Abstract
Alcohol is a well-established cause of esophageal carcinoma, but its effect on survival is little known and contradictory. To clarify whether drinking is an independent predictor of survival in esophageal carcinoma, 2151 Chinese patients, receiving surgical resection from January 1997 to December 2008, were followed until March 2014. Cox proportional hazards analysis was applied to evaluate the prognostic effect of alcohol consumption. The median follow-up was 64 months. The median overall survival (OS; 42 months) and disease-free survival (DFS; 33 months) for never-drinkers were significantly higher than ever-drinkers (27 and 22 months, respectively). In the multivariate Cox model that was adjusted for age, weight loss, stage according to criteria set by the American Joint Committee on Cancer, radicality of surgery, adjuvant treatment, smoking status, and gender, the hazard ratios of ever-drinking were 1.22 (1.06–1.41, P = 0.005) on OS, and 1.16 (1.01–1.34, P = 0.037) on DFS. The hazardous effect on OS and DFS of drinking grew statistically significantly in a dose-dependent manner with increasing amount of alcohol consumption per day (both P-value for trend < 0.05). The predictive effect of drinking on OS (P = 0.596) or DFS (P = 0.207) was not significant in the subgroup with esophageal adenocarcinoma (n = 195). The current study revealed that the survival is shortened, of those patients who consume alcohol before diagnosis of esophageal squamous cell carcinoma, which are not attributable to differences in stage, smoking status, and gender. Alcohol control should be emphasized to reduce mortality of esophageal carcinoma, and further outcome studies should include alcohol as a potential prognosticator.
Keywords: Alcohol, esophageal cancer, esophagectomy, prognosis, survival
Based on the GLOBOCAN2012, esophageal carcinoma is the eighth most common cancer worldwide, and the sixth most common cause of death from cancer; however, it is one of the least studied.(1) Despite the advance in the diagnosis, staging, and treatment in recent years, the overall survival (OS) of esophageal carcinoma is still unsatisfactory.
Esophageal squamous cell carcinoma (ESCC) accounts for most esophageal malignancy in East Asia, including China,(2) and southern Africa, and its incidence remains relatively constant. Esophageal adenocarcinoma (EAC), including adenocarcinoma of the esophagogastric junction, has increased rapidly in North America and Europe.(3) Substantial alcohol intake as a main contributor for ESCC has been proved by a large number of well-designed epidemiological studies.(4–9) According to a recent World Cancer Research Fund report,(10) alcohol is considered a “convincing” risk factor for esophageal carcinoma. Alcohol causes chronic irritation and inflammation of the esophageal mucosa, and consequently induces a series of molecular changes, triggering carcinogenesis.(11) Alcohol may promote the development of specific types of esophageal carcinoma, and it is possible that alcohol influences the behavior and course of the disease and has an effect on outcomes. However, the association between alcohol consumption and increased risk of EAC was not observed by etiology studies,(9,12,13) and this reflects the different biological behavior of these two subtypes. Previous studies have proved that alcohol consumption shortened survival in patients with head and neck cancer.(14–16) So far, data that elucidate the prognostic role of alcohol in patients with esophageal carcinoma have been limited; moreover, they are contradictory.(17–21)
Alcohol drinking is associated with many factors that potentially contribute to poorer cancer survival: male gender,(22,23) cigarette smoking,(24,25) body size,(26) sporadic incidence, malnutrition, comorbidity, advanced stage at diagnosis, insufficient treatment, impaired immune function,(27) and an increased molecular biological alteration that could lead to accelerated carcinogenesis and progression.(11) Herein, we report the findings of a large cohort study to determine whether alcohol drinking predicts survival independently, and whether survival effects are mediated through stage disparity, cigarette smoking, and/or gender inequality.
Materials and Methods
Study population
An esophageal carcinoma database was prospectively created for cohort study sponsored by the Guangdong Esophageal Cancer Institute (Guangzhou, China). All patients, who underwent esophagectomy between January 1997 and December 2008 at Sun Yat-sen University Cancer Center (Guangzhou, China), representative of the Chinese population, were enrolled at discharge. The database includes information regarding sociodemographic data, disease extent, treatment given, and follow-up status. Excluding 18 patients with unavailable information on drinking status and/or alcohol consumption amount, and 5 patients with distant metastasis at diagnosis, 2151 patients finally constituted our study cohort. This study was approved by the Ethics Committee of the Sun Yat-sen University Cancer Center. Informed consent was obtained from all patients.
Data collection
Patients were asked to report their lifetime history of drinking and smoking, including status, frequency, average consumption amount, and type of alcohol, at the time of admission. We calculated alcohol drinks per day from patients' responses for usual frequency and portion size of wine, spirit, and beer consumption. One drink corresponded to one serving of the US Department of Agriculture's food guide pyramid: one 12-fluid ounce beer, one 5-fluid ounce glass of wine, or one 1.5-ounce shot of spirit (each approximately 13 g of alcohol).(9) According to the average amount of alcohol consumption per day, drinkers were classified as light drinkers (0–0.99 drinks per day), moderate drinkers (1–2.99 drinks per day), or heavy drinkers (≥3 drinks per day).(28)
The most common surgical approaches included left transthoracic and right transthoracic procedures (including Ivor Lewis and McKeown esophagectomy). Lymph node dissection including standard, extended, and total dissection of thoracic and abdominal lymph nodes was carried out in patients with no evidence of metastatic disease that included cervical or coeliac lymph node metastases. Tumors were staged according to the 7th edition of the American Joint Committee on Cancer (AJCC) staging system.(29) Patients were classified according to Asian-specific body mass index cut-off values(30) as follows: underweight, <18.5 kg/m2; normal weight, 18.5–22.9 kg/m2; and overweight and obese, ≥23.0 kg/m2.
Follow-up
The patients were followed every 3 months for the first year and then every 6 months for the next 2 years, and finally annually. The diagnostic examinations consisted of esophagography, computed tomography, chest X-ray, abdominal ultrasonography, and bone scan when necessary to detect recurrence and/or metastasis. Follow-up time was calculated from the date of surgery to the date of the last contact. The primary endpoint was OS, which was calculated from the time of surgery to the time of death from any cause. The secondary endpoint was disease-free survival (DFS), which was calculated from the time from surgery to the first recurrence or metastasis of cancer, or to esophageal carcinoma-specific survival.
Statistical analysis
All statistical analyses were carried out using spss 16.0 for Windows software (SPSS, Chicago, IL, USA). The distributions of demographic, epidemiological, and clinicopathologic parameters between never- and ever-drinkers, were obtained through the χ2-test or Fisher's exact test. Overall and stratified survival was estimated and plotted using the Kaplan–Meier method and log–rank test. Cox proportional hazards regression modeling was applied to calculate the crude and adjusted hazard ratios (HR) and 95% confidence intervals (CI). We tested for linear trends by modeling the median of each category as a continuous variable and testing the significance of the term in a likelihood ratio test. Statistical significance was set at 0.05, and all tests were two-sided.
Results
Patient characteristics by drinking status
The population consisted of 1500 (69.7%) never-drinkers and 651 (30.3%) ever-drinkers, including former or current drinkers. As Table 1 shows, the majority of ever-drinkers were male (98.6%), younger (65.5%) and ever-smokers (92.8%). At the time of diagnosis, ever-drinkers were less likely to be overweight or obese, and had a higher likelihood of weight loss at the time of diagnosis. Drinking status correlated significantly with histology and stage distribution, with ever-drinkers having more ESCC and stage III disease (both P < 0.001).
Table 1.
Clinical and pathologic characteristics of esophageal cancer patients at baseline, stratified by alcohol drinking status
| Parameter | Overall, n = 2151† | Never drinkers, n = 1500 (%) | Ever drinkers, n = 651 (%) | P-value, χ2-test |
|---|---|---|---|---|
| Gender | ||||
| Male | 1653 | 1011 (67.4) | 642 (98.6) | <0.001 |
| Female | 498 | 489 (32.6) | 9 (1.4) | |
| Age, years | ||||
| ≤60 | 1290 | 864 (57.6) | 426 (65.5) | 0.001 |
| >60 | 861 | 636 (42.4) | 225 (34.5) | |
| BMI, kg/m2 | ||||
| <18.5 | 331 | 233 (15.6) | 98 (15.1) | 0.012 |
| 18.5–22.9 | 1150 | 772 (51.6) | 378 (58.1) | |
| ≥23 | 666 | 491 (32.8) | 175 (26.9) | |
| Weight loss | ||||
| No | 1146 | 838 (55.9) | 308 (47.5) | <0.001 |
| Yes | 1001 | 660 (44.1) | 341 (52.5) | |
| Smoking status | ||||
| Never-smokers | 771 | 724 (48.3) | 47 (7.2) | <0.001 |
| Ever-smokers | 1380 | 776 (51.7) | 604 (92.8) | |
| Histology | ||||
| SCC | 1851 | 1251 (83.4) | 600 (92.2) | <0.001 |
| AC | 195 | 168 (11.2) | 27 (4.1) | |
| Other | 105 | 81 (5.4) | 24 (3.7) | |
| AJCC stage | ||||
| 0+ I | 182 | 140 (9.5) | 42 (6.6) | <0.001 |
| II | 951 | 695 (47.1) | 256 (40.4) | |
| III | 978 | 642 (43.5) | 336 (53.0) | |
| No. of comorbidities | ||||
| 0 | 1541 | 1084 (72.3) | 457 (70.2) | 0.477 |
| ≥1 | 608 | 414 (27.6) | 194 (29.8) | |
| Surgical procedure | ||||
| Left transthoracic | 1460 | 1004 (66.9) | 456 (70.0) | 0.096 |
| Right transthoracic | 500 | 359 (23.9) | 141 (21.7) | |
| Others | 191 | 137 (9.1) | 54 (8.3) | |
| Radicality of surgery | ||||
| R0 | 1885 | 1325 | 560 | 0.134 |
| R1+ R2 | 146 | 94 | 52 | |
| Preoperative treatment | ||||
| None | 2016 | 1416 (94.5) | 600 (92.4) | 0.298 |
| Chemotherapy | 49 | 31 (2.1) | 18 (2.8) | |
| Radiotherapy | 40 | 25 (1.7) | 15 (2.3) | |
| Chemoradiotherapy | 42 | 26 (1.7) | 16 (2.5) | |
| Adjuvant treatment | ||||
| None | 1424 | 982 (79.5) | 442 (79.9) | 0.966 |
| Chemotherapy | 238 | 167 (13.5) | 71 (12.8) | |
| Radiotherapy | 89 | 60 (4.9) | 29 (5.2) | |
| Chemoradiotherapy | 37 | 26 (2.1) | 11 (2.0) | |
AC, adenocarcinoma; AJCC, American Joint Committee on Cancer; BMI, body mass index; SCC, squamous cell carcinoma.
Numbers may not sum to the total because of missing data.
Survival analysis according to drinking status
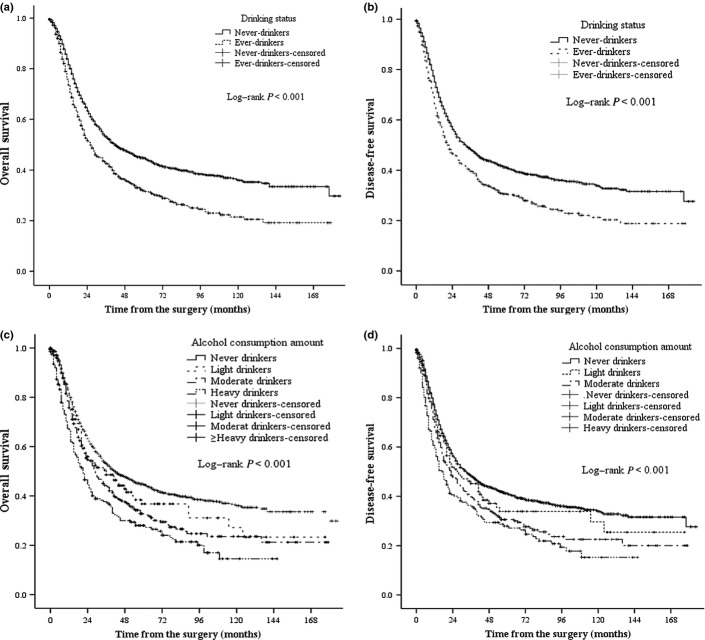
The median follow-up was 64 months with a follow-up rate of 83.9%, and the median OS and DFS was 36 months (95% CI, 32.6–39.4) and 29 months (95% CI, 25.7–32.3). The median OS for never-drinkers and ever-drinkers was 42 months (95% CI, 35.7–48.2) and 27 months (95% CI, 22.8–31.2), and median DFS was 33 months (95% CI, 28.5–37.5) and 22 months (95% CI, 18.5–25.5), respectively. The crude Kaplan–Meier survival curves in Figure 1(a,b) showed worse prognosis among ever-drinkers in terms of OS and DFS (both log–rank P < 0.001), and the univariate HR for ever-drinkers was 1.43 (95% CI, 1.27–1.61; P < 0.001) and 1.34 (95% CI, 1.21–1.53; P < 0.001) for OS and DFS, respectively.
Fig. 1.
Kaplan–Meier survival curves are shown for overall survival (a) and disease-free survival (b) in patients with esophageal carcinoma according to alcohol drinking status. Kaplan–Meier survival curves are shown for overall survival (c) and disease-free survival (d) in patients with esophageal carcinoma according to average alcohol consumption per day. P-values were calculated using the unadjusted log–rank test.
In addition, gender (P = 0.004), age (P = 0.002), weight loss (P = 0.002), stage (P < 0.001), radicality of surgery (P < 0.001), and adjuvant treatment (P = 0.011) were independent predictors of OS in multivariate Cox regression analysis adjusted for the baseline parameters (Table 2). Ever-drinking was associated with deleterious levels of four of the six aforementioned predictors, except age and adjuvant treatment. Younger age at the time of diagnosis was the only protective association that ever-drinkers had.
Table 2.
Prognostic significance of baseline parameters in esophageal cancer patients by univariate and multivariate Cox regression analysis
| Parameter | No. of events/No. at risk | Univariate analysis | Multivariate analysis† | ||
|---|---|---|---|---|---|
| HR (95% CI) | P–value | HR (95% CI) | P–value | ||
| Gender | |||||
| Male | 1011/642 | 1.00 | 1.00 | 0.004 | |
| Female | 250/248 | 0.71 (0.61–0.81) | <0.001 | 0.80 (0.68–0.93) | |
| Age, years | |||||
| ≤60 | 720/570 | 1.00 | 1.00 | 0.002 | |
| >60 | 541/320 | 1.14 (1.02–1.28) | 0.018 | 1.86 (1.07–1.33) | |
| BMI, kg/m2 | |||||
| <18.5 | 228/103 | 1.18 (1.01–1.37) | 0.034 | – | – |
| 18.5–22.9 | 669/481 | 1.00 | – | ||
| ≥23 | 358/308 | 0.86 (0.76–0.98) | 0.021 | – | |
| Weight loss | |||||
| No | 622/524 | 1.00 | 1.00 | 0.002 | |
| Yes | 639/362 | 1.31 (1.17–11.46) | <0.001 | 1.21 (1.07–1.37) | |
| Smoking status | |||||
| Never–smokers | 403/368 | 1.00 | – | ||
| Ever–smokers | 858/522 | 1.36 (1.20–1.53) | <0.001 | – | |
| Histology | 0.033 | ||||
| SCC | 1087/764 | 1.00 | – | – | |
| AC | 131/64 | 1.25 (1.04–1.51) | 0.018 | – | |
| Other | 63/42 | 1.20 (0.92–1.56) | 0.182 | – | |
| AJCC stage | <0.001 | ||||
| 0+ I | 50/132 | 1.00 | 1.00 | <0.001 | |
| II | 494/457 | 2.07 (1.55–2.76) | <0.001 | 2.09 (1.52–2.86) | |
| III | 716/262 | 4.71 (3.54–6.26) | <0.001 | 4.44 (3.24–6.08) | |
| No. of comorbidities | |||||
| 0 | 909/634 | 1.00 | – | ||
| ≥1 | 352/256 | 1.02 (0.90–1.150) | 0.775 | – | |
| Surgical procedure | |||||
| Left transthoracic | 883/577 | 1.00 | – | – | |
| Right transthoracic | 266/234 | 0.87 (0.76–0.99) | 0.040 | – | |
| Others | 112/79 | 0.96 (0.79–1.18) | 0.717 | – | |
| Radicality of surgery | |||||
| R0 | 1075/810 | 1.00 | <0.001 | ||
| R1+ R2 | 125/21 | 2.91 (2.43–3.48) | <0.001 | 2.29 (1.84–2.86) | |
| Preoperative treatment | |||||
| None | 1185/831 | 1.00 | – | – | |
| Chemotherapy | 30/21 | 1.53 (1.05–2.24) | 0.029 | – | |
| Radiotherapy | 25/16 | 1.26 (0.84–1.88) | 0.266 | – | |
| Chemoradiotherapy | 21/22 | 0.95 (0.61–1.47) | 0.81 | – | |
| Adjuvant treatment | |||||
| None | 847/573 | 1.00 | 1.00 | 0.011 | |
| Chemotherapy | 124/115 | 0.97 (0.80–1.17) | 0.757 | 0.71 (0.58–0.87) | |
| Radiotherapy | 73/16 | 1.83 (1.44–2.32) | <0.001 | 0.96 (0.73–1.25) | |
| Chemoradiotherapy | 29/10 | 1.54 (1.05–2.24) | 0.026 | 0.86 (0.57–1.28) | |
AC, adenocarcinoma; AJCC, American Joint Committee on Cancer; BMI, body mass index; CI, confidence interval; HR, hazard ratio; SCC, squamous cell carcinoma.
Multivariate analysis included all these baseline parameters. –, not included in the multivariate analysis.
To determine whether the crude hazard association between drinking status and OS was mediated by other important prognostic factors, Cox proportional hazard models were used, adjusted for baseline covariates (model A), additionally adjusted for smoking status (model B), and then additionally adjusted for gender (model C). In the Cox model that was adjusted for the baseline covariates, which includes age, weight loss prior to diagnosis, AJCC stage, radicality of surgery, and adjuvant treatment, the HR for ever-drinkers was 1.30 (95% CI, 1.14–1.47; P < 0.001) (Table 3, model A). The multivariate HR for ever-drinkers declined by 30.2% (1.43 vs 1.30) following adjustment for baseline covariates. Additionally adjusted for smoking status, the HR for ever-drinkers was 1.24 (95% CI, 1.08–1.43; P = 0.002) (Table 3, model B). Adjusted for baseline covariates, smoking status, and gender, the prognostic effect of drinking remained significant, and the HR was 1.22 (95% CI, 1.06–1.41; P = 0.005) (Table 3, model C). Ever-drinking was also shown to be an independent prognostic factor of DFS (Table 3).
Table 3.
Three multivariate Cox models for alcohol drinking status on overall survival and disease–free survival among patients with esophageal carcinoma
| Model A adjusted for baseline covariates† | Model B adjusted for baseline covariates† and smoking | Model C adjusted for baseline covariates,†smoking, and gender | |
|---|---|---|---|
| Overall survival | |||
| Drinking status (ever vs never) | 1.30 (1.14–1.47, P < 0.001) | 1.24 (1.08–1.43, P = 0.002) | 1.22 (1.06–1.41, P = 0.005) |
| Age, years (>60 vs ≤60) | 1.22 (1.08–1.38,P = 0.002) | 1.22 (1.08–1.39, P = 0.001) | 1.23 (1.08–1.39, P = 0.001) |
| Weight loss (yes vs no) | 1.18 (1.04–1.33, P = 0.009) | 1.18 (1.04–1.33, P = 0.009) | 1.18 (1.05–1.34, P = 0.007) |
| AJCC stage | |||
| 0+ I | 1.00 | 1.00 | 1.00 |
| II | 2.08 (1.52–2.85, P < 0.001) | 2.08 (1.52–2.85, P < 0.001) | 2.09 (1.53–2.86, P < 0.001) |
| III | 4.49 (3.28–6.14, P < 0.001) | 4.46 (3.26–6.10, P < 0.001) | 4.46 (3.26–6.11, P < 0.001) |
| Radicality of surgery (R1 + R2 vs R0) | 2.14 (1.72–2.66, P < 0.001) | 2.16 (1.74–2.68, P < 0.001) | 2.17 (1.75–2.70, P < 0.001) |
| Adjuvant treatment | |||
| None | 1.00 | 1.00 | 1.00 |
| Chemotherapy | 0.71 (0.59–0.87, P = 0.001) | 0.72 (0.59–0.87, P = 0.001) | 0.71 (0.58–0.86, P = 0.708) |
| Radiotherapy | 1.04 (0.80–1.35, P = 0.792) | 1.03 (0.79,1.34, P = 0.812) | 1.02 (0.78–1.32, P = 0.907) |
| Chemoradiotherapy | 0.88 (0.60–1.30, P = 0.521) | 0.89 (0.60–1.31, P = 0.131) | 0.88 (0.60–1.30, P = 0.525) |
| Smoking status (ever vs never) | 1.12 (0.97–1.29, P = 0.131) | 1.02 (0.85–1.22, P = 0.836) | |
| Gender (female vs male) | 0.85 (0.69–1.05, P = 0.124) | ||
| Disease-free survival | |||
| Drinking status (ever vs never) | 1.22 (1.07–1.39, P = 0.002) | 1.17 (1.012–1.35, P = 0.027) | 1.16 (1.01–1.34, P = 0.037) |
| Age, years (>60 vs ≤60) | 1.19 (1.05–1.34, P = 0.006) | 1.19 (1.05–1.34, P = 0.006) | 1.19 (1.05–1.34, P = 0.004) |
| Weight loss (yes vs no) | 1.12 (0.99–1.26, P = 0.073) | 1.12 (0.99–1.26, P = 0.072) | 1.12 (0.99–1.27, P = 0.067) |
| AJCC stage | |||
| 0+ I | 1.00 | 1.00 | 1.00 |
| II | 2.21 (1.62–3.00, P < 0.001) | 2.21 (1.63–3.01, P < 0.001) | 2.22 (1.63–3.01, P < 0.001) |
| III | 4.75 (3.49–6.46, P < 0.001) | 4.72 (3.47–6.43, P < 0.001) | 4.73 (3.47–6.43, P < 0.001) |
| Radicality of surgery (R1+ R2 vs R0) | 1.78 (1.43–2.22, P < 0.001) | 1.78 (1.43–2.22, P < 0.001) | 1.79 (1.44–2.24, P < 0.001) |
| Adjuvant treatment | |||
| None | 1.00 | 1.00 | 1.00 |
| Chemotherapy | 0.83 (0.68–1.01, P = 0.056) | 0.83 (0.69–1.01, P = 0.062) | 0.83 (0.68–1.00, P = 0.055) |
| Radiotherapy | 1.12 (0.86–1.45, P = 0.409) | 1.11 (0.86–1.45, P = 0.426) | 1.10 (0.85–1.44, P = 0.466) |
| Chemoradiotherapy | 0.87 (0.59–1.30, P = 0.508) | 0.89 (0.0–1.32, P = 0.548) | 0.88 (0.59–1.31, P = 0.533) |
| Smoking status (ever vs never) | 1.11 (0.97–1.28, P = 0.144) | 1.06 (0.88–1.27, P = 0.563) | |
| Gender (female vs male) | 0.91 (0.74–1.12, P = 0.392) | ||
AJCC, American Joint Committee on Cancer.
Baseline covariates include age, weight loss, stage, radicality of surgery, and adjuvant treatment.
Survival analysis according to the average amount of alcohol consumption
According to the average amount of alcohol consumption per day, patients were classified as never drinkers (n = 1500, 69.7%), light drinkers (n = 139, 6.5%), moderate drinkers (n = 309, 14.4%), or heavy drinkers (n = 202, 9.4%). The crude OS and DFS curves by these four categories are shown in Figure 1(c,d). Compared to never-drinkers, the univariate HRs for light, moderate, and heavy drinkers were 1.19 (95% CI, 0.94–1.51; P = 0.147), 1.36 (95% CI, 1.17–1.58; P < 0.001) and 1.76 (95% CI, 1.47–2.10; P < 0.001) in terms of OS. These univariate HRs were 1.14 (95% CI, 0.89–1.44; P = 0.298), 1.33 (95% CI, 1.14–1.54; P < 0.001), and 1.61 (95% CI, 1.34–1.93; P < 0.001) in terms of DFS. The hazardous effect of drinking on OS and DFS increased markedly across alcohol consumption categories (both P-value for trend <0.001), indicating the dose–response relationship between alcohol consumption and survival. Instead of drinking status, the category of alcohol consumption amount was included in the three multivariate Cox models successively (Table 4). The independent effect of heavy drinking remained in all three models. We observed an association between moderate drinking and poor OS in both model A and B, however, this association become marginally significant (P = 0.057), adjusted for baseline covariates, smoking status, and gender. The dose–response relationship remained statistically significant in all three models.
Table 4.
Univariate analysis and three multivariate Cox models for alcohol consumption amount per day on overall survival and disease-free survival among patients with esophageal carcinoma
| Average alcohol consumption | Crude HR (95% CI, P-value) | Model A, adjusted for baseline covariates† | Model B, adjusted for baseline covariates† and smoking | Model C, adjusted for baseline covariates,† smoking, and gender |
|---|---|---|---|---|
| Overall survival | ||||
| Never (n = 1500) | 1.00 | 1.00 | 1.00 | 1.00 |
| Light (n = 139) | 1.19 (0.94–1.51, P = 0.147) | 1.04 (0.80–1.37, P = 0.751) | 1.00 (0.76–1.31, P = 0.993) | 0.99 (0.75–1.30, P = 0.928) |
| Moderate (n = 309) | 1.36 (1.17–1.58, P < 0.001) | 1.25 (1.06–1.48, P = 0.008) | 1.20 (1.01–1.4328, P = 0.041) | 1.19 (1.00–1.41, P = 0.057) |
| Heavy (n = 202) | 1.76 (1.47–2.10, P < 0.001) | 1.55 (1.27–1.88, P < 0.001) | 1.48 (1.21–1.82, P < 0.001) | 1.46 (1.19–1.79, P < 0.001) |
| P value for trend2 | 0.005 | 0.004 | 0.013 | 0.016 |
| Disease-free survival | ||||
| Never (n = 1500) | 1.00 | 1.00 | 1.00 | 1.00 |
| Light (n = 139) | 1.14 (0.89–1.44, P = 0.298) | 1.02 (0.78–1.33, P = 0.907) | 0.97 (0.74–1.28, P = 0.849) | 0.97 (0.74–1.20, P = 0.811) |
| Moderate (n = 309) | 1.33 (1.14–1.54, P < 0.001) | 1.20 (1.02–1.42, P = 0.027) | 1.15 (0.97–1.37, P = 0.108) | 1.14 (0.96–1.36, P = 0.127) |
| Heavy (n = 202) | 1.61 (1.34–1.93, P < 0.001) | 1.43 (1.17–1.75, P < 0.001) | 1.37 (1.12–1.88, P = 0.003) | 1.36 (1.11–1.67, P = 0.004) |
| P-value for trend‡ | 0.002 | 0.009 | 0.025 | 0.026 |
Baseline covariates include age, weight loss, stage, radicality of surgery, and adjuvant treatment.
P-value for trend was calculated by modeling the median of each categories as a continuous variable.
Subgroup analysis
Overall survival analyses stratified by covariates were carried out using the Kaplan–Meier method. Compared with never-drinkers, the survival of ever-drinkers was significantly shorter in males, younger patients, all three body mass index categories, both weight-loss subgroups, both never- and ever-smokers, patients with ESCC, stage II and III diseases, patients with or without pretreatment comorbidities, and the R0 subgroup (Table S1).
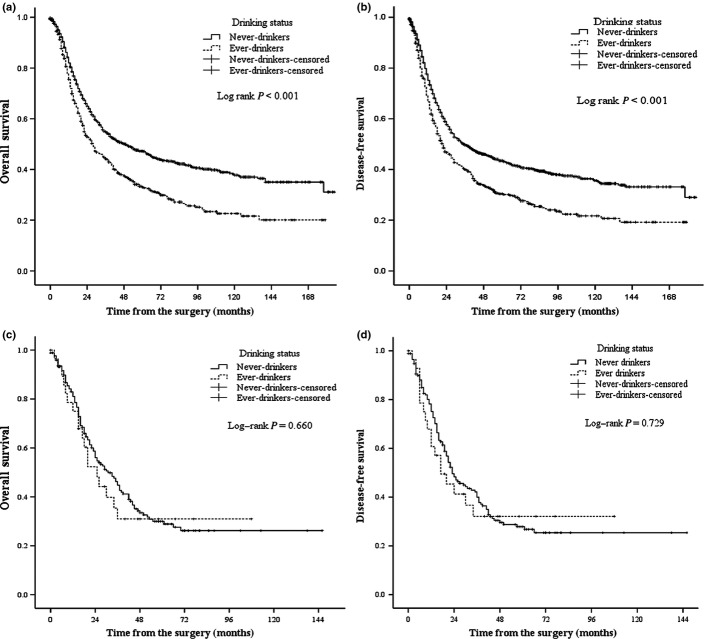
In patients with ESCC, ever-drinkers had significantly poorer OS (median, 49 months vs 28 months, P < 0.001) (Fig. 2a) and DFS (median, 36 months vs 22 months, P < 0.001) (Fig. 2b). Among patients with EAC, including adenocarcinoma of the esophagogastric junction, there was no significant difference between never- and ever-drinkers in OS (median, 31 months vs 25 months, P = 0.660) (Fig. 2c) or DFS (median, 23 months vs 17 months, P = 0.729) (Fig. 2d). In model C, adjusted for baseline covariates, smoking status, and gender, drinking status was found to be an independent prognostic factor for poorer OS (HR, 1.19; 95% CI, 1.03–1.38; P = 0.022) and DFS (HR, 1.18; 95% CI, 1.02–1.36; P = 0.023) in patients with ESCC. Among patients with EAC, drinking status was not a significant prognostic factor for OS (HR, 1.27; P = 0.596) or DFS (HR, 1.77; P = 0.207) (Table S2). In addition, the dose–response relationship also existed in the ESCC subgroup (Table S3).
Fig. 2.
Kaplan–Meier survival curves are shown for overall survival (a) and disease-free survival (b) in patients with esophageal squamous cell carcinoma according to alcohol drinking status. Kaplan–Meier survival curves are shown for overall survival (c) and disease-free survival (d) in patients with esophageal adenocarcinoma according to alcohol drinking status. P-values were calculated using the unadjusted log–rank test.
Discussion
Our large-scale population study of patients with esophageal cancer revealed that prediagnosis drinking increased the hazard of dying by over 20% compared to never-drinkers. Adjusted for other important prognostic factors, the adverse association between drinking and survival remained statistically significant. Moreover, the hazardous effects of drinking grew statistically significantly in a dose-dependent manner with increasing amount of alcohol consumption per day, which was not described by previous studies.
In recent years, growing attention in cancer research has been paid to the impacts of host-related factors, including lifestyle. Goodwin(31) asserted that host-related factors have characteristics that are associated with certain prognoses, so that they appear to influence prognosis. Furthermore, caution should be exercised in attributing prognostic effects to sociodemographic factors that cause delay in diagnosis and advanced tumor stage. Indeed, drinking status is associated with other prognostic factors in our study, as mentioned above. Although the injurious effects on survival attenuate after adjustment for age, weight loss at diagnosis, tumor stage, radicality of surgery, smoking status, and gender, alcohol drinking remains an important and independent predictor of unfavorable outcome. The dose–response relationship further confirms the effects.
There was no significant difference in preoperative comorbidity between never- and ever-drinkers, and comorbidity had no influence on prognosis. This might result from the fact that patients receiving surgical treatment constituted our study cohort. Patients with severe and alcohol-abuse related comorbidities were more likely to have poor cardiac, pulmonary and hepatic function, and therefore to be excluded during critical preoperative evaluation. The fact that the prevalence of comorbidity in our cohort was markedly lower than others(32,33) including non-surgical treatments, supported this contention. Anyway, this revealed that the prognostic effect of alcohol was not mediated through comorbidity. Alcohol drinking is thought to correlate with lower socioeconomic status (SES), which reduces the possibility of receiving reasonable multimodality treatment. In our study, treatment modality was similar between the two groups, suggesting that it was unlikely to introduce bias. Insufficient treatment shouldn't be the explanation of poor survival in ever-drinkers.
In the subgroup analysis, the hazardous effect of drinking existed broadly in most subgroups, but the subgroup with adenocarcinoma was an exception. The small sample size of drinkers with adenocarcinoma (n = 27) might restrict the power to distinguish the difference. This could be an explanation; however, this is not necessarily the case. In studies by Sundelof et al.,(18) Trivers et al.,(19) and Thrift et al.,(34) alcohol drinking did not predict the survival of EAC patients, either. It seems plausible to conclude that alcohol is not a risk factor for both incidence and mortality of patients EAC. However, further validation is needed to draw the final conclusion.
There are several strengths and limitations of our study that should be considered in interpreting the results. To our knowledge, the current study has been the largest, to date, in elucidating the prognostic role of alcohol drinking in esophageal cancer. The clinicopathologic data were retrieved from medical records, and the follow-up continued over a long period of time. Patients who had an esophageal resection constitute our cohort, reducing the heterogeneity of subjects and avoiding some potential confounding factors deriving from definitive chemoradiotherapy or other therapies. In addition, this is the first to report dose-relationship between the amount of alcohol consumption and poor survival, further confirming the adverse prognostic effects of alcohol drinking.
One limitation is that our study does not evaluate behavioral change in drinking after diagnosis, which may have ongoing effects on survival. It is an important component of patient education to give strong advice on abstinence from admission to postoperative follow-up. The majority of patients would possibly comply with this advice postoperatively for fear of destroying the esophagus replacement and aggravating complications. In addition, the HRs for former- and current-drinkers were pooled during analysis. We failed to analyze the effects of former-drinkers alone and elucidate whether giving up drinking could reduce the risk of mortality.
If further investigations prove the current study results to be accurate, these finding have significant public health, clinical, and research implications. Reducing alcohol consumption is an important and underemphasized cancer prevention strategy, yet it receives little attention, especially when compared with efforts related to other cancer prevention topics such as screening, tobacco, and obesity. Given the dose–response relationship between alcohol intake and risk of mortality in esophageal carcinoma, control of heavy drinking remains the main target not only for cancer control, but also mortality reduction, at least in ESCC. Clinical investigations and trials in esophageal carcinoma should obtain detailed data on drinking status, consider drinking as a potential confounding factor of survival, and adjust for it in analysis, or stratify by drinking status. More intensive treatment and/or surveillance may be needed in patients with esophageal carcinoma who are alcohol drinkers.
Molecular biological research has revealed that alcohol and its metabolites trigger many genetic and epigenetic alterations,(35,36) leading to more aggressive biological behavior, cancer progression, and alcohol-associated reduced immune surveillance. For instance, alcohol consumption coupled with genetic variants in ADH1B and ALDH2, two alcohol metabolizing genes, has effects on early ESCC diagnosis and tumor dissemination.(36) It is urgent to develop a better understanding of the underlying mechanisms to provide insight into esophageal carcinogenesis and uncover novel therapeutic approaches.
In summary, we carried out a large-scale investigation with long-term follow-up to evaluate the effects of prediagnosis drinking on survival in esophageal carcinoma. We observed that alcohol drinking was associated with decreased survival in ESCC, which appears not to be attributable to differences in stage, smoking status, gender, comorbidity, or treatment. The hazardous effects of alcohol grew significantly with increased alcohol consumption. Nevertheless, alcohol consumption might not be a prognostic factor in patients with EAC. The findings presented here warrant confirmation in further studies, with an emphasis on elucidating mechanisms.
Acknowledgments
This work was funded by the National Natural Science Foundation of China General Program (Grant No. 81272635), the Guangdong Science and Technology Program (Grant No. 2012A030400007), and the Guangzhou Science and Technology Program (Grant No. 2014Y2-00143). Thanks are also extended to LetPub Company for professional language improvement.
Disclosure Statement
The authors have no conflict of interest.
Supporting Information
Additional supporting information may be found in the online version of this article:
Table S1. Survival of esophageal cancer patients according to alcohol drinking status stratified by covariates, estimated by the Kaplan–Meier method.
Table S2. Prognostic factors in subgroups of patients with esophageal squamous cell carcinoma and esophageal adenocarcinoma in multivariate Cox model C, adjusted for age, weight loss, stage according to the American Joint Committee on Cancer, radicality of surgery, adjuvant treatment, smoking status, and gender.
Table S3. Univariate analysis and three multivariate Cox models for effect of alcohol consumption amount per day on overall survival and disease-free survival among patients with esophageal squamous cell carcinoma.
References
- 1.Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241–52. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Devesa SS, Blot WJ, Fraumeni JF., Jr Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;83:2049–53. [PubMed] [Google Scholar]
- 4.Nakachi K, Imai K, Hoshiyama Y, Sasaba T. The joint effects of two factors in the aetiology of oesophageal cancer in Japan. J Epidemiol Community Health. 1988;42:355–64. doi: 10.1136/jech.42.4.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao YT, McLaughlin JK, Blot WJ, et al. Risk factors for esophageal cancer in Shanghai, China. I. Role of cigarette smoking and alcohol drinking. Int J Cancer. 1994;58:192–6. doi: 10.1002/ijc.2910580208. [DOI] [PubMed] [Google Scholar]
- 6.Hanaoka T, Tsugane S, Ando N, et al. Alcohol consumption and risk of esophageal cancer in Japan: a case-control study in seven hospitals. Jpn J Clin Oncol. 1994;24:241–6. [PubMed] [Google Scholar]
- 7.Yang CX, Wang HY, Wang ZM, et al. Risk factors for esophageal cancer: a case-control study in South-western China. Asian Pac J Cancer Prev. 2005;6:48–53. [PubMed] [Google Scholar]
- 8.Zambon P, Talamini R, La Vecchia C, et al. Smoking, type of alcoholic beverage and squamous-cell oesophageal cancer in northern Italy. Int J Cancer. 2000;86:144–9. doi: 10.1002/(sici)1097-0215(20000401)86:1<144::aid-ijc23>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 9.Freedman ND, Abnet CC, Leitzmann MF, et al. A prospective study of tobacco, alcohol, and the risk of esophageal and gastric cancer subtypes. Am J Epidemiol. 2007;165:1424–33. doi: 10.1093/aje/kwm051. [DOI] [PubMed] [Google Scholar]
- 10.Wiseman M. The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Proc Nutr Soc. 2008;67:253–6. doi: 10.1017/S002966510800712X. [DOI] [PubMed] [Google Scholar]
- 11.Toh Y, Oki E, Ohgaki K, et al. Alcohol drinking, cigarette smoking, and the development of squamous cell carcinoma of the esophagus: molecular mechanisms of carcinogenesis. Int J Clin Oncol. 2010;15:135–44. doi: 10.1007/s10147-010-0057-6. [DOI] [PubMed] [Google Scholar]
- 12.Gammon MD, Schoenberg JB, Ahsan H, et al. Tobacco, alcohol, and socioeconomic status and adenocarcinomas of the esophagus and gastric cardia. J Natl Cancer Inst. 1997;89:1277–84. doi: 10.1093/jnci/89.17.1277. [DOI] [PubMed] [Google Scholar]
- 13.Wu AH, Wan P, Bernstein L. A multiethnic population-based study of smoking, alcohol and body size and risk of adenocarcinomas of the stomach and esophagus (United States) Cancer Causes Control. 2001;12:721–32. doi: 10.1023/a:1011290704728. [DOI] [PubMed] [Google Scholar]
- 14.Bundgaard T, Bentzen SM, Wildt J. The prognostic effect of tobacco and alcohol consumption in intra-oral squamous cell carcinoma. Eur J Cancer B Oral Oncol. 1994;30B:323–8. doi: 10.1016/0964-1955(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 15.Deleyiannis FW, Thomas DB, Vaughan TL, Davis S. Alcoholism: independent predictor of survival in patients with head and neck cancer. J Natl Cancer Inst. 1996;88:542–9. doi: 10.1093/jnci/88.8.542. [DOI] [PubMed] [Google Scholar]
- 16.Johnston WD, Ballantyne AJ. Prognostic effect of tobacco and alcohol use in patients with oral tongue cancer. Am J Surg. 1977;134:444–7. doi: 10.1016/0002-9610(77)90374-9. [DOI] [PubMed] [Google Scholar]
- 17.Shitara K, Matsuo K, Hatooka S, et al. Heavy smoking history interacts with chemoradiotherapy for esophageal cancer prognosis: a retrospective study. Cancer Sci. 2010;101:1001–6. doi: 10.1111/j.1349-7006.2009.01466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sundelof M, Lagergren J, Ye W. Patient demographics and lifestyle factors influencing long-term survival of oesophageal cancer and gastric cardia cancer in a nationwide study in Sweden. Eur J Cancer. 2008;44:1566–71. doi: 10.1016/j.ejca.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Trivers KF, De Roos AJ, Gammon MD, et al. Demographic and lifestyle predictors of survival in patients with esophageal or gastric cancers. Clin Gastroenterol Hepatol. 2005;3:225–30. doi: 10.1016/s1542-3565(04)00613-5. [DOI] [PubMed] [Google Scholar]
- 20.Park SM, Lim MK, Shin SA, Yun YH. Impact of prediagnosis smoking, alcohol, obesity, and insulin resistance on survival in male cancer patients: National Health Insurance Corporation Study. J Clin Oncol. 2006;24:5017–24. doi: 10.1200/JCO.2006.07.0243. [DOI] [PubMed] [Google Scholar]
- 21.Thrift AP, Nagle CM, Fahey PP, et al. The influence of prediagnostic demographic and lifestyle factors on esophageal squamous cell carcinoma survival. Int J Cancer. 2012;131:E759–68. doi: 10.1002/ijc.27420. [DOI] [PubMed] [Google Scholar]
- 22.Younes M, Henson DE, Ertan A, Miller CC. Incidence and survival trends of esophageal carcinoma in the United States: racial and gender differences by histological type. Scand J Gastroenterol. 2002;37:1359–65. doi: 10.1080/003655202762671215. [DOI] [PubMed] [Google Scholar]
- 23.Baquet CR, Commiskey P, Mack K, Meltzer S, Mishra SI. Esophageal cancer epidemiology in blacks and whites: racial and gender disparities in incidence, mortality, survival rates and histology. J Natl Med Assoc. 2005;97:1471–8. [PMC free article] [PubMed] [Google Scholar]
- 24.Lin CS, Chang SC, Wei YH, et al. Prognostic variables in thoracic esophageal squamous cell carcinoma. Ann Thorac Surg. 2009;87:1056–65. doi: 10.1016/j.athoracsur.2008.11.051. [DOI] [PubMed] [Google Scholar]
- 25.Lin Y, Su X, Su H, et al. Prediagnostic smoking and postoperative survival in lymph node-negative esophagus squamous cell carcinoma patients. Cancer Sci. 2012;103:1985–8. doi: 10.1111/cas.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang SS, Yang H, Luo KJ, et al. The impact of body mass index on complication and survival in resected oesophageal cancer: a clinical-based cohort and meta-analysis. Br J Cancer. 2013;109:2894–903. doi: 10.1038/bjc.2013.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MacGregor RR. Alcohol and immune defense. JAMA. 1986;256:1474–9. [PubMed] [Google Scholar]
- 28.Jiao L, Silverman DT, Schairer C, et al. Alcohol use and risk of pancreatic cancer: the NIH-AARP Diet and Health Study. Am J Epidemiol. 2009;169:1043–51. doi: 10.1093/aje/kwp034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC cancer staging manual: esophagus and esophagogastric junction. Ann Surg Oncol. 2010;17:1721–4. doi: 10.1245/s10434-010-1024-1. [DOI] [PubMed] [Google Scholar]
- 30.Choi Y, Park B, Jeong BC, et al. Body mass index and survival in patients with renal cell carcinoma: a clinical-based cohort and meta-analysis. Int J Cancer. 2013;132:625–34. doi: 10.1002/ijc.27639. [DOI] [PubMed] [Google Scholar]
- 31.Goodwin PJ. Host-related factors in breast cancer: an underappreciated piece of the puzzle? J Clin Oncol. 2008;26:3299–300. doi: 10.1200/JCO.2007.15.4526. [DOI] [PubMed] [Google Scholar]
- 32.Fang FM, Tsai WL, Chiu HC, Kuo WR, Hsiung CY. Quality of life as a survival predictor for esophageal squamous cell carcinoma treated with radiotherapy. Int J Radiat Oncol Biol Phys. 2004;58:1394–404. doi: 10.1016/j.ijrobp.2003.09.100. [DOI] [PubMed] [Google Scholar]
- 33.Janssen-Heijnen ML, Houterman S, Lemmens VE, Louwman MW, Maas HA, Coebergh JW. Prognostic impact of increasing age and co-morbidity in cancer patients: a population-based approach. Crit Rev Oncol Hematol. 2005;55:231–40. doi: 10.1016/j.critrevonc.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 34.Thrift AP, Nagle CM, Fahey PP, Smithers BM, Watson DI, Whiteman DC. Predictors of survival among patients diagnosed with adenocarcinoma of the esophagus and gastroesophageal junction. Cancer Causes Control. 2012;23:555–64. doi: 10.1007/s10552-012-9913-1. [DOI] [PubMed] [Google Scholar]
- 35.van Engeland M, Weijenberg MP, Roemen GM, et al. Effects of dietary folate and alcohol intake on promoter methylation in sporadic colorectal cancer: the Netherlands cohort study on diet and cancer. Cancer Res. 2003;63:3133–7. [PubMed] [Google Scholar]
- 36.Lee CH, Wu DC, Wu IC, et al. Genetic modulation of ADH1B and ALDH2 polymorphisms with regard to alcohol and tobacco consumption for younger aged esophageal squamous cell carcinoma diagnosis. Int J Cancer. 2009;125:1134–42. doi: 10.1002/ijc.24357. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Survival of esophageal cancer patients according to alcohol drinking status stratified by covariates, estimated by the Kaplan–Meier method.
Table S2. Prognostic factors in subgroups of patients with esophageal squamous cell carcinoma and esophageal adenocarcinoma in multivariate Cox model C, adjusted for age, weight loss, stage according to the American Joint Committee on Cancer, radicality of surgery, adjuvant treatment, smoking status, and gender.
Table S3. Univariate analysis and three multivariate Cox models for effect of alcohol consumption amount per day on overall survival and disease-free survival among patients with esophageal squamous cell carcinoma.