Abstract
Although there have been multiple advances in the development of novel anticancer agents and operative procedures, prognosis of patients with advanced gastric cancer remains poor, especially in patients with peritoneal metastasis. In this study, we established nanoparticles loaded with indocyanine green (ICG) derivatives: ICG loaded lactosomes (ICGm) and investigated the diagnostic and therapeutic value of photodynamic therapy (PDT) using ICGm for experimental peritoneal dissemination of gastric cancer. Experimental peritoneal disseminated xenografts of human gastric cancer were established in nude mice. Three weeks after intraperitoneal injection of the cancer cells, either ICGm (ICGm-treated mice) or ICG solution (ICG-treated mice) was injected through the tail vein. Forty-eight hours after injection of the photosensitizer, in vivo and ex vivo imaging was carried out. For PDT, 48 h after injection of the photosensitizer, other mice were irradiated through the abdominal wall, and the body weight and survival rate were monitored. In vivo imaging revealed that peritoneal tumors were visualized through the abdominal wall in ICGm-treated mice, whereas only non-specific fluorescence was observed in ICG-treated mice. The PDT reduced the total weight of the disseminated nodules and significantly improved weight loss and survival rate in ICGm-treated mice. In conclusion, ICGm can be used as a novel diagnostic and therapeutic nanodevice in peritoneal dissemination of gastric cancer.
Keywords: Drug delivery system, gastric cancer, nanoparticle, peritoneal dissemination, photodynamic therapy
Gastric cancer remains a worldwide health problem and accounts for 10% of all new cancer diagnoses, and 12% of all cancer-related deaths.(1) In Japan, a nation-wide screening program has resulted in early diagnosis and prompt surgical intervention, resulting in improved prognosis of patients with primary gastric cancer.(2,3) However, prognosis of patients with advanced gastric cancer remains poor, with 5-year survival rates of 12.4% in patients with peritoneal metastasis and 15.8% in patients with stage IV disease.(4) The most frequent cause of death in patients with stage IV disease was peritoneal metastasis (48.8%).
There have been numerous clinical trials aimed at decreasing and treating peritoneal metastases. These trials have evaluated treatment methods such as systemic adjuvant chemotherapy,(5,6) i.p. chemoperfusion,(7) and chemoradiotherapy;(8,9) however, several meta-analyses have shown only a marginally significant benefit for some of the above therapies.(10) With these findings, most clinicians believe that there are currently no standard effective therapies for preventing and/or treating peritoneal metastasis in gastric cancer.
In photodynamic therapy (PDT), a systemically administered photosensitizing agent is activated by the laser light of a specific wavelength delivered by an optical fiber. Light-activated photosensitizer molecules react with endogenous oxygen, resulting in the generation of singlet oxygen, thereby initiating a series of intracellular events that result in a direct necrotic and apoptotic effect on tumor cells.(11) With respect to side-effect profiles and damage to normal tissue, PDT has advantages over radiation and chemotherapy because the photosensitizing agents used in PDT specifically accumulates in cancer cells as opposed to normal cells and PDT does not induce any chemoresistance. Photodynamic therapy has thus become more widely accepted as a treatment option for early lung cancer, gastric cancer, esophageal cancer, and other diseases.(12,13) This treatment has also been clinically applied in poor surgical candidates with advanced cancers.(14,15) Because laser energy penetrates to a depth of only 2 mm in tissues,(16,17) PDT cytotoxicity has been limited to the treatment of superficial lesions. Therefore, disseminated small nodules on the peritoneal surface could potentially be suitable targets for PDT.
In this study, we established nanoparticles loaded with indocyanine green (ICG) derivatives, ICG-loaded lactosomes (ICGm), and investigated the diagnostic and therapeutic values in PDT using ICGm for experimental peritoneal dissemination of gastric cancer.
Materials and Methods
Cancer cell lines, animals, and ethics
A luciferases stably expressing human gastric adenocarcinoma cell line (MKN45-luc) was purchased from the Riken Bioresource Center (Cell Bank, Ibaraki, Japan). The cancer cells were cultured in RPMI-1640 (Sigma-Aldrich, St. Louis, MO, USA) containing 10% heat-inactivated FBS (Life Technologies, Carlsbad, CA, USA), 100 U/mL penicillin, 100 μg/mL streptomycin, and 0.25 μg/mL amphotericin B (Antibiotic–Antimycotic; Life Technologies) at 37°C in 5% CO2 with 95% humidity. Male nude mice (BALB/c nu/nu) that were 8 weeks of age (CLEA Japan, Tokyo, Japan) were fed under specific pathogen-free conditions. All animal procedures followed the guidelines approved by the National Defense Medical College Animal Care and Use Committee.
Development of peritoneal dissemination of human gastric cancer
To develop peritoneally disseminated xenografts of human gastric cancer, mice were anesthetized with sevoflurane, and i.p. injected with 6 × 106 MKN45-luc cells in 500 μL PBS using a 27-gauge needle.
Development of ICGm
We successfully synthesized the “lactosome”.(18) The lactosome is a micelle, assembled from block copolymers, poly(sarcosine)–poly(L-lactic acid) (PS–PLLA) that was synthesized as previously reported.(18) Because PS–PLLA is composed of a biodegradable polypeptide, its toxicity is negligible, thus suggesting its safe use in humans.
The ICG-loaded lactosome (ICGm) used in this study contains 22% ICG–PLLA and 78% PS–PLLA. Regarding the synthesis of ICG–PLLA, the terminal end of PLLA was chemically modified using ICG as follows: 1.0 mg ICG–OSu was added to the dimethylformamide solution of the free amino group bearing PLLA (2.46 mg), whose amino group is designed as an indicator of sacrosine N-carboxyanhydride polymerization during the synthesis of amphiphilic PLLA–PS-block copolymers. The reaction mixture was stirred at room temperature overnight under light-shielding conditions. The reaction mixture was purified by size exclusion chromatography using the Sephadex LH-20 column (GE Healthcare, Tokyo, Japan) and dimethylformamide as the eluent.
Chloroform solutions of PS–PLLA and ICG–PLAA were mixed at a ratio of 0.78:0.22. The solvent was removed under a reduced pressure environment and the formed thin film was dissolved in 10 mM Tris–HCl buffer (pH 7.4). The resulting aqueous solution was purified by Sephacryl S-100 size exclusion chromatography by elution with 10 mM Tris-HCl buffer (pH 7.4) to obtain ICGm (Fig. 1). Dynamic light-scattering analysis revealed that the hydrodynamic diameter of ICGm was 40–50 nm and the zeta potential of ICGm was −0.51 mV (Fig. S1).
Fig. 1.
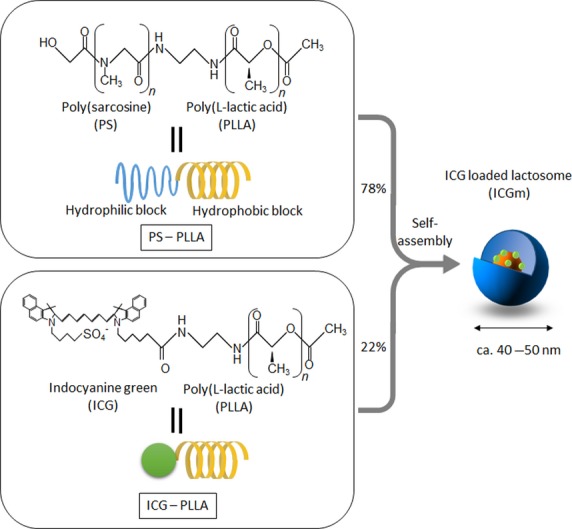
Structure of indocyanine green (ICG) loaded lactosome (ICGm). ICGm is a molecular assembly composed of hydrophobic helical poly(L-lactic acid) (PLLA) and hydrophilic poly(sarcosine) (PS) amphiphilic block polydepsipeptide including ICG-labeled PLLA in the hydrophobic inner core.
In vivo imaging
For the visualization of i.p. tumors in mice, either 100 μL ICGm in which the ICG concentration was 281 μM or 100 μL ICG solution (281 μM) was injected through the tail vein, and macroscopic in vivo fluorescence and luminescence imaging was carried out using an IVIS system (PerkinElmer, Waltham, MA, USA) as described previously.(19)
For bioluminescence imaging, 48 h after administration of the photosensitizer, mice were anesthetized with pentobarbital and subsequently received i.p. injection with 200 μL PBS containing D-luciferin (OZ Biosciences, San Diego, CA, USA) (15 mg/mL). Images were acquired 10–15 min after luciferin administration. A photographic image of the animal was taken in the chamber under dim illumination, followed by acquisition and overlay of the pseudocolor image representing the spatial distribution of photon counts produced by active luciferase within the animal. An integration time of 1 min with a binning of 4 was used for luminescent image acquisition.
After obtaining luminescent images, fluorescence imaging was implemented. The abdomen of mice or disseminated nodules after laparotomy was illuminated with a 780-nm excitation light and the fluorescence was captured using an 845-nm filter. The exposure time was 2000 ms.
Photodynamic therapy
Three weeks after i.p. tumor injection, either 100 μL ICGm, in which the ICG concentration was 281 μM, or 100 μL ICG solution (281 μM) was injected through the tail vein. Forty-eight hours after the administration of the photosensitizer, photoirradiation was carried out using a fiber-coupled laser system with an 808-nm laser diode (model FC-W-808, maximum output 10 W; Changchun New Industries Optoelectronics Technology, Jilin, China). The fiber probe was placed just above the abdomen so that the irradiation spot on the abdominal skin was 3.14 cm2 (diameter, 2.0 cm) in size, which covered almost the entire abdomen. The fluence rate and irradiation period was set to 500 mW/cm2 and 1000 s, respectively, corresponding to a fluence of 500 J/cm2.
Two weeks after PDT, the mice were killed and the number and the weight of the total disseminated nodules were measured in the two groups (n = 5, each group). In addition, the survival rate was monitored after PDT (n = 8, each group).
Statistical analysis
The data are represented as the mean ± standard deviation. Statistical analyses were carried out using the Mann–Whitney U-test or χ2-test with Fisher's exact test, whichever was considered appropriate. Survival curves were generated using the Kaplan–Meier method and the significance of the difference in survival was determined by the log–rank test. Statistical calculations were carried out using StatView version 5.0 (SAS Institute, Inc., Cary, NC, USA). P-values <0.05 were considered statistically significant.
Results
Development of peritoneal dissemination of human gastric cancer cells
Three weeks after the i.p. tumor injection, peritoneal nodules and bloody ascites were evident. Microscopic examination confirmed these nodules to be disseminated adenocarcinoma (data not shown).
Imaging of peritoneal dissemination and nodules
Forty-eight hours after injection of ICG or ICGm, mice were anesthetized with pentobarbital, and in vivo and ex vivo imaging was carried out, because the strongest contrast between tumor site and normal tissue was obtained between 48 and 72 h after ICGm injection, by preliminary experiments (data not shown).
We detected luminescent signals through the abdominal wall as well as in the post-laparotomy abdominal cavity in both treatment groups (Fig. 2). Fluorescence imaging revealed that no specific fluorescence was observed in ICG-treated mice through the abdominal wall or post-laparotomy abdominal cavity (Fig. 2a), whereas we detected fluorescence in the location identical to the site of luminescence in ICGm-treated mice (Fig. 2b). Although we detected luminescent signals in disseminated nodules in both groups, disseminated nodules in ICGm-treated mice, but not ICG-treated mice, were visualized in ex vivo fluorescence imaging (Fig. 3).
Fig. 2.
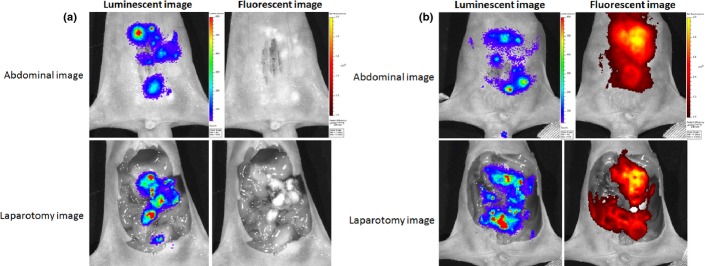
Luminescence and fluorescence imaging of mice with peritoneal dissemination. Luminescence originating from tumors is seen through the abdominal wall as well as in the post-laparotomy abdominal cavity in both treatment groups. No specific fluorescence is seen in indocyanine green (ICG)-treated mice (a), whereas obvious fluorescence signals identical to luminescence sites are seen through the abdominal wall and in the post-laparotomy abdominal cavity in ICG loaded lactosome (ICGm)-treated mice (b). Relative light units/pixel are indicated as color scale bars.
Fig. 3.
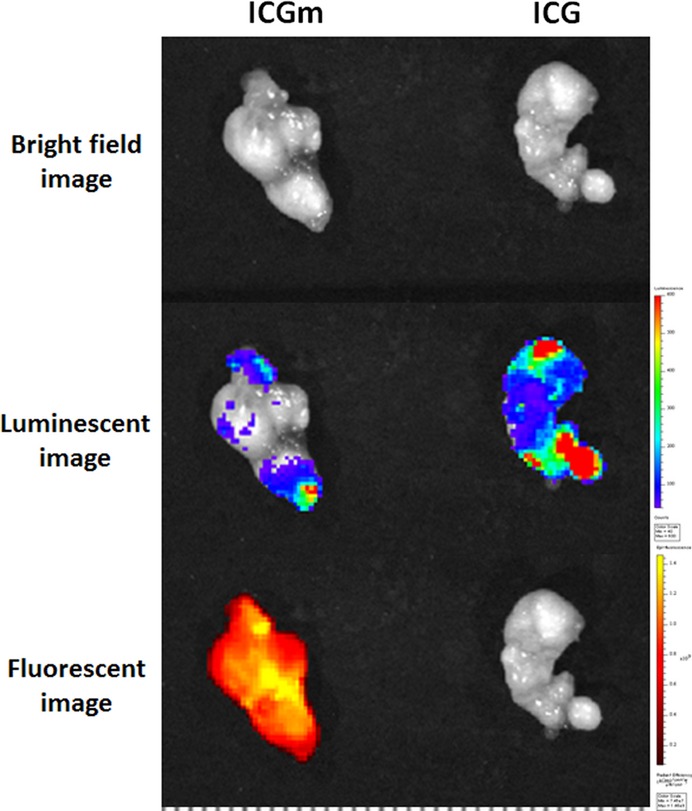
Luminescence and fluorescence imaging of disseminated nodules in mice treated with indocyanine green (ICG) or ICG loaded lactosome (ICGm). Although luminescent signals in disseminated nodules in both groups are seen, fluorescence is seen only in ICGm-treated mice. Relative light units/pixel are indicated as color scale bars (right), and a scale bar at the bottom of the figure.
Photodynamic therapy for peritoneal dissemination model
Although the overall body weight of ICG-treated mice gradually decreased throughout the investigation period, this weight loss was not observed in ICGm-treated mice (Fig. 4). The weight of the total disseminated nodules in ICGm-treated mice was significantly lower than that of ICG-treated mice, and there were fewer disseminated nodules in ICGm-treated mice than in ICG-treated mice (Fig. 5). Photodynamic therapy using ICGm significantly improved survival rate compared to that using ICG (log–rank, P < 0.05); the median survival time of ICGm-treated mice after PDT was 32 days (n = 8) and that of ICG-treated mice was 17 days after PDT (n = 8) (Fig. 6).
Fig. 4.
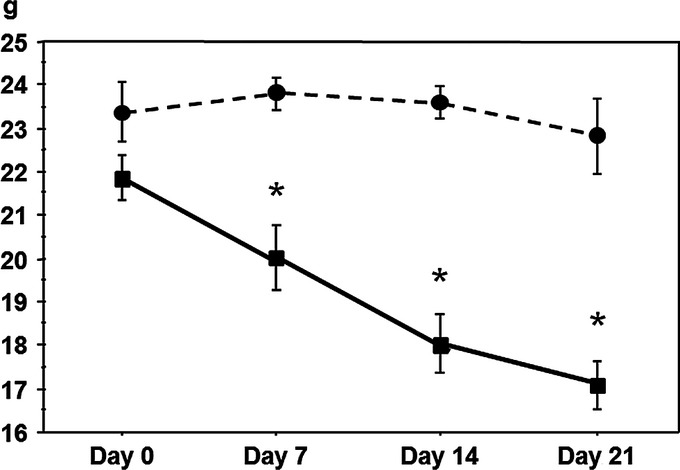
Change in body weight after photodynamic therapy in mice treated with indocyanine green (ICG; ▪) or ICG loaded lactosome (ICGm; ○). *P < 0.05 versus ICGm-treated mice.
Fig. 5.
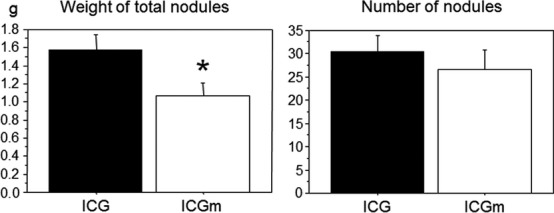
Total weight and number of disseminated nodules in mice treated with indocyanine green (ICG) or ICG loaded lactosome (ICGm). *P < 0.05 versus ICG-treated mice.
Fig. 6.
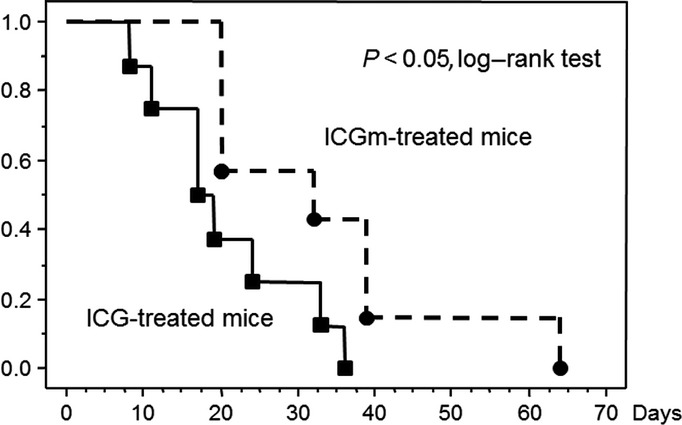
Survival rate after photodynamic therapy (PDT) in mice treated with indocyanine green (ICG) or ICG loaded lactosome (ICGm). PDT using ICGm significantly improved the survival rate compared to PDT using ICG (log–rank, P < 0.05). The median survival time of ICGm-treated mice was 32 days (n = 8) and that of ICG-treated mice was 17 days (n = 8) after PDT.
Discussion
In this study, we showed that in vivo and ex vivo imaging using ICGm clearly visualized peritoneal dissemination through the abdominal wall in mice with disseminated xenografts of human gastric cancer. In addition, PDT using ICGm significantly improved the survival rate in this model.
In the clinical setting, it has been common practice to irradiate a wide field that often includes healthy tissues, to treat potential microscopic malignant foci in these areas; under these conditions, the tumoritropic characteristics of photosensitizers become crucial. Many photosensitizing agents have been developed; however, very few have made it to clinical trials due to factors such as poor selectivity for target and healthy tissues, low extinction coefficients, and absorption spectra at relatively short wavelengths. Each of the photosensitizers that are commercially available has specific characteristics, but none incorporates all the properties of an ideal photosensitizer.
In this study, we used ICG as a photosensitizer. A tricarbocyanine dye, ICG has been approved by the United States Food and Drug Administration for medical diagnostic studies and is widely used for the evaluation of cardiac output, liver function, and visualization of the retinal and choroidal vasculature. The recent interest in using ICG as a photosensitizer in PDT comes from the fact that this dye has a strong absorption band (between 700 and 800 nm) which allows deeper tissue penetration without causing significant heating.(20)
The ideal photosensitizer has to preferentially accumulate in the target tumor cells. The ICGm used in this study was 40–50 nm in diameter and selectively accumulated in the tumor tissues. In tumor tissues, submicron-sized defects exist on the vascular wall because of rapid angiogenesis, enabling the permeation of macromolecules through the wall. Furthermore, the lymphatic system around the tumor grows too slowly to remove foreign compounds from the tumor region and nanocarriers in the range of 30–100 nm in size can passively accumulate into tumors; this is known as the enhanced permeation and retention effect.(21,22) Our preliminary study revealed the fluorescent intensity of ICGm was approximately threefold that of ICG solution (Fig. S2) and provided direct evidence regarding cancer cell death induced by PDT with ICGm (Fig. S3). Thus, ICGm may be a safe and ideal photosensitizer of PDT for the treatment of disseminated nodules.
Recently, we developed a novel homogenous irradiation fiber probe for whole bladder wall PDT.(23) This fiber probe provides a fluence distribution which approximates the 3-D shape of the bladder cavity. For clinical use, laparoscopic PDT for peritoneal disseminations may be available. Thus, we are carrying out an experiment regarding laparoscopic PDT that uses the rat peritoneal dissemination model to examine the feasibility for clinical application.
In conclusion, ICGm selectively accumulated in tumor tissues as a result of the enhanced permeation and retention effect, and PDT using ICGm reduced the total amount of dissemination and improved the survival rate. These findings suggested that ICGm could be useful as a diagnostic and novel therapeutic tool for peritoneal disseminations. Photodynamic therapy has the advantage of the ability to repeat treatments without inducing resistance, unlike chemotherapy and radiotherapy. In this regard, laparoscopic PDT using ICGm may be a novel and promising therapeutic approach for peritoneal disseminations in the clinical setting.
Acknowledgments
This work was supported by the Japan Society for the Promotion of Science (Kakenhi) Grant Number 24591892. The authors would like to thank Enago for the English language review.
Disclosure Statement
The authors have no conflict of interest.
Supporting Information
Additional supporting information may be found in the online version of this article:
Fig. S1. Zeta potential of indocyanine green loaded lactosome (ICGm).
Fig. S2. Relative fluorescent intensity of indocyanine green (ICG) loaded lactosome (ICGm) and ICG solution.
Fig. S3. Cancer cell death induced by photodynamic therapy (PDT) with indocyanine green (ICG) loaded lactosome (ICGm).
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Maruyama K, Kaminishi M, Hayashi K, et al. Gastric cancer treated in 1991 in Japan: data analysis of nationwide registry. Gastric Cancer. 2006;9:51–66. doi: 10.1007/s10120-006-0370-y. [DOI] [PubMed] [Google Scholar]
- 3.Tsujimoto H, Sugasawa H, Ono S, Ichikura T, Yamamoto J, Hase K. Has the accuracy of preoperative diagnosis improved in cases of early-stage gastric cancer? World J Surg. 2010;34:1840–6. doi: 10.1007/s00268-010-0587-0. [DOI] [PubMed] [Google Scholar]
- 4.Isobe Y, Nashimoto A, Akazawa K, et al. Gastric cancer treatment in Japan: 2008 annual report of the JGCA nationwide registry. Gastric Cancer. 2011;14:301–16. doi: 10.1007/s10120-011-0085-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao SL, Fang JY. The role of postoperative adjuvant chemotherapy following curative resection for gastric cancer: a meta-analysis. Cancer Invest. 2008;26:317–25. doi: 10.1080/07357900701834686. [DOI] [PubMed] [Google Scholar]
- 6.Bajetta E, Buzzoni R, Mariani L, et al. Adjuvant chemotherapy in gastric cancer: 5-year results of a randomised study by the Italian Trials in Medical Oncology (ITMO) Group. Ann Oncol. 2002;13:299–307. doi: 10.1093/annonc/mdf040. [DOI] [PubMed] [Google Scholar]
- 7.Yan TD, Cao CQ, Munkholm-Larsen S. A pharmacological review on intraperitoneal chemotherapy for peritoneal malignancy. World J Gastrointest Oncol. 2011;2:109–16. doi: 10.4251/wjgo.v2.i2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baeza MR, Giannini TO, Rivera SR, et al. Adjuvant radiochemotherapy in the treatment of completely resected, locally advanced gastric cancer. Int J Radiat Oncol Biol Phys. 2001;50:645–50. doi: 10.1016/s0360-3016(01)01467-5. [DOI] [PubMed] [Google Scholar]
- 9.Petit T, Velten M, d'Hombres A, et al. Long-term survival of 106 stage III ovarian cancer patients with minimal residual disease after second-look laparotomy and consolidation radiotherapy. Gynecol Oncol. 2007;104:104–8. doi: 10.1016/j.ygyno.2006.07.034. [DOI] [PubMed] [Google Scholar]
- 10.Bijelic L, Sugarbaker PH. The role of intraperitoneal chemotherapy in the treatment of patients with advanced gastric cancer. Ann Ital Chir. 2012;83:224–31. [PubMed] [Google Scholar]
- 11.Oleinick NL, Morris RL, Belichenko I. The role of apoptosis in response to photodynamic therapy: what, where, why, and how. Photochem Photobiol Sci. 2002;1:1–21. doi: 10.1039/b108586g. [DOI] [PubMed] [Google Scholar]
- 12.Mimura S, Ito Y, Nagayo T, et al. Cooperative clinical trial of photodynamic therapy with photofrin II and excimer dye laser for early gastric cancer. Lasers Surg Med. 1996;19:168–72. doi: 10.1002/(SICI)1096-9101(1996)19:2<168::AID-LSM7>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 13.Prosst RL, Wolfsen HC, Gahlen J. Photodynamic therapy for esophageal diseases: a clinical update. Endoscopy. 2003;35:1059–68. doi: 10.1055/s-2003-44604. [DOI] [PubMed] [Google Scholar]
- 14.Ortner MA. Photodynamic therapy for cholangiocarcinoma: overview and new developments. Curr Opin Gastroenterol. 2009;25:472–6. doi: 10.1097/MOG.0b013e32832e6e1f. [DOI] [PubMed] [Google Scholar]
- 15.Takita H, Mang TS, Loewen GM, et al. Operation and intracavitary photodynamic therapy for malignant pleural mesothelioma: a phase II study. Ann Thorac Surg. 1994;58:995–8. doi: 10.1016/0003-4975(94)90443-x. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi W, Liu Q, Nakagawa H, et al. Photodynamic therapy with mono-L-aspartyl chlorin e6 can cause necrosis of squamous cell carcinoma of tongue: experimental study on an animal model of nude mouse. Oral Oncol. 2006;42:46–50. doi: 10.1016/j.oraloncology.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Grant WE, Speight PM, Hopper C, Bown SG. Photodynamic therapy: an effective, but non-selective treatment for superficial cancers of the oral cavity. Int J Cancer. 1997;71:937–42. doi: 10.1002/(sici)1097-0215(19970611)71:6<937::aid-ijc4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 18.Makino A, Yamahara R, Ozeki E, Kimura S. Preparation of novel polymer assemblies, “lactosome”, composed of poly(L-lactic acid) and poly(sarcosine) Chem Lett. 2007;36:1220–1. [Google Scholar]
- 19.Vooijs M, Jonkers J, Lyons S, Berns A. Noninvasive imaging of spontaneous retinoblastoma pathway-dependent tumors in mice. Cancer Res. 2002;62:1862–7. [PubMed] [Google Scholar]
- 20.Skrivanova K, Skorpikova J, Svihalek J, Mornstein V, Janisch R. Photochemical properties of a potential photosensitiser indocyanine green in vitro. J Photochem Photobiol, B. 2006;85:150–4. doi: 10.1016/j.jphotobiol.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Lukyanov AN, Hartner WC, Torchilin VP. Increased accumulation of PEG-PE micelles in the area of experimental myocardial infarction in rabbits. J Control Release. 2004;94:187–93. doi: 10.1016/j.jconrel.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 22.Torchilin VP. Micellar nanocarriers: pharmaceutical perspectives. Pharm Res. 2007;24:1–16. doi: 10.1007/s11095-006-9132-0. [DOI] [PubMed] [Google Scholar]
- 23.Miyazaki K, Morimoto Y, Nishiyama N, et al. A novel homogeneous irradiation fiber probe for whole bladder wall photodynamic therapy. Lasers Surg Med. 2012;44:413–20. doi: 10.1002/lsm.22010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Zeta potential of indocyanine green loaded lactosome (ICGm).
Fig. S2. Relative fluorescent intensity of indocyanine green (ICG) loaded lactosome (ICGm) and ICG solution.
Fig. S3. Cancer cell death induced by photodynamic therapy (PDT) with indocyanine green (ICG) loaded lactosome (ICGm).


