLARSTAR study was a non-interventional surveillance study analyzing all NSCLC patients receiving erlotinib in Japan, therefore it was not registered as a phase II/III clinical trial would be.
References
- 1.Shepherd F, Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. New Engl J Med. 2005;353:123–32. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 2.Kubota K, Nishiwaki Y, Tamura T, et al. Efficacy and safety of erlotinib monotherapy for Japanese patients with advanced non-small cell lung cancer: a phase II study. J Thorac Oncol. 2008;3:1439–45. doi: 10.1097/JTO.0b013e31818d6702. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi T, Yamamoto N, Nukiwa T, et al. Phase II study of erlotinib in Japanese patients with advanced non-small cell lung cancer. Anticancer Res. 2010;30:557–63. [PubMed] [Google Scholar]
- 4.Yamamoto N, Horiike A, Fujisaka Y, et al. Phase I dose-finding and pharmacokinetic study of the oral epidermal growth factor receptor tyrosine kinase inhibitor Ro50–8231 (erlotinib) in Japanese patients with solid tumors. Cancer Chemother Pharmacol. 2008;61:489–96. doi: 10.1007/s00280-007-0494-8. [DOI] [PubMed] [Google Scholar]
- 5.Yoshida S. The results of gefitinib prospective investigation. Med Drug J. 2005;41:772–89. [Google Scholar]
- 6.Kudoh S, Kato H, Nishiwaki Y, et al. Interstitial lung disease in Japanese patients with lung cancer: a cohort and nested case-control study. Am J Respir Crit Care Med. 2008;117:1348–57. doi: 10.1164/rccm.200710-1501OC. [DOI] [PubMed] [Google Scholar]
- 7.Hotta K, Kiura K, Tabata M, et al. Interstitial lung disease in Japanese patients with non-small cell lung cancer receiving gefitinib: an analysis of risk factors and treatment outcomes in Okayama Lung Cancer Study Group. Cancer J. 2005;11:417–24. doi: 10.1097/00130404-200509000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Hotta K, Kiura K, Takigawa N, et al. Comparison of the incidence and pattern of interstitial lung disease during erlotinib and gefitinib treatment in Japanese patients with non-small cell lung cancer: the Okayama Lung Cancer Study Group experience. J Thorac Oncol. 2010;5:179–84. doi: 10.1097/JTO.0b013e3181ca12e0. [DOI] [PubMed] [Google Scholar]
- 9.Nakagawa K, Kudoh S, Ohe Y, et al. Postmarketing surveillance study of erlotinib in Japanese patients with non-small-cell lung cancer (NSCLC): an interim analysis of 3488 patients (POLARSTAR) J Thorac Oncol. 2012;7:1296–303. doi: 10.1097/JTO.0b013e3182598abb. [DOI] [PubMed] [Google Scholar]
- 10.Inoue Y, Fukuoka M, Kudoh S, et al. Tarceva tablet non-small-cell lung cancer special drug use-results survey final analysis about targeted numbers (3000 pts) Proc Japan Lung Cancer Society. 2010;50:0–184. [Google Scholar]
- 11.Travis W, Costabel U, Hansell D, et al. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188:733–48. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collard H, Moore B, Flaherty K, et al. Acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2007;176:636–43. doi: 10.1164/rccm.200703-463PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Azuma A, Hagiwara K, Kudoh S. Basis of acute exacerbation of idiopathic pulmonary fibrosis in Japanese patients. Am J Respir Crit Care Med. 2008;177:1397–8. doi: 10.1164/ajrccm.177.12.1397a. [DOI] [PubMed] [Google Scholar]