Abstract
Angiopoietin-like protein 2 (ANGPTL2) plays an important role in inflammatory carcinogenesis and tumor metastasis by activating tumor angiogenesis and tumor cell chemotaxis and invasiveness. However, it is unclear whether ANGPTL2 expression has an effect on tumor cell survival. Here, we explored that possibility by determining whether ANGPTL2 expression altered survival of human colorectal cancer cell lines treated with antineoplastic drugs. To do so, we generated SW480 cells expressing ANGPTL2 (SW480/ANGPTL2) and control (SW480/Ctrl) cells. Apoptosis induced by antineoplastic drug treatment was significantly decreased in SW480/ANGPTL2 compared to control cells. Expression of anti-apoptotic BCL-2 family genes was upregulated in SW480/ANGPTL2 compared to SW480/Ctrl cells. To assess signaling downstream of ANGPTL2 underlying this effect, we carried out RNA sequencing analysis of SW480/ANGPTL2 and SW480/Ctrl cells. That analysis, combined with in vitro experiments, indicated that Syk-PI3K signaling induced expression of BCL-2 family genes in SW480/ANGPTL2 cells. Furthermore, ANGPTL2 increased its own expression in a feedback loop by activating the spleen tyrosine kinase–nuclear factor of activated T cells (Syk–NFAT) pathway. Finally, we observed a correlation between higher ANGPTL2 expression in primary unresectable tumors from colorectal cancer patients who underwent chemotherapy with a lower objective response rate. These findings suggest that attenuating ANGPTL2 signaling in tumor cells may block tumor cell resistance to antineoplastic therapies.
Keywords: ANGPTL2, apoptosis, BCL-2, chemoresistance, Syk
Colorectal cancer (CRC) is the third most common cancer in the world. An estimated 1.36 million people worldwide were diagnosed with CRC in 2012, accounting for approximately 10% of total cancer cases.(1) Surgery, chemotherapy, and radiotherapy are the primary treatments for CRC.(2) However, long-term survival rates remain extremely poor. In particular, development of drug resistance by human CRC cells is a primary cause of therapy failure, an outcome that usually leads to death.(3) Therefore, it is critical to define mechanisms underlying development of drug resistance by tumor cells.
Inflammation plays a key role at various stages of CRC development, including initiation, growth, proliferation, survival, invasion, and metastasis. Recently, we reported that angiopoietin-like protein 2 (ANGPTL2) acts as a chronic inflammatory mediator in various oncogenic settings.(4–6) For example, we showed that increased ANGPTL2 expression in skin tissues promotes inflammation and accelerates carcinogenesis in a chemically-induced skin squamous cell carcinoma mouse model by increasing susceptibility to “preneoplastic change” and “malignant conversion.”(7,8) We have also shown that lung and breast tumor cells expressing ANGPTL2 show high metastatic potential and acquire invasive and high motility phenotypes.(9) However, it remains unclear whether ANGPTL2 functions in development of resistance to antitumor chemotherapy.
The spleen tyrosine kinase (Syk) is a 72-kDa non-receptor tyrosine kinase that is most highly expressed in hematopoietic cells,(10) and Syk signaling was initially thought to be restricted to the immune response. However, Syk-deficient embryos show defects in lymphatic vascular development, and Syk is now believed to function in multiple activities.(10) Syk activation also initiates cell survival signals, including phosphoinositide 3-kinase (PI3K), nuclear factor-κB (NF-κB), ERK–MAPK, and nuclear factor of activated T cells (NFAT) pathways.(10,12,13)
In the present study, we investigated whether ANGPTL2 expression affects tumor cell death by treating CRC cells with antineoplastic drugs. We found that ANGPTL2 overexpression in SW480 cells reduced apoptotic cell death induced by treatment with the cytotoxic drug 5-fluorouracil (5-FU). Furthermore, expression of anti-apoptotic BCL-2 and BCL-XL was upregulated in SW480/ANGPTL2 cells in response to Syk-dependent activation of PI3K and NF-κB. Collectively, our findings provide strong evidence that ANGPTL2 activates an anti-apoptotic pathway in tumor cells treated with cytotoxic drugs, suggesting that ANGPTL2 overexpression in those cells constitutes a mechanism underlying drug resistance.
Materials and Methods
Cell culture
The human CRC cell line SW480, which was purchased from the ATCC (Manassas, VA, USA), was cultured in Leibovitz's medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% FCS at 37°C in a humidified 5% CO2 atmosphere.
Plasmid transfection
To create stably transfected SW480 cells, ANGPTL2 or control vectors(9) were transfected into cells using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. Transfected lines were selected in 400 μg/mL G418 (Merck KGaA, Darmstadt, Germany).
Quantitation of ANGPTL2 protein by ELISA
Human CRC cell lines were grown to confluency. The medium was then changed, cells were maintained for 24 h, and then medium was collected to quantify ANGPTL2 protein by ELISA. ANGPTL2 concentrations in the medium were estimated using an ANGPTL2 Assay Kit (IBL, Fujioka, Japan) according to the manufacturer's instructions.
Proliferation assay
A viability assay was carried out using the Cell Counting Kit-8 (Dojindo, Kumamoto, Japan). Five thousand cells were added to a 96-well plate and incubated for 24 h. To investigate cell growth in vitro at 24, 48 and 72 h after seeding, 10 μL Cell Counting Kit-8 reagent was added to each well, plates were incubated for 3 h, and the optical density at 450 nm was measured. For the growth inhibition assay, the experiment was done in a similar manner after treatment with or without 10 μg/mL the following reagents: 5-FU (Wako, Osaka, Japan), CPT-11 (irinotecan; ChromaDex, Irvine, CA, USA), CDDP (cisplatin; Wako), or MMC (mitomycin C; Wako).
Flow cytometry analysis of apoptosis
Cells were plated for 24 h before induction of apoptosis. After treatment with or without 5-FU, CPT-11, CDDP, or MMC (10 μg/mL each), cells were detached with Accutase (Sigma-Aldrich, St. Louis, MO, USA) and double-stained with annexin V–FITC (eBioscience, San Diego, CA, USA) and 7-amino-actinomycin D (7-AAD; Beckman Coulter, Brea, CA, USA), according to the manufacturer's protocol. Samples were immediately analyzed by FACSCalibur using CellQuest (BD Biosciences, Franklin Lakes, NJ, USA) and FlowJo software (Tree Star, Ashland, OR, USA). The apoptosis proportion was defined as the percentage of the annexin V–FITC-positive cells among total cells.
Real-time quantitative RT-PCR
Total RNA was isolated from cells using TRIzol reagent (Invitrogen). DNase-treated RNA was reverse-transcribed with a PrimeScript RT reagent Kit (Takara Bio, Otsu, Japan). The PCR products were analyzed using a Thermal Cycler Dice Real Time System (Takara Bio), and relative transcript abundance was normalized to that of 18S mRNA. Oligonucleotides used for PCR are listed in Table S1.
Immunoblot analysis and antibodies
Cells were homogenized in lysis buffer (10 mM NaF, 1 mM Na3VO4, 1 mM Na4P2O7, 1 mM EDTA, 150 mM NaCl, 20 mM HEPES-KOH, 1% Triton X-100, pH 7.4). Extracts derived from supernatants were subjected to SDS-PAGE, and proteins were electrotransferred to nitrocellulose membranes. Immunodetection was carried out using an ECL kit (GE Healthcare, Little chalfont, Buckingham shire, UK) according to the manufacturer's protocol. The following antibodies were purchased: goat anti-hANGPTL2 polyclonal antibody from R&D Systems (Minneapolis, MN, USA), mouse anti-Hsc70 mAb from Santa Cruz Biotechnology (Dallas, TX, USA), and rabbit anti-Syk, mouse anti-phospho-Syk, rabbit anti-BCL-2, and rabbit anti-BCL-XL mAbs from Cell Signaling Technology (Danvers, MA, USA). A rabbit anti-GAPDH polyclonal antibody was purchased from Imgenex (San Diego, CA, USA).
Immunohistochemical staining
For NF-κB p65 staining, cells were fixed in acetone and ethanol (1:1) for 20 min, and then non-specific binding was minimized by blocking with 2% BSA. Cells were incubated with anti-NF-κB p65 polyclonal antibodies or anti-NFATc3 polyclonal antibodies (both from Santa Cruz Biotechnology) at 1 μg/mL and then with Alexa 488-conjugated anti-rabbit antibodies (Invitrogen). Nuclei were counterstained with DAPI (Funakoshi, Tokyo, Japan). The proportion of cells showing nuclear staining for the NF-κB subunit p65 was calculated as a percentage of DAPI-positive total cells in four random fields of view per cell line.
Nuclear factor-κB and PI3K inhibition assay
For treatment with the NF-κB inhibitor BAY11-7085 (Sigma-Aldrich), cells were incubated with 10 μM BAY11-7085 under serum-free conditions for 1 h and then cultured in normal growth medium for 24 h. For studies using the PI3K inhibitor LY294002 (Sigma-Aldrich), cells were incubated with 50 μM LY294002 for 24 h in normal growth medium.
RNA sequencing
Sample libraries from SW480/ANGPTL2-1 and SW480/Ctrl cells were prepared using a TruSeq RNA Sample Prep Kit (Illumina, San Diego, CA, USA), and sequencing runs were carried out on an Illumina Genome Analyzer IIx; 38-bp reads were mapped to the human genome (hg19 from the UCSC genome browser database at https://genome.ucsc.edu/) using TopHat version 2.0.0.(14) Only reads with a Phred quality score ≥25 were analyzed. For filtering rRNA and tRNA, the BEDtools package(15) was used with rRNA and tRNA annotations downloaded from the UCSC table browser.
To evaluate differential expression, expression data was normalized, and gene annotations were added using RegionMiner with Genomatix Genome Analyzer software (Genomatix, Munich, Germany) software. The normalized expression value (NE-value) is based on the following formula:(16)
To summarize NE-value information in individual genes, the mean of NE-values were calculated from all transcripts in a gene. Then we calculated the log2-transformed fold changes between SW480/ANGPTL2-1 and SW480/Ctrl cells.
Syk knockdown
SW480 cells were reseeded in six-well plates (AGC Techno Glass, Yoshida, Japan) with Syk siRNA (SYK [ID 6850] Trilencer-27 human siRNA; OriGene, Rockville, MD, USA). As a control, we used Trilencer-27 Universal Scrambled Negative Control (OriGene). Apoptosis was then analyzed by FACSCalibur using CellQuest and FlowJo software after 5-FU treatment (10 g/mL). Total protein was extracted for immunoblot analysis, and Syk protein expression after 72 h of incubation was analyzed.
Flow cytometry analysis of integrin expression
Cells were incubated with 10 μg/mL anti-α5β1 (JBS5), anti-αvβ3 (LM609), anti-αvβ5 (P1F6) (all Millipore, Temecula, CA, USA), anti-α4 (2B4) and anti-β2 (both R&D Systems), or respective isotype-matched control IgG for 30 min at 4°C. After washing, cells were incubated with 5 μg/mL FITC-conjugated goat anti-mouse IgG for another 30 min at 4°C, washed twice, and then analyzed by FACS using CellQuest software.
Adhesion assay
Assays were carried out as described.(4) In brief, 96-well flat-bottomed plates (MaxiSorp; Nunc, Roskilde, Denmark) were coated overnight at 4°C with various concentrations of ANGPTL2 protein and blocked with 3% BSA in PBS for 1 h at 37°C. Cells were diluted to 105 cells/mL in serum-free medium and pre-incubated for 30 min at 37°C with or without the following inhibitors: anti-α5β1 (JBS5), anti-αvβ5 (P1F6) (Millipore), RGD or RGE peptide (Sigma-Aldrich). Cell suspensions were seeded into wells of coated plates and incubated at 37°C for 2 h. Non-adherent cells were removed by gentle washing with PBS, and then adherent cells were fixed with 4% paraformaldehyde in PBS for 30 min and stained with 0.5% crystal violet in 25% methanol for 30 min. Plates were rinsed with tap water, stained cells were solubilized in 1% SDS, and the OD595 values were determined.
Characterization of patient samples
A total of 232 consecutive patients with unresectable CRC underwent systemic chemotherapy at Kumamoto University Hospital (Kumamoto, Japan) between April 2005 and December 2013. Patients received oxaliplatin- or irinotecan-based chemotherapy. We then obtained paraffin-embedded tumor samples from biopsy specimens of primary tumors from 92 patients enrolled in the study. Immunohistochemical staining of ANGPTL2 was performed as described.(9) Data collected from inpatient and outpatient records included demographic data (age and sex), tumor-specific data (tumor location, timing of metastases, and carcinoembryonic antigen levels at diagnosis), pathologic data (differentiation of primary tumor), and survival data (relapse-free survival and overall survival). We assessed the objective response according (ORR) to RECIST version 1.1 (PAREXEL, Waltham, MA, USA), and progression-free and overall survival using the Kaplan–Meier method, and compared them with log–rank tests using a significance level of 0.05. This study was approved by the Ethics Committees of Kumamoto University. Written informed consent was obtained from each subject.
Results
Expression of ANGPTL2 decreases 5-FU-induced apoptosis of colorectal cancer cells
To assess a potential role for ANGPTL2 in enhancing survival of CRC cells treated with antineoplastic drugs, we established two independent SW480 lines constitutively expressing ANGPTL2 (SW480/ANGPTL2-1 and -2) and a control line expressing vector only (SW480/Ctrl). Western blotting and ELISA analyses confirmed that both SW480/ANGPTL2-1 and -2 cells showed similarly enhanced expression of ANGPTL2 protein in both cell lysates and in culture medium relative to SW480/Ctrl cells (Fig. 1a,b). However, we observed no significant difference in proliferation among all three lines at 24, 48 and 72 h after plating (Fig. 1c), indicating that ANGPTL2 protein secreted by CRC cells does not alter proliferation under normal growth conditions. Next, we carried out a cell viability assay 24 h after treatment of CRC cells with the apoptosis-inducing drug 5-FU.(17) Constitutive ANGPTL2 expression in SW480/ANGPTL2-1 and -2 cells increased cell viability following 5-FU treatment relative to control drug-treated control cells (SW480/Ctrl) (Fig. 1d). Thus, we used flow cytometry to evaluate the proportion of apoptosis following 5-FU treatment using double-staining with annexin V and 7-AAD. That proportion significantly decreased in SW480/ANGPTL2-1 or -2 compared to SW480/Ctrl cells (Fig. 1e). We also examined cell viability after treatment of cells with other antineoplastic drugs used to treat CRC, including irinotecan (CPT11), cisplatin (CDDP), and mitomycin C (MMC),(2,18) and observed similar results (Fig. S1). These findings suggest that constitutive expression of ANGPTL2 in SW480 cells increases their chemoresistance by inhibiting apoptosis.
Fig. 1.
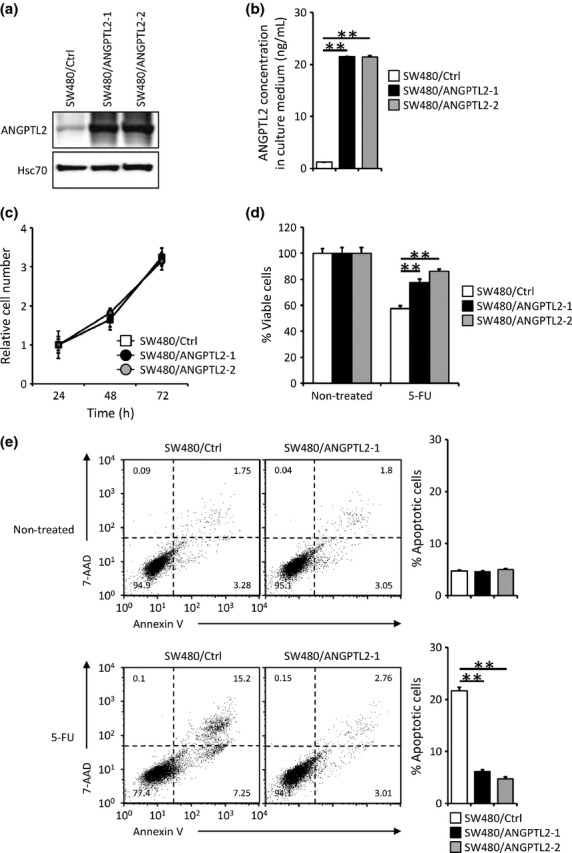
Angiopoietin-like protein 2 (ANGPTL2) expression decreases 5-fluorouracil (5-FU)-induced apoptosis in colorectal cancer cells. (a) Representative images showing Western blot analysis of SW480 control cells (SW480/Ctrl) and ANGPTL2-expressing SW480 cells (SW480/ANGPTL2-1 and -2) with an ANGPTL2 antibody. Hsc70 serves as an internal control. (b) ANGPTL2 protein concentrations in the culture medium of SW480/Ctrl, SW480/ANGPTL2-1, and SW480/ANGPTL2-2 cells (n = 3). (c) The relative number of proliferating cells (SW480/Ctrl, SW480/ANGPTL2-1, and SW480/ANGPTL2-2) at indicated time points during in vitro culture (n = 3). (d) Percentage of viable cells among non-treated (left) or 5-FU treated (right) SW480/Ctrl, SW480/ANGPTL2-1, or SW480/ANGPTL2-2 cells (n = 4). (e) FACS analysis of apoptotic cells among non-treated (upper) or 5-FU-treated (lower) SW480/Ctrl, SW480/ANGPTL2-1, or SW480/ANGPTL2-2 cells based on analysis of 7-aminoactinomycin D (7-AAD) and annexin V. Right panels show quantitative analysis of the percentage of apoptotic (annexin V-positive) cells (n = 3). Error bars show SEM. **P < 0.01.
Expression of anti-apoptotic BCL-2 family genes upregulated by ANGPTL2
The BCL-2 family is a key regulator of programmed cell death.(11) It is comprised of both pro- and anti-apoptotic proteins, and the latter (BCL-2, BCL-XL and MCL-1) reportedly inhibit 5-FU-induced apoptosis in CRC cells.(19) Therefore, we examined the expression of BCL-2 family mRNAs in SW480/ANGPTL2-1 and -2 cells or SW480/Ctrl cells. Real-time PCR and Western blot analyses revealed upregulated expression of anti-apoptotic BCL-2 and BCL-XL in SW480/ANGPTL2-1 and -2 cells relative to SW480/Ctrl cells; however, we observed no significant difference in expression of MCL-1 or pro-apoptotic BAX or BAD among groups (Figs 2a,S2). Recently, several reports suggested that a high BCL-2/BAX or BCL-XL/BAX ratio is positively correlated with progression of several diseases or malignant tumors and that the BCL-2/BAX ratio may serve as a prognostic marker for patients with rectal carcinomas.(19,20) Therefore, we analyzed that ratio in SW480/ANGPTL2-1, SW480/ANGPTL2-2, and SW480/Ctrl cells and found that BCL-2/BAX and BCL-XL/BAX ratios were greater in both ANGPTL2-overexpressing lines than they were in controls (Fig. 2b).
Fig. 2.
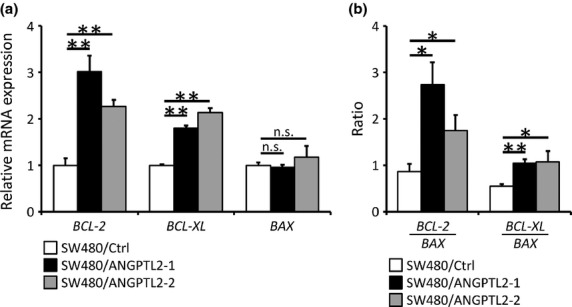
Angiopoietin-like protein 2 (ANGPTL2) induces expression of anti-apoptotic BCL-2 family members. (a) Relative mRNA expression of the anti-apoptotic BCL-2 family members BCL-2 (left) and BCL-XL (middle) and of pro-apoptotic BAX (right) in SW480/ANGPTL2-1, SW480/ANGPTL2-2, or SW480/Ctrl cells. Data from SW480/Ctrl was set at 1 (n = 3). (b) BCL-2/BAX and BCL-XL/BAX ratios in SW480/Ctrl, SW480/ANGPTL2-1, or SW480/ANGPTL2-2 cells (n = 3). Error bars show SEM. *P < 0.05; **P < 0.01. n.s., no statistically significant difference.
ANGPTL2 antagonizes apoptosis by increasing Syk expression
To examine signaling downstream of ANGPTL2 that might induce anti-apoptotic genes, we carried out RNA sequencing analysis of SW480/ANGPTL2-1 and SW480/Ctrl cells (Fig. 3a). We found that levels of 10 transcripts, including ANGPTL2 itself, were markedly increased in SW480/ANGPTL2-1 compared with SW480/Ctrl cells. As BCL-2 and BCL-XL were induced more robustly in SW480/ANGPTL2 compared to SW480/Ctr cells (Fig. 2), we checked their expression by RNA sequencing but did not observe significant induction of either gene using this method. However, we observed upregulation of Syk, which plays a role in cell survival,(10,12,13) in SW480/ANGPTL2 cells. Real-time PCR analysis validated that Syk expression levels in SW480/ANGPTL2-1 and -2 cells increased compared with levels seen in control SW480/Ctrl cells (Fig. 3b). Tyrosine phosphorylation is required to activate Syk kinase activity and function.(21) Thus, we examined Syk activity in SW480/ANGPTL2-1 and -2 versus control cells by Western blotting with a phospho-Syk antibody. Syk expression and phosphorylation was markedly increased in SW480/ANGPTL2-1 and -2 cells compared with SW480/Ctrl cells (Fig. 3c), suggesting that the enhanced cell survival activity seen in both lines is due to Syk upregulation and activation. Next, we asked whether Syk knockdown by siRNA in ANGPTL2-expressing SW480 cells would reduce cell viability or induce cell apoptosis following 5-FU treatment. We confirmed Syk knockdown levels in SW480/ANGPTL2-1 cells by Western blot analysis. Total Syk and phospho-Syk protein were concomitantly reduced by knockdown (Fig. 3d). We observed no significant difference in proliferation between knockdown versus scrambled control cells at 24, 48 or 72 h after plating in normal growth conditions (Fig. 3e). However, Syk knockdown cells (siRNA/A, B, and C) showed decreased viability following 5-FU treatment compared with scrambled control (siRNA Scr) cells (Fig. 3f). In addition, the proportion of apoptosis significantly increased in siRNA/A, B, and C cells compared to siRNA Scr cells (Fig. 3g). Thus, we conclude that Syk upregulation and activation enhances cell survival in ANGPTL2-overexpressing CRC cells.
Fig. 3.
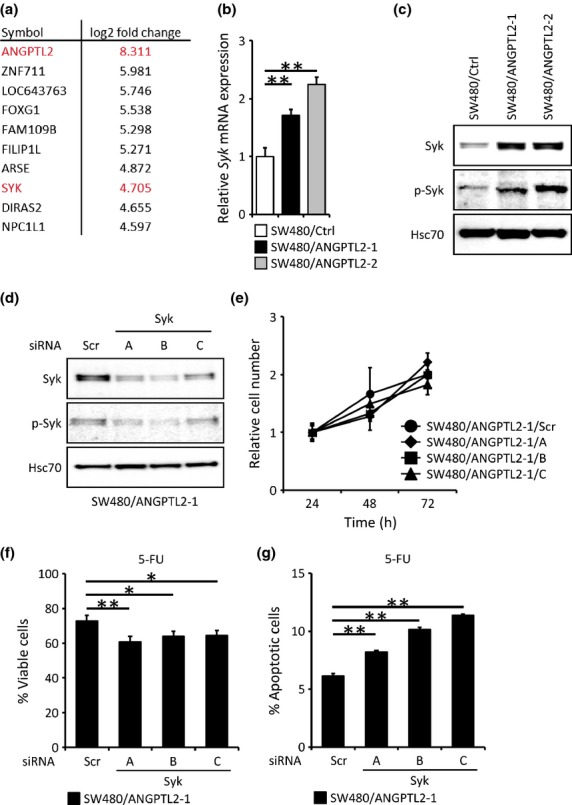
Angiopoietin-like protein 2 (ANGPTL2) blocks SW480 cell apoptosis by increasing spleen tyrosine kinase (Syk) expression. (a) Genes increased in SW480/ANGPTL2-1 cells based on RNA sequencing analysis. Data are log2 fold-change relative to SW480/Ctrl cells. (b) Relative Syk mRNA expression in SW480/Ctrl, SW480/ANGPTL2-1, or SW480/ANGPTL2-2 cells. Data from SW480/Ctrl cells was set at 1 (n = 3). (c) Western blotting analysis of Syk, phosphorylated Syk (p-Syk), and Hsc70 in SW480/Ctrl, SW480/ANGPTL2-1, and SW480/ANGPTL2-2 cells. Hsc70 serves as an internal control. (d) Western blot analysis of Syk, p-Syk, and Hsc70 in SW480/ANGPTL2-1 cells transduced with three Syk siRNAs (A, B, or C) or with control siRNA (Scr). Hsc70 serves as an internal control. (e) The relative number of proliferating cells (SW480/ANGPTL2-1/Scr, SW480/ANGPTL2-1/A, SW480/ANGPTL2-1/B, or SW480/ANGPTL2-1/C cells) at indicated time points of in vitro culture (n = 4). (f) Percentage of viable cells remaining after 5-fluorouracil (5-FU) treatment of SW480/ANGPTL2-1/Scr, SW480/ANGPTL2-1/A, SW480/ANGPTL2-1/B, or SW480/ANGPTL2-1/C cells (n = 4). (g) Quantitative analysis of the proportion of apoptotic cells following 5-FU treatment of SW480/ANGPTL2-1/Scr, SW480/ANGPTL2-1/A, SW480/ANGPTL2-1/B, or SW480/ANGPTL2-1/C cells based on annexin V staining (n = 3). Error bars show SEM. *P < 0.05; **P < 0.01.
ANGPTL2 activates the PI3K–NF-κB pathway and induces expression of anti-apoptotic BCL-2 family members
Nuclear factor-κB induces BCL-2 family member genes(22) and is known to be activated by Syk signaling.(10) To further investigate molecular mechanisms underlying ANGPTL2 anti-apoptotic activity, we examined NF-κB activation in SW480/ANGPTL2-1 cells and SW480/Ctrl cells by staining with a p65 antibody (Fig. 4a). Analysis of staining distribution indicated that the proportion of NF-κB subunit p65 found in the nucleus increased in SW480/ANGPTL2 cells compared with control cells, which showed largely cytoplasmic staining (Fig. 4a,b). It has been reported that PI3K activates the NF-κB pathway and induces survival signals.(23) To investigate whether NF-κB activation in SW480/ANGPTL2 cells was due to activation of the PI3K pathway, we assessed p65 translocation to the nucleus in the presence of the PI3K inhibitor LY294002. Treatment of SW480/ANGPTL2-1 cells with LY294002 significantly decreased the proportion of nuclear NF-κB relative to untreated cells (Fig. 4a,c). When we asked whether Syk knockdown would alter NF-κB nuclear translocation in SW480/ANGPTL2-1 cells, we found that knockdown decreased NF-κB nuclear translocation relative to that seen in Scr control cells (Fig. S3a). Taken together, these results suggest that ANGPTL2 overexpression promotes nuclear translocation of NF-κB through the Syk–PI3K pathway in CRC cells.
Fig. 4.
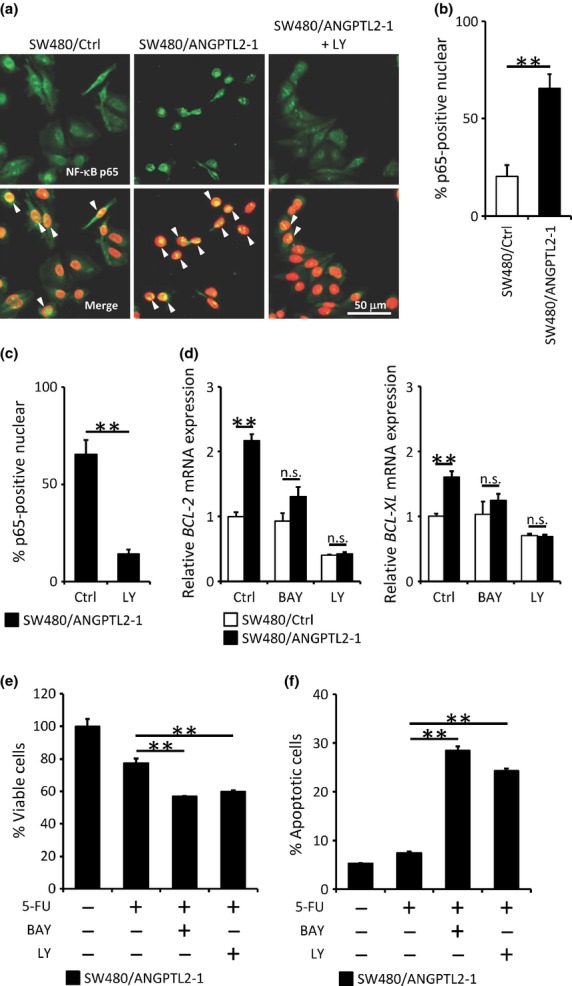
Induction of anti-apoptotic BCL-2 family members occurs through phosphoinositide 3-kinase–nuclear factor-κB (PI3K–NF-κB) signaling. (a) Nuclear translocation of NF-κB p65 in SW480/Ctrl and SW480/ANGPTL2-1 cells. Nuclei were counterstained with DAPI. Arrowheads indicate overlap of p65 and DAPI staining. Scale bar = 50 μm. (b,c) Quantification of p65-positive nuclei in (a). The proportion of cells showing nuclear staining for the NF-κB subunit p65 was calculated as a percentage of DAPI-positive total cells in four random fields of view per cell line (n = 4). (d) Relative mRNA expression of the anti-apoptotic BCL-2 family members BCL-2 (left) and BCL-XL (right) in SW480/Ctrl or SW480/ANGPTL2-1cells, either non-treated (Ctrl) or treated with NF-κB (BAY) or PI3K (LY) inhibitors. Data from non-treated SW480/Ctrl cells was set at 1 (n = 3). (e) Percentage of viable SW480/ANGPTL2-1 cells treated with or without various combinations of 5-fluorouracil (5-FU), BAY11-7085 (BAY), and LY294002 (LY) (n = 4). (f) Quantitative analysis of the proportion of apoptotic cells seen among SW480/ANGPTL2-1cells treated with or without various combinations of 5-FU, BAY, and LY based on FACS analysis (n = 3). Error bars show SEM. **P < 0.01. n.s., no statistically significant difference.
To determine whether upregulation of BCL-2 and BCL-XL in ANGPTL2-overexpressing cells correlates positively with PI3K and NF-κB activation, we treated SW480/ANGPTL2-1 cells with the NF-κB inhibitor BAY11-7085 or the PI3K inhibitor LY294002 and assessed levels of BCL-2 and BCL-XL mRNAs by real-time PCR. We confirmed that BAY11-7085 treatment suppresses expression of the NF-κB targets interleukin-1β and tumor necrosis factor-α using real-time PCR analysis (Fig. S3b). We observed that BCL-2 and BCL-XL upregulation in SW480/ANGPTL2 cells was suppressed by treatment with either BAY11-7085 or LY294002 (Figs 4d,S3c). Furthermore, treatment of SW480/ANGPTL2-1 cells with BAY11-7085 or LY294002 decreased cell viability following 5-FU treatment (Fig. 4e), and the proportion of apoptosis induced by 5-FU significantly increased in the presence of either inhibitor (Fig. 4f). These results suggest that ANGPTL2 activates PI3K/NF-κB signaling to induce expression of the anti-apoptotic BCL-2 family members BCL-2 and BCL-XL.
Next, we examined how ANGPTL2 activates Syk-PI3K-NF-κB signaling. We previously reported that ANGPTL2 binds to integrin α5β1 and activates NF-κB signaling.(4) In addition, another group reported that ANGPTL2 binds to leukocyte Ig-like receptor B2 (LILRB2) and promotes expansion of human hematopoietic stem cells.(24) Thus, we asked whether SW480 cells express integrins or LILRB2. Although LILRB2 was not expressed in SW480 cells (Fig. S4a), integrin α5β1 and αvβ5 were (Fig. S4b). Thus, we analyzed cell adhesion in the presence of a series of function-blocking antibodies for specific integrins. Neutralizing antibodies for integrin α5β1 and αvβ5 inhibited SW480 cell adhesion to ANGPTL2-coated plates, as did RGD peptide, which blocks RGD-dependent integrins (Fig. S4c). These results confirm that ANGPTL2 binds integrin α5β1 and αvβ5 in SW480 cells. However, when we tested whether neutralizing antibody for integrin α5β1 or αvβ5 blocked Syk induction in SW480/ANGPTL2 cells, we did not observed suppression of Syk induction. We conclude, therefore, that an unknown ANGPTL2 receptor mediates Syk induction in CRC.
ANGPTL2 positively regulates itself by activating the Syk–NFAT pathway in colorectal cancer cells
Syk reportedly activates NFAT signaling.(25) In addition, we previously reported that NFATc induces ANGPTL2 expression in tumor cells.(10) These findings suggest that ANGPTL2 increases its own expression through Syk–NFAT signaling. To examine this possibility, we evaluated transcript levels of NFATc family genes in SW480/ANGPTL2-1 or SW480/Ctrl cells by real-time PCR. Our analysis showed that SW480 cells express NFATc3 mRNA rather than other NFATcs (Fig. 5a). In normal conditions, inactive NFATc proteins are cytoplasmic.(26) When cells are activated, intracellular Ca2+ concentrations increase and NFATc proteins move to the nucleus to activate target genes, including ANGPTL2.(9) Therefore, we examined intracellular localization of NFATc3 in SW480/ANGPTL2-1 or SW480/Ctrl cells. NFATc3 nuclear staining was more apparent in SW480/ANGPTL2 cells compared to control cells (Fig. 5b), suggesting that NFATc3 is activated in ANGPTL2-overexpressing cells.
Fig. 5.
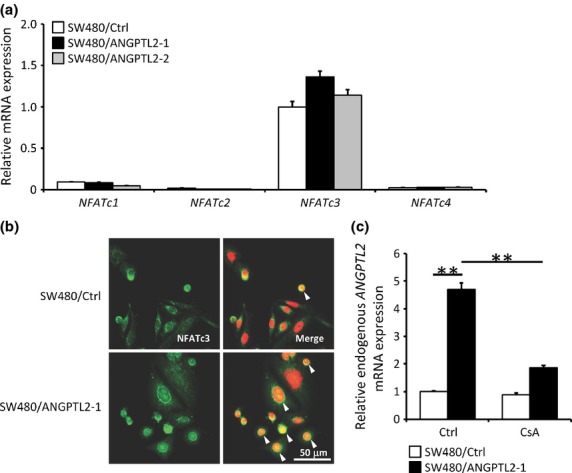
Angiopoietin-like protein 2 (ANGPTL2) positively regulates itself by activating the spleen tyrosine kinase–nuclear factor of activated T cells (Syk–NFAT) pathway in colorectal cancer cells. (a) Relative mRNA expression of NFAT family members in SW480/Ctrl, SW480/ANGPTL2-1, or SW480/ANGPTL2-2 cells (n = 3). (b) Nuclear translocation of NFATc3 in SW480/Ctrl or SW480/ANGPTL2-1 cells. Nuclei were counterstained with DAPI. Arrowheads indicate overlap of NFATc3 and DAPI staining. Scale bar = 50 μm. (c) Relative expression of endogenous ANGPTL2 mRNA in SW480/Ctrl or SW480/ANGPTL2-1 cells, either non-treated (control) or treated with the NFAT inhibitor cyclosporin A (CsA) (n = 3). Data from non-treated SW480/Ctrl cells was set at 1. Error bars show SEM. **P < 0.01.
We next examined whether ANGPTL2 autoregulates its own expression. To detect endogenous ANGPTL2 mRNA, we used primers recognizing the ANGPTL2 3′-UTR, as those regions are absent in the ANGPTL2 expression vector. We found that expression of endogenous ANGPTL2 in SW480/ANGPTL2-1 cells was 4.7-times greater than it was in SW480/Ctrl cells (Fig. 5c, left columns). Increased expression of endogenous ANGPTL2 seen in those cells was effectively suppressed by treatment with cyclosporin A, which inhibits calcineurin and suppresses NFAT activation (Fig. 5c, right columns). BCL-2 mRNA expression was minimally affected by cyclosporin A treatment (Fig. S5). Overall, these results suggest that ANGPTL2 increases expression of itself through a feedback loop by activating the Syk–NFAT pathway in CRC cells.
Higher ANGPTL2 expression in primary tumor tissues from colorectal cancer patients reflects a lower ORR
We next carried out ANGPTL2 immunostaining using tissues from 92 CRC patients with unresectable tumors in order to correlate ANGPTL2 expression with the ORR to chemotherapy (Fig. 6, Table S2). Patients were placed into high- and low-ANGPTL2 groups: the high group showed ≥50% of ANGPTL2-positive tumor cells and the low showed <50% (Fig. 6a). The percentage of patients who showed a response to chemotherapy was significantly lower in the high-ANGPTL2 than the low-ANGPTL2 group (Fig. 6b, P < 0.05), although progression-free survival and overall survival did not differ significantly between groups (data not shown). This analysis suggests that high ANGPTL2 expression in CRC cells is associated with chemoresistance.
Fig. 6.
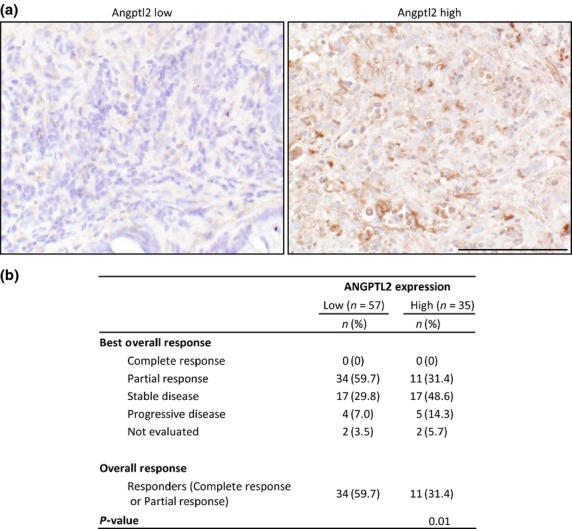
Higher angiopoietin-like protein 2 (ANGPTL2) expression in primary tumor tissues from colorectal cancer patients correlates with a lower objective response rate. ANGPTL2 immunostaining in primary tumors from CRC patients. (a) Representative images of ANGPTL2 low (<50% ANGPTL2-positive tumor cells; left) and ANGPTL2-high (≥50% ANGPTL2-positive tumor cells; right) specimens. Scale bar = 100 μm. (b) Summary of overall responses to chemotherapy and ANGPTL2 expression in CRC patient specimens. P < 0.05 (Fisher's exact test).
Discussion
We previously showed that ANGPTL2 enhances acquisition of aggressive phenotypes underlying invasion and metastasis of tumor cells by activating Rac-dependent tumor cell migration,(7) increasing monocyte and macrophage infiltration, and enhancing tumor angiogenesis and lymphangiogenesis.(4,6) In this study, we show that ANGPTL2 also enhances resistance of CRC cells to cytotoxic, antineoplastic drugs by antagonizing apoptosis and that higher ANGPTL2 expression in primary tumor tissues from CRC patients is associated with a lower ORR. We also showed that ANGPTL2 increases BCL-2 and BCL-XL expression through Syk-dependent PI3K–NF-κB activation. This is the first report of a function for ANGPTL2 in promoting survival of tumor cells treated with antineoplastic drugs.
Cytotoxic drugs exert antitumor effects primarily by promoting apoptosis.(17,27) The tumor suppressor p53 promotes cell cycle arrest and/or apoptosis and is a molecular target of some of these drugs.(27) Several reports indicated that 5-FU-induced apoptosis requires p53 activity.(27,28) However, there are several reports that apoptosis occurs in mutant p53 cell lines by an unknown mechanism.(29–31) SW480 cells, examined here, harbor a loss-of-function mutation in p53, indicating that ANGPTL2-induced anti-apoptotic effects seen in these cells are p53-independent. In addition, it has been reported that epithelial–mesenchymal transition (EMT), which is regulated primarily by transforming growth factor (TGF)-β, contributes to drug resistance.(32) SW480 cells do not undergo an EMT and show attenuated TGF-β signaling due to mutations in TGF receptor 2.(33) Therefore, it seems unlikely that the EMT and/or TGF-β signaling contribute to ANGPTL2-induced anti-apoptotic effects in SW480 cells.
High BCL-2/BAX and BCL-XL/BAX ratios are inversely correlated with 5-FU sensitivity, independent of p53 status.(28) Accordingly, ANGPTL2-overexpressing SW480 cells show increased expression of BCL-2 and BCL-XL relative to untransfected SW480 cells and may become resistant to cell death because BCL-2 or BCL-XL levels relative to BAX are now sufficient to antagonize 5-FU-induced cytotoxicity. The PI3K pathway is a major signaling pathway that promotes cell survival.(34) Here, we show that the induction of BCL-2 and BCL-XL was strongly suppressed by treatment of cells with a PI3K inhibitor, indicating that ANGPTL2 induces expression of BCL-2 and BCL-XL transcripts through PI3K signaling, a conclusion consistent with our previous report that ANGPTL2 activates the PI3K–Akt pathway.(35)
The tyrosine kinase Syk, which is expressed in hematopoietic lineage cells, regulates downstream PI3K-AKT signaling pathways.(36) In this study, we also found abundant expression of Syk in SW480/ANGPTL2-1 cells, which may underlie upregulation of cell survival genes.
Syk also reportedly activates NFATc.(10) We previously showed that NFATc activation increased ANGPTL2 expression in human lung and breast cancer cells. Thus, Syk activation may increase ANGPTL2 expression. In agreement with this idea, we found that ANGPTL2 increases its own expression in a positive feedback loop through activating the Syk–NFAT pathway. Recently, we reported that properties of the tumor microenvironment, such as hypoxia and undernutrition, induce ANGPTL2 expression in tumor cells by demethylation of the ANGPTL2 promoter.(37) Taken together, these findings raise the possibility that tumor microenviromental changes drive resistance of tumor cells to antineoplastic drugs by accelerating ANGPTL2-induced anti-apoptotic effects.
In summary, we have shown that ANGPTL2 promotes CRC cell survival after antineoplastic drug treatment by regulating anti-apoptotic BCL-2 family genes through Syk–PI3K signaling. Furthermore, ANGPTL2 positively autoregulated its own expression through the Syk–NFAT pathway (Fig. 7). We also showed that CRC cells expressing relatively high levels of ANGPTL2 may develop resistance to chemotherapy. These findings possibly suggest novel approaches to counteracting chemoresistance based on attenuating ANGPTL2 signaling in tumor cells.
Fig. 7.
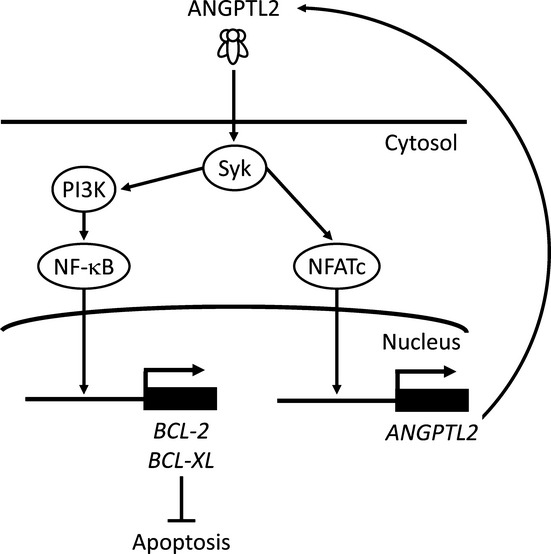
Model of tumor cell resistance to antineoplastic therapy through angiopoietin-like protein 2 (ANGPTL2) expression. Tumor cell-secreted ANGPTL2 induces spleen tyrosine kinase (Syk), which activates phosphoinositide 3-kinase (PI3K) and nuclear factor of activated T cells c (NFATc). The PI3K pathway activates nuclear factor-κB (NF-κB) signaling and induces expression of anti-apoptotic BCL-2 family members to inhibit apoptosis. In addition, ANGPTL2 upregulates itself through NFATc.
Acknowledgments
We thank K. Tabu, M. Nakata, and N. Shirai for technical assistance. This work was supported by JSPS KAKENHI Grant Number 25461192, 25870543 and 26116722, a research program of the Project for Development of Innovative Research on Cancer Therapeutics, the Ministry of Education, Culture, Sports, Science and Technology of Japan, and the Core Research for Evolutional Science and Technology program of the Japan Science and Technology Agency, and by grants from the Takeda Science Foundation and the Kobayashi Foundation for Cancer Research.
Disclosure Statement
The authors have no conflict of interest.
Supporting Information
Additional supporting information may be found in the online version of this article:
Fig. S1. Angiopoietin-like protein 2 (ANGPTL2) expression decreases apoptosis of colorectal cancer cells in response to various antineoplastic drugs.
Fig. S2. Induction of BCL-2 family members in angiopoietin-like protein 2 (ANGPTL2)-expressing or control cells.
Fig. S3. Spleen tyrosine kinase (Syk) knockdown attenuates nuclear factor-κB (NF-κB) activation induced by angiopoietin-like protein 2 (ANGPTL2).
Fig. S4. Integrins α5β1 and αvβ5 are expressed in SW480 cells but do not mediate spleen tyrosine kinase–phosphoinositide 3-kinase–nuclear factor κB (Syk–PI3K–NF-κB) signaling.
Fig. S5. BCL-2 induction is not dependent on the nuclear factor of activated T cells (NFAT) pathway.
Table S1. Sequences of primers used in real-time PCR analysis.
Table S2. Patient characteristics.
References
- 1.Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet] Lyon, France: International Agency for Research on Cancer; 2012. Available from URL: http://globocan.iarc.fr. [Google Scholar]
- 2.Andre T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–51. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 3.Longley D, Allen W, Johnston P. Drug resistance, predictive markers and pharmacogenomics in colorectal cancer. Biochim Biophys Acta. 2006;2:184–96. doi: 10.1016/j.bbcan.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Tabata M, Kadomatsu T, Fukuhara S, et al. Angiopoietin-like protein 2 promotes chronic adipose tissue inflammation and obesity-related systemic insulin resistance. Cell Metab. 2009;10:178–88. doi: 10.1016/j.cmet.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Okada T, Tsukano H, Endo M, et al. Synoviocyte-derived angiopoietin-like protein 2 contributes to synovial chronic inflammation in rheumatoid arthritis. Am J Pathol. 2010;176:2309–19. doi: 10.2353/ajpath.2010.090865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tazume H, Miyata K, Tian Z, et al. Macrophage-derived angiopoietin-like protein 2 accelerates development of abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. 2012;32:1400–9. doi: 10.1161/ATVBAHA.112.247866. [DOI] [PubMed] [Google Scholar]
- 7.Aoi J, Endo M, Kadomatsu T, et al. Angiopoietin-like protein 2 is an important facilitator of inflammatory carcinogenesis and metastasis. Cancer Res. 2011;71:7502–12. doi: 10.1158/0008-5472.CAN-11-1758. [DOI] [PubMed] [Google Scholar]
- 8.Aoi J, Endo M, Kadomatsu T, et al. Angiopoietin-like protein 2 accelerates carcinogenesis by activating chronic inflammation and oxidative stress. Mol Cancer Res. 2014;28:28. doi: 10.1158/1541-7786.MCR-13-0336. [DOI] [PubMed] [Google Scholar]
- 9.Endo M, Nakano M, Kadomatsu T, et al. Tumor cell-derived angiopoietin-like protein ANGPTL2 is a critical driver of metastasis. Cancer Res. 2012;72:1784–94. doi: 10.1158/0008-5472.CAN-11-3878. [DOI] [PubMed] [Google Scholar]
- 10.Mocsai A, Ruland J, Tybulewicz V. The SYK tyrosine kinase: a crucial player in diverse biological functions. Nat Rev Immunol. 2010;10:387–402. doi: 10.1038/nri2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Juin P, Geneste O, Gautier F, Depil S, Campone M. Decoding and unlocking the BCL-2 dependency of cancer cells. Nat Rev Cancer. 2013;13:455–65. doi: 10.1038/nrc3538. [DOI] [PubMed] [Google Scholar]
- 12.Sada K, Takano T, Yanagi S, Yamamura H. Structure and function of Syk protein-tyrosine kinase. J Biochem. 2001;130:177–86. doi: 10.1093/oxfordjournals.jbchem.a002970. [DOI] [PubMed] [Google Scholar]
- 13.Chen C, Martin V, Gorenstein N, Geahlen R, Post C. Two closely spaced tyrosines regulate NFAT signaling in B cells via Syk association with Vav. Mol Cell Biol. 2011;31:2984–96. doi: 10.1128/MCB.05043-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trapnell C, Pachter L, Salzberg S. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25:1105–11. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quinlan A, Hall I. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics. 2010;26:841–2. doi: 10.1093/bioinformatics/btq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Audic S, Claverie J. The significance of digital gene expression profiles. Genome Res. 1997;7:986–95. doi: 10.1101/gr.7.10.986. [DOI] [PubMed] [Google Scholar]
- 17.Parker W, Cheng Y. Metabolism and mechanism of action of 5-fluorouracil. Pharmacol Ther. 1990;48:381–95. doi: 10.1016/0163-7258(90)90056-8. [DOI] [PubMed] [Google Scholar]
- 18.Douillard J, Cunningham D, Roth A, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355:1041–7. doi: 10.1016/s0140-6736(00)02034-1. [DOI] [PubMed] [Google Scholar]
- 19.Violette S, Poulain L, Dussaulx E, et al. Resistance of colon cancer cells to long-term 5-fluorouracil exposure is correlated to the relative level of Bcl-2 and Bcl-X(L) in addition to Bax and p53 status. Int J Cancer. 2002;98:498–504. doi: 10.1002/ijc.10146. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, Yu J, Park B, Kinzler K, Vogelstein B. Role of BAX in the apoptotic response to anticancer agents. Science. 2000;290:989–92. doi: 10.1126/science.290.5493.989. [DOI] [PubMed] [Google Scholar]
- 21.de Castro R, Zhang J, Jamur M, Oliver C, Siraganian R. Tyrosines in the carboxyl terminus regulate Syk kinase activity and function. J Biol Chem. 2010;285:26674–84. doi: 10.1074/jbc.M110.134262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vogler M. BCL2A1: the underdog in the BCL2 family. Cell Death Differ. 2012;19:67–74. doi: 10.1038/cdd.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoesel B, Schmid J. The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer. 2013;12:1476–4598. doi: 10.1186/1476-4598-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng J, Umikawa M, Cui C, et al. Inhibitory receptors bind ANGPTLs and support blood stem cells and leukaemia development. Nature. 2012;485:656–60. doi: 10.1038/nature11095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goodridge H, Simmons R, Underhill D. Dectin-1 stimulation by Candida albicans yeast or zymosan triggers NFAT activation in macrophages and dendritic cells. J Immunol. 2007;178:3107–15. doi: 10.4049/jimmunol.178.5.3107. [DOI] [PubMed] [Google Scholar]
- 26.Pan M, Xiong Y, Chen F. NFAT gene family in inflammation and cancer. Curr Mol Med. 2013;13:543–54. doi: 10.2174/1566524011313040007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Longley D, Harkin D, Johnston P. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330–8. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 28.Nita M, Nagawa H, Tominaga O, et al. 5-Fluorouracil induces apoptosis in human colon cancer cell lines with modulation of Bcl-2 family proteins. Br J Cancer. 1998;78:986–92. doi: 10.1038/bjc.1998.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petak I, Tillman D, Houghton J. p53 dependence of Fas induction and acute apoptosis in response to 5-fluorouracil-leucovorin in human colon carcinoma cell lines. Clin Cancer Res. 2000;6:4432–41. [PubMed] [Google Scholar]
- 30.Backus H, Wouters D, Ferreira C, et al. Thymidylate synthase inhibition triggers apoptosis via caspases-8 and -9 in both wild-type and mutant p53 colon cancer cell lines. Eur J Cancer. 2003;39:1310–7. doi: 10.1016/s0959-8049(03)00204-1. [DOI] [PubMed] [Google Scholar]
- 31.Bunz F, Hwang P, Torrance C, et al. Disruption of p53 in human cancer cells alters the responses to therapeutic agents. J Clin Invest. 1999;104:263–9. doi: 10.1172/JCI6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29:4741–51. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coffey RJ, Shipley G, Moses H. Production of transforming growth factors by human colon cancer lines. Cancer Res. 1986;46:1164–9. [PubMed] [Google Scholar]
- 34.Fulda S. Modulation of mitochondrial apoptosis by PI3K inhibitors. Mitochondrion. 2013;13:195–8. doi: 10.1016/j.mito.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 35.Kubota Y, Oike Y, Satoh S, et al. Cooperative interaction of Angiopoietin-like proteins 1 and 2 in zebrafish vascular development. Proc Natl Acad Sci USA. 2005;102:13502–7. doi: 10.1073/pnas.0501902102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen L, Monti S, Juszczynski P, et al. SYK inhibition modulates distinct PI3K/AKT- dependent survival pathways and cholesterol biosynthesis in diffuse large B cell lymphomas. Cancer Cell. 2013;23:826–38. doi: 10.1016/j.ccr.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Odagiri H, Kadomatsu T, Endo M, et al. The secreted protein ANGPTL2 promotes metastasis of osteosarcoma cells through integrin α5β1, p38 MAPK, and matrix metalloproteinases. Sci Signal. 2014;7:2004612. doi: 10.1126/scisignal.2004612. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Angiopoietin-like protein 2 (ANGPTL2) expression decreases apoptosis of colorectal cancer cells in response to various antineoplastic drugs.
Fig. S2. Induction of BCL-2 family members in angiopoietin-like protein 2 (ANGPTL2)-expressing or control cells.
Fig. S3. Spleen tyrosine kinase (Syk) knockdown attenuates nuclear factor-κB (NF-κB) activation induced by angiopoietin-like protein 2 (ANGPTL2).
Fig. S4. Integrins α5β1 and αvβ5 are expressed in SW480 cells but do not mediate spleen tyrosine kinase–phosphoinositide 3-kinase–nuclear factor κB (Syk–PI3K–NF-κB) signaling.
Fig. S5. BCL-2 induction is not dependent on the nuclear factor of activated T cells (NFAT) pathway.
Table S1. Sequences of primers used in real-time PCR analysis.
Table S2. Patient characteristics.


