Abstract
Recent studies have reported that stromal cells contribute to tumor progression. We previously demonstrated that tumor endothelial cells (TEC) characteristics were different from those of normal endothelial cells (NEC). Furthermore, we performed gene profile analysis in TEC and NEC, revealing that suprabasin (SBSN) was upregulated in TEC compared with NEC. However, its role in TEC is still unknown. Here we showed that SBSN expression was higher in isolated human and mouse TEC than in NEC. SBSN knockdown inhibited the migration and tube formation ability of TEC. We also showed that the AKT pathway was a downstream factor of SBSN. These findings suggest that SBSN is involved in the angiogenic potential of TEC and may be a novel TEC marker.
Keywords: Angiogenesis, suprabasin, suprabasin signaling, tumor endothelial cell marker, tumor endothelial cells
Tumor angiogenesis is necessary for the progression of tumor growth and metastasis.(1,2) Because tumor blood vessels supply tumor cells with nutrients and oxygen, anti-angiogenesis treatment is recognized as a new cancer therapy.(3) Bevacizumab, anti-vascular endothelial growth factor (VEGF) antibody,(4) and sorafenib or sunitinib, a VEGF receptor kinase inhibitor, have been used as anti-angiogenic drugs.(5) However, there are negative reports regarding side effects and increases in metastasis have been observed. To overcome these problems, a new anti-angiogenic drug is required.(6) The morphology of tumor blood vessels is different from that of normal blood vessels.(7–9) Differences between tumor endothelial cells (TEC) and normal endothelial cells (NEC) in aspects, such as gene expression and biological behavior, have also been reported.(7,8,10) Recently, we revealed that TEC were more resistant to anti-cancer drugs compared with NEC.(11) In addition, inhibition of cyclooxygenase-2 or lysyl oxidase in TEC suppressed tumor growth and lung metastasis in vivo.(12,13) These findings indicate that TEC may be a good target for anti-cancer therapy. To identify specific TEC markers, we performed DNA microarray analysis and reported that some molecules were upregulated in TEC.(14–16) Among these molecules, suprabasin (SBSN) showed very high expression levels in several TEC.
Suprabasin has been identified as an epidermal differentiation marker and has been detected in the suprabasal layers of the epithelia in the epidermis, stomach and tongue in mice.(17,18) SBSN participates in the proliferation of normal small cell lung carcinoma cells.(19) The SBSN expression is also correlated with the growth and invasiveness of salivary gland adenoid cystic carcinoma and glioblastoma.(20,21) However, the details of SBSN's involvement in tumor malignancy and tumor angiogenesis are unknown.
In this study, we examined the SBSN expression and its function in TEC to determine whether SBSN is a potential TEC marker.
Materials and Methods
Cell lines and culture conditions
The human renal clear cell carcinoma cell OS-RC-2 was purchased from the RIKEN Cell Bank (Tsukuba, Japan) and cultured in RPMI1640 medium (Sigma–Aldrich, St. Louis, MO, USA) supplemented with 10% heat-inactivated FBS, 100 U/mL penicillin and 100 μg/mL streptomycin. A375SM cells, a super-metastatic human melanoma cell line, were a gift from Dr Isaiah J Fidler (MD Anderson Cancer Center, Houston, TX, USA). The cells were cultured in minimum essential medium (Gibco, Grand Island, NY, USA) supplemented with 10% FBS, 100 U/mL penicillin and 100 μg/mL streptomycin, as described previously.(15) In the growth factor experiments, NEC were treated with human EGF (AF-100-15; PeproTech, Rocky Hill, NJ, USA) at final concentrations of 5 and 15 ng/mL and human VEGF (100-20; PeproTech, Rocky Hill, NJ, USA) at final concentrations of 15 and 30 ng/mL for 12 h. These cells were cultured at 37°C in a humidified atmosphere of 5% CO2 and 95% air.
Isolation of tumor endothelial cells and normal endothelial cells
All procedures for animal experiments were approved by the local animal research authorities, and animal care was performed in accordance with institutional guidelines. Mouse TEC (mTEC) and NEC (mNEC) were isolated as previously described(12) with some modifications. Diphtheria toxin (500 ng/mL; Calbiochem, San Diego, CA, USA) was added to mTEC subcultures to kill any human tumor cells and to mNEC subcultures for technical consistency. Using an anti-human CD31 antibody, human TEC (hTEC) and NEC (hNEC) were isolated from excised renal cell carcinoma (RCC) or colon cancer tissues from patients at Hokkaido University Hospital. Normal renal or colon tissues were obtained from areas that were adjacent to the tumor in the same patient. Clinical background information is described in Supplementary Table S1. These protocols were approved by the Ethics Committee of Hokkaido University, and written informed consent was obtained from each patient before surgery. Endothelial cells (EC) were cultured as previously described.(22,23)
Reverse transcription and quantitative PCR
Total RNA was extracted from cells and human tumor and normal tissue samples using the ReliaPrep RNA Cell Miniprep System (Promega Corporation, Madison, WI, USA). Complementary DNA (cDNA) was synthesized using a ReverTra-Plus kit (Toyobo, Osaka, Japan). For relative quantification of target mRNA, we used SsoFast EvaGreen Supermix (CFX 96 Real-Time PCR Detection System; Bio-Rad, Hercules, CA, USA) for mouse EC and SYBR Green Real-time PCR Master Mix-Plus (Bio-Rad) for human EC (in triplicate) according to the manufacturer's instructions.(24) The quantitative PCR amplification program was performed at 95°C for 3 min and 45 cycles at 95°C for 10 s and 60°C for 30 s. Data were analyzed with CFX Manager software (Bio-Rad). The primers used are described in Supplementary Table S2. Each experiment included four PCR reactions, and each experiment was performed three times.
Western blotting
Western blotting analysis was performed as described previously.(25) This analysis used antibodies specific for total AKT, phosphorylated AKT (Cell Signaling Technology, Beverly, MA, USA), total Erk, phosphorylated Erk (Cell Signaling Technology), beta actin and an HRP-conjugated secondary antibody.
Immunostaining
Human tissue samples were obtained from excised RCC, normal renal tissue, colon cancer and normal colon tissues of patients at Hokkaido University Hospital. Frozen sections of excised tissues were prepared as previously described.(14,26) Human sections were double-stained with anti-human CD31/Alexa Fluor 594 rat anti-mouse IgG and anti-SBSN/Alexa Fluor 488 goat anti-rabbit IgG. All samples were counterstained with DAPI (Roche Diagnostics, Mannheim, Germany) and examined using an Olympus FluoView FV10i confocal microscope (Olympus, Tokyo, Japan).
For AKT staining, formalin-fixed paraffin-embedded specimens from two cases of colon cancer were prepared. Immunohistochemical analysis was performed using serial sections that were stained with anti-SBSN (1:250 dilution), AKT (1:100 dilution) and CD31 (Leica Microsystem, UK; 1:500 dilution), followed by antibody detection using a peroxidase-conjugated streptavidin-diaminobenzidine (DAB) readout system (DAKO), and counterstaining with DAPI. Images were randomly captured using a nanozoomer slide scanner and NDPViewer (Hamamatsu, Japan).
Suprabasin knockdown
siSBSN was transfected into cells using Lipofectamine transfection reagent (Invitrogen, Tokyo, Japan) according to the manufacturer's instructions. The sequence of siSBSN was 5′-UAUUGAUGCCUUCAAGGGCCUUGCC-3′ (siSBSN1) and 5′-UUCCCUUCCAGCUUGAGUGAUUCCG-3′ (siSBSN2). A nontargeting control siRNA was used (Invitrogen).
Cell migration assay
Cell migration toward VEGF-A was analyzed using a Boyden chamber (Neuro Probe, Gaithersburg, MD, USA), as previously described.(27) VEGF-A (10 ng/mL) was added to the lower chamber as a chemoattractant. TEC were treated with the control siRNA (10 nM) or siSBSN (10 nM) in endothelial basal medium (EBM)-2 supplemented with 0.5% FBS for 24 h. In total, 1.5 × 104cells were seeded in the upper chamber and incubated for 4 h at 37°C. The assays were independently performed three times.
Tube formation assay
A tube formation assay was performed as previously described.(26) EC were seeded at a density of 1.0 × 105 cells per well and incubated at 37°C on Matrigel (BD Biosciences, San Jose, CA, USA). Tube formation was observed using an inverted microscope by measuring the junction number of endothelial tubes. For inhibition experiments using the PI3 kinase inhibitor LY294002, TEC were preincubated for 2 h at 37°C in EBM2 supplemented with 0.5% FBS. To investigate the involvement of AKT in TEC tube formation, assays were performed with or without LY294002 (0, 10 or 20 μM). The assays were independently performed three times.
Cell proliferation assay
Cell proliferation was assessed with an MTS assay as described previously.(11) TEC were treated with the control siRNA (10 nM) or siSBSN (10 nM) in EBM2 supplemented with 0.5% FBS for 24 h. After siRNA transfection, 1.0 × 103 cells per well were seeded into 96-well plates in EBM2 supplemented with 5% FBS. Cell proliferation was measured daily for 3 days by the MTS assay. The assays were independently performed three times.
Statistical analysis
Results are given as mean ± SD. Group comparisons were made by one-way anova with the Tukey–Kramer multiple comparison test. When only two groups were compared, a two-sided Student's t-test was used. P < 0.05 was considered significant, and P < 0.01 was considered highly significant.
Results
Suprabasin was highly expressed in human tumor endothelial cells
To analyze the SBSN expression in hTEC and hNEC, we isolated hTEC from tissues of four cases of RCC and two cases of colon cancer. Furthermore, hNEC were isolated from the tissues of normal renal tissue and colon in the same patients.(14,28) The SBSN mRNA expression levels in hTEC isolated from RCC and colon cancer tissues were higher than those of hNEC (Fig. 1a,b). Double-immunofluorescence staining with anti-SBSN and anti-CD31 antibodies revealed that SBSN was markedly expressed in tumor blood vessels both in RCC and colon cancer, whereas the SBSN expression was low in normal blood vessels (Fig. 1c,d). In addition, SBSN mRNA expression levels were higher in human renal tumor tissues than those in normal tissues (Suppl. Fig. S1). These findings showed that SBSN was upregulated in hTEC from several tumor types.
Fig. 1.
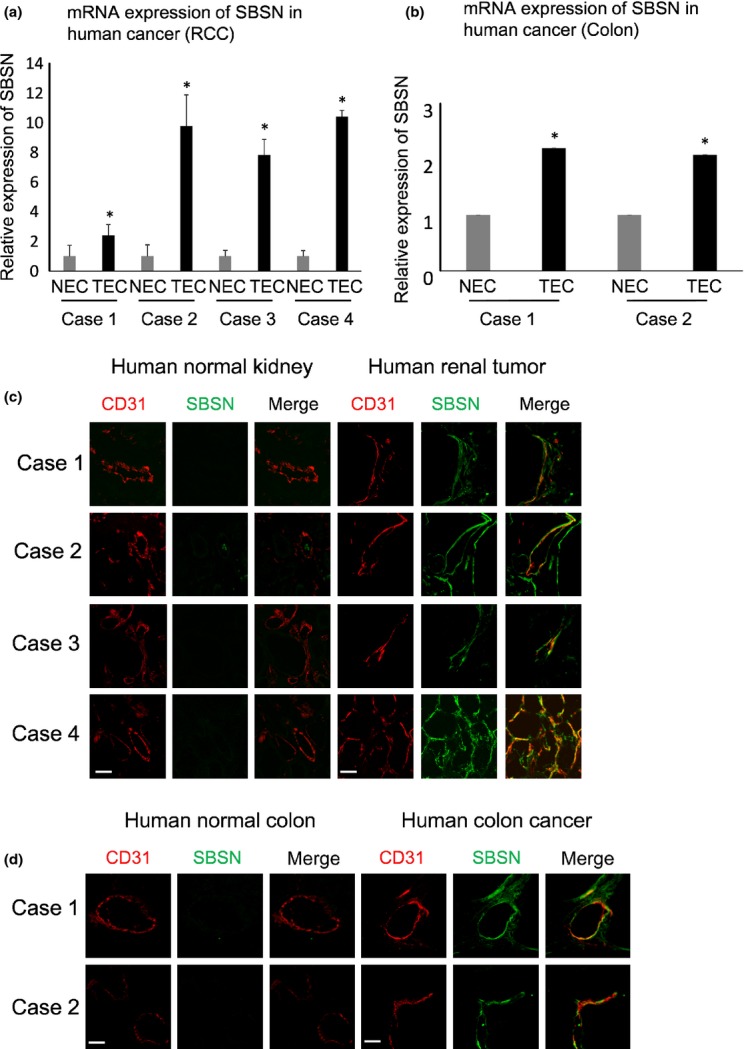
Suprabasin (SBSN) expression in human tumor endothelial cells (hTEC). (a, b) Relative SBSN mRNA expression levels in hNEC and hTEC evaluated by quantitative PCR (a, RCC, n = 4; b, colon tumor, n = 2). *P < 0.01 versus control; two-sided Student's t-test. (c, d) Clinical samples of renal cell carcinoma (RCC) and colon cancer-derived tumor endothelial cells were double-stained with anti-CD31 and anti-SBSN antibodies. Scale bar: 50 μm.
Suprabasin knockdown inhibited migration and tube formation of mouse tumor endothelial cells
To clarify the role of SBSN in TEC, we used mTEC isolated from human tumor xenografts (A375SM and OS-RC-2). mNEC were isolated from mouse dermis as a normal control. We verified that mNEC and mTEC had the characteristics of EC using an RT-PCR assay (Suppl. Fig. S2). The SBSN mRNA expression levels were upregulated in mTEC from melanoma and renal carcinoma compared with mNEC (Fig. 2a) and other mouse normal tissues (Suppl. Fig. S3). To evaluate the SBSN function in TEC, we examined the migration ability and tube formation of mTEC following the SBSN knockdown. The efficacy of RNA interference (RNAi) was confirmed using quantitative real-time PCR, which showed that siSBSN, unlike control siRNA, decreased the SBSN mRNA level in mTEC and mNEC (Fig. 2b). We next demonstrated that the SBSN knockdown significantly suppressed cell migration toward VEGF-A in mTEC but not in mNEC (Fig. 2c). However, siSBSN had no effect on cell proliferation in either mTEC or mNEC (Suppl. Fig. S4). In this study, we used two types of siRNA and obtained similar results. This suggests that the results are not off-target effects of the nucleic acids. In addition, the junction number of endothelial tubes in mTEC was reduced by siSBSN treatment (Fig. 2d). These findings revealed that SBSN contributed to the angiogenic phenotype, such as migration and tube formation in mTEC.
Fig. 2.
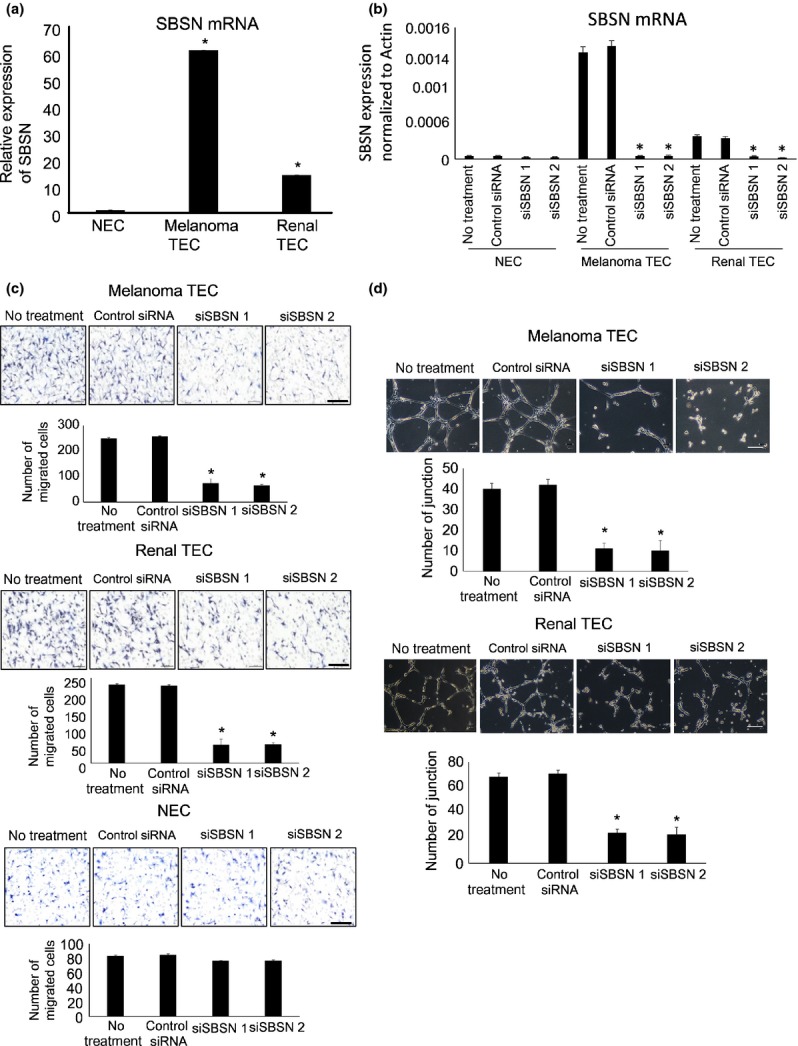
Effect of SBSN knockdown on cell migration and tube formation in mouse tumor endothelial cells (mTEC). (a) Relative SBSN mRNA expression levels in mouse normal endothelial cells (mNEC) and mTEC (melanoma and renal) evaluated by quantitative PCR. (b) SBSN mRNA expression levels in mTEC and mNEC transfected with the control siRNA or siSBSN, determined by quantitative PCR. (c) Migration toward vascular endothelial growth factor (VEGF) of mTEC and mNEC transfected with control siRNA or siSBSN analyzed using a Boyden chamber. Scale bar: 100 μm. (d) Tube number of mTEC transfected with control siRNA or siSBSN. Scale bar: 50 μm. *P < 0.01 versus control; one-way anova with the Tukey–Kramer multiple comparison test (mean ± SD, n = 3).
Suprabasin knockdown suppressed AKT pathway in mouse tumor endothelial cells
The PI3K/AKT pathway plays an essential role in the survival of TEC.(29) We previously reported that activation of AKT was involved in cell migration of mTEC;(13,23) and, therefore, we explored the interaction between the AKT pathway and SBSN. Phosphorylation of AKT in mTEC was suppressed by the PI3K inhibitor LY294002 treatment (Suppl. Fig. S5a). Moreover, we showed that the protein level of phosphorylated AKT was reduced by siSBSN treatment compared with control siRNA in both types of mTEC (melanoma and renal) (Fig. 3a), but not in NEC or in other cell types (Suppl. Fig. S5b). Moreover, we demonstrated that LY294002 inhibited tube formation in mTEC in a concentration-dependent manner (Fig. 3b). These findings indicate that SBSN regulated the migration and tube formation of mTEC via the AKT pathway. In addition, SBSN-positive blood vessels in human colon cancer tissues were positively stained by anti-AKT, but not those of normal colon tissues (Fig. 3c). This result suggests that SBSN may also be involved in AKT activation in human tumor blood vessels. To address how SBSN expression is regulated, endothelial cells were treated with growth factors such as endothelial growth factor (EGF), VEGF and fibroblast growth factor-2 (FGF-2). Among these growth factors, EGF significantly induced SBSN mRNA expression in NEC (Fig. 4).
Fig. 3.
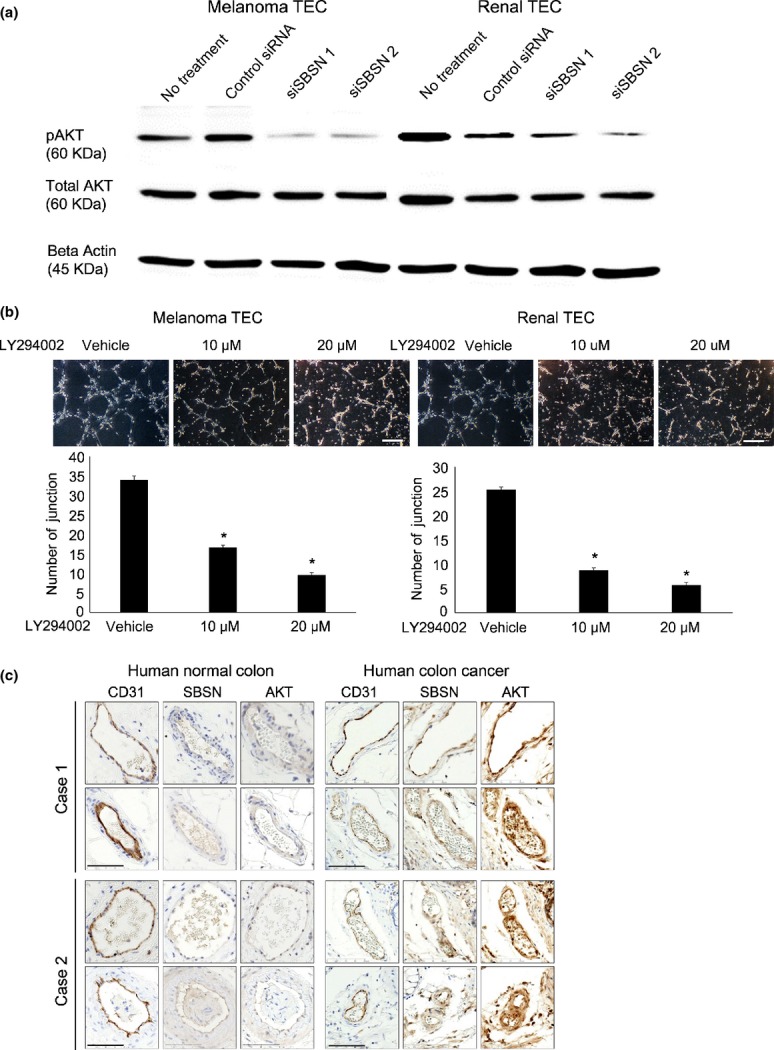
Relationship between suprabasin (SBSN) knockdown and AKT activation in mouse tumor endothelial cells (mTEC) in vitro and in vivo. (a) Total AKT, phosphorylated AKT (p-AKT), and beta actin protein levels in mTEC treated with control siRNA or siSBSN, determined by western blotting. (b) Tube number of mTEC treated with or without LY294002 (10 or 20 μM) evaluated by the tube formation assay. Scale bar: 100 μm. *P < 0.05 versus control; two-sided Student's t-test (mean ± SD, n = 3). (c) SBSN and AKT expression levels were determined by immunohistochemical analysis. CD31-positive blood vessels were stained with anti-SBSN and anti-AKT antibodies in two cases of human colon cancer (Cases 1 and 2), whereas those of normal tissues were weakly stained in vivo. Scale bar: 80 μm.
Fig. 4.
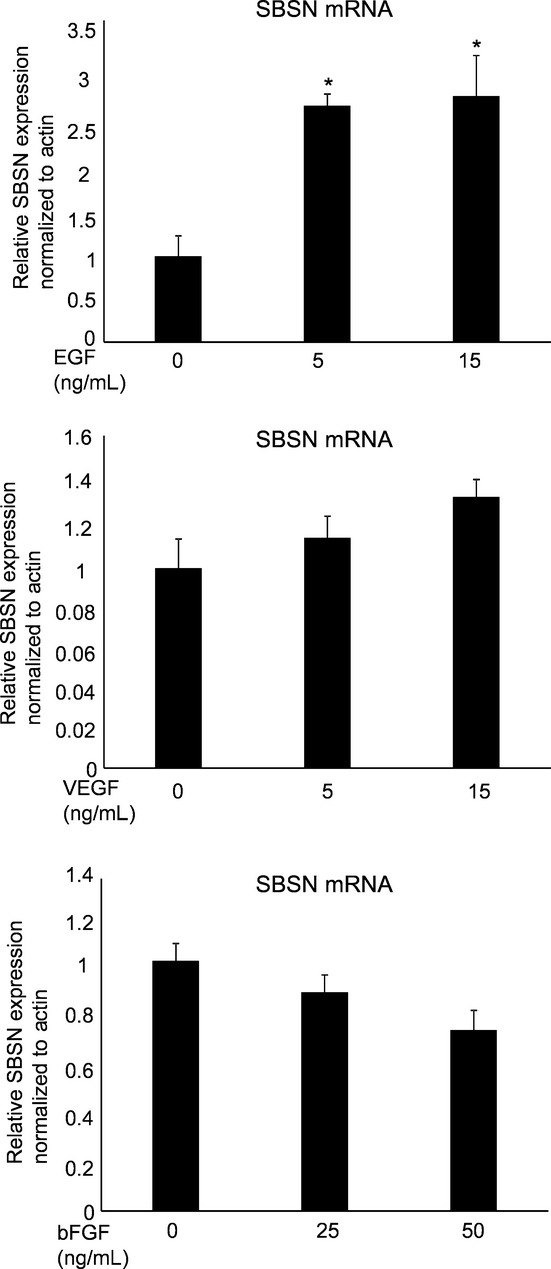
Suprabasin (SBSN) expression after growth factor treatment. NEC were incubated in 0.5% EBM2 medium for 12 h, followed by treatment with endothelial growth factor (EGF), vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) for 12 h. The cells were cultured at 37°C in a humidified atmosphere of 5% CO2. *P < 0.05 versus control; two-sided student's t-test. After 12 h of incubation, mRNA was extracted from the cells and used in the RT-PCR analysis of SBSN expression.
Discussion
In this study, we demonstrated that the SBSN expression was markedly increased in human TEC (renal carcinoma and colon carcinomas) as well as mTEC (melanoma and renal carcinoma). These findings indicate that SBSN may be used as a common marker of TEC.
The SBSN mRNA expression levels tended to be higher in hTEC (isolated from renal carcinoma) with higher T classifications under the tumor-node-metastasis system (order: case 4 > 2 > 3 > 1) (Suppl. Table S1). In this study, because the number of clinical samples was small, further studies are required to explore the relationship between the SBSN expression and clinical background in larger numbers of patients.
Previously, we reported that mTEC demonstrate a pro-angiogenic phenotype compared with mNEC.(11,30,31) SBSN plays a role in epidermal differentiation(18) and the growth and invasiveness of tumors.(19–21) For example, Shao et al.(21) report that SBSN was upregulated because the SBSN gene promoter in adenocystic carcinoma was demethylated. However, our preliminary analysis of epigenetics showed that methylation levels in TEC did not differ from those in NEC, which suggests that there may be another mechanism that is responsible for the enhanced expression of the SBSN gene in TEC. We found that EGF upregulated the expression of SBSN in NEC. However, its mechanism of transcriptional regulation or its function in tumor angiogenesis is unknown. In this study, we demonstrated that the SBSN knockdown inhibited cell migration and tube formation in mTEC. These findings revealed the role of SBSN in tumor angiogenesis.
We previously reported that the VEGF receptor-2 (VEGFR-2) expression was high in TEC and that TEC were more sensitive to VEGF than NEC.(27) The SBSN knockdown had no significant effect on the VEGFR mRNA expression in mTEC, suggesting that involvement of SBSN in the angiogenic phenotype of mTEC is independent of VEGF/VEGFR-2 signaling.
There has been no report of SBSN signaling. We showed that the activation of AKT was suppressed by siSBSN. However, activation of the ERK pathway, which is related to angiogenesis, was not affected (Suppl. Fig. S6). Our finding revealed at least a part of downstream signaling of SBSN in TEC. Thus, these findings enhanced our understanding of TEC function.
Our data demonstrate that the number of tube junctions in TEC was decreased more by siSBSN than by a PI3K inhibitor.
These results suggest that other molecules besides AKT are involved in SBSN-related tube formation in mTEC. Additional studies are required to determine whether the AKT is directly involved in the downstream of SBSN.
In this study, to the best of our knowledge, we demonstrated for the first time that SBSN is upregulated in TEC and that SBSN plays significant roles in the pro-angiogenic phenotype in TEC, but not in NEC. In this study, we showed that SBSN could be a potential TEC marker. Thus, SBSN may be a novel target for anti-angiogenic therapy, which is specific for tumor blood vessels.
Acknowledgments
We thank Dr I. J. Fidler for providing the A375SM super-metastatic human malignant melanoma cell line and Dr Aya Matsuda, Ms. Yuko Suzuki, and Ms. Tomomi Takahashi for their technical assistance in the experiments. This article was supported in part by a Grant-in-Aid for scientific research from the Ministry of Education, Science and Culture of Japan (20390506 and 23112501 to Kyoko Hida). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure Statement
The authors have no conflict of interest to declare.
Supporting Information
Additional supporting information may be found in the online version of this article:
Fig. S1. Suprabasin (SBSN) expression in human tumor tissues. Relative SBSN mRNA expression levels in both human colon normal and cancer tissues were analyzed by quantitative RT-PCR. *P < 0.05 versus control; two-sided Student's t-test. Clinical samples from three patients were collected.
Fig. S2. Characterization of isolated mouse tumor endothelial cells (mTEC) and mouse normal endothelial cells (mNEC). mRNA levels of CD31, CD105, VEGFR-1 (VR1), VEGFR-2 (VR2), CD11b, CD45, human HB-EGF (hHB-EGF) and GAPDH in mTEC and mNEC were evaluated by RT-PCR.
Fig. S3. SBSN expression in mouse tumor endothelial cells (mTEC) and other various tissue of mouse organs. Relative SBSN mRNA expression levels in various tissue of mouse organs besides ECs analyzed by quantitative PCR. *P < 0.01 versus control. (ND, not detected.)
Fig. S4. Effect of siSBSN on proliferation of mouse tumor endothelial cells (mTEC) transfected with control siRNA or siSBSN was analyzed using the MTS assay.
Fig. S5. (a) Effect of LY294002 treatment on mouse tumor endothelial cells (mTEC). (a) Total AKT, phosphorylated AKT (p-AKT), and beta actin protein levels in mTEC treated or not treated with LY294002 (10 or 20 μM) were determined by western blotting. (b) Total AKT, phosphorylated AKT (p-AKT), and beta actin protein levels in mTEC were compared with those of the NEC, NIH3T3 and B16F10 cell lines transfected with control siRNA or siSBSN. Approximately 20 μg of total protein was loaded into each lane for western blot analysis.
Fig. S6. Effect of ERK activation by suprabasin (SBSN) knockdown. ERK activation was determined by western blot analysis. Total ERK and phosphorylated ERK (p-ERK) protein expression levels were detected in mouse tumor endothelial cells (mTEC) transfected with control siRNA or siSBSN in melanoma tumor endothelial cells (TEC) and renal TEC. Approximately 20 μg of total protein was loaded into each lane for western blot analysis. Beta actin antibody was used as internal control.
Table S1. Clinical background of renal cell carcinoma (RCC) and colon cancer specimens. M/F, male/female; †according to 1997 tumor-node-metastasis (TNM) staging guidelines; ††according to the Fuhrman system.
Table S2. List of primers. Primer sequences for RT-PCR and quantitative PCR.
References
- 1.Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29:15–8. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- 2.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273–86. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 3.Welti J, Loges S, Dimmeler S, Carmeliet P. Recent molecular discoveries in angiogenesis and antiangiogenic therapies in cancer. J Clin Invest. 2013;123:3190–200. doi: 10.1172/JCI70212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Willett CG, Boucher Y, di Tomaso E, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10:145–7. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ivy SP, Wick JY, Kaufman BM. An overview of small-molecule inhibitors of VEGFR signaling. Nat Rev Clin Oncol. 2009;6:569–79. doi: 10.1038/nrclinonc.2009.130. [DOI] [PubMed] [Google Scholar]
- 6.Jain RK, Duda DG, Clark JW, Loeffler JS. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat Clin Pract Oncol. 2006;3:24–40. doi: 10.1038/ncponc0403. [DOI] [PubMed] [Google Scholar]
- 7.McDonald DM, Baluk P. Significance of blood vessel leakiness in cancer. Cancer Res. 2002;62:5381–5. [PubMed] [Google Scholar]
- 8.Morikawa S, Baluk P, Kaidoh T, Haskell A, Jain RK, McDonald DM. Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am J Pathol. 2002;160:985–1000. doi: 10.1016/S0002-9440(10)64920-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–93. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 10.St Croix B, Rago C, Velculescu V, et al. Genes expressed in human tumor endothelium. Science. 2000;289:1197–202. doi: 10.1126/science.289.5482.1197. [DOI] [PubMed] [Google Scholar]
- 11.Akiyama K, Ohga N, Hida Y, et al. Tumor endothelial cells acquire drug resistance by MDR1 up-regulation via VEGF signaling in tumor microenvironment. Am J Pathol. 2012;180:1283–93. doi: 10.1016/j.ajpath.2011.11.029. [DOI] [PubMed] [Google Scholar]
- 12.Osawa T, Ohga N, Akiyama K, et al. Lysyl oxidase secreted by tumour endothelial cells promotes angiogenesis and metastasis. Br J Cancer. 2013;109:2237–47. doi: 10.1038/bjc.2013.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muraki C, Ohga N, Hida Y, et al. Cyclooxygenase-2 inhibition causes antiangiogenic effects on tumor endothelial and vascular progenitor cells. Int J Cancer. 2012;130:59–70. doi: 10.1002/ijc.25976. [DOI] [PubMed] [Google Scholar]
- 14.Maishi N, Ohga N, Hida Y, et al. CXCR7: a novel tumor endothelial marker in renal cell carcinoma. Pathol Int. 2012;62:309–17. doi: 10.1111/j.1440-1827.2012.02792.x. [DOI] [PubMed] [Google Scholar]
- 15.Osawa T, Ohga N, Hida Y, et al. Prostacyclin receptor in tumor endothelial cells promotes angiogenesis in an autocrine manner. Cancer Sci. 2012;103:1038–44. doi: 10.1111/j.1349-7006.2012.02261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamamoto K, Ohga N, Hida Y, et al. Biglycan is a specific marker and an autocrine angiogenic factor of tumour endothelial cells. Br J Cancer. 2012;106:1214–23. doi: 10.1038/bjc.2012.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsui T, Hayashi-Kisumi F, Kinoshita Y, et al. Identification of novel keratinocyte-secreted peptides dermokine-alpha/-beta and a new stratified epithelium-secreted protein gene complex on human chromosome 19q13.1. Genomics. 2004;84:384–97. doi: 10.1016/j.ygeno.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 18.Park GT, Lim SE, Jang SI, Morasso MI. Suprabasin, a novel epidermal differentiation marker and potential cornified envelope precursor. J Biol Chem. 2002;277:45195–202. doi: 10.1074/jbc.M205380200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glazer CA, Smith IM, Ochs MF, et al. Integrative discovery of epigenetically derepressed cancer testis antigens in NSCLC. PLoS ONE. 2009;4:e8189. doi: 10.1371/journal.pone.0008189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Formolo CA, Williams R, Gordish-Dressman H, MacDonald TJ, Lee NH, Hathout Y. Secretome signature of invasive glioblastoma multiforme. J Proteome Res. 2011;10:3149–59. doi: 10.1021/pr200210w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shao C, Tan M, Bishop JA, et al. Suprabasin is hypomethylated and associated with metastasis in salivary adenoid cystic carcinoma. PLoS ONE. 2012;7:e48582. doi: 10.1371/journal.pone.0048582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akino T, Hida K, Hida Y, et al. Cytogenetic abnormalities of tumor-associated endothelial cells in human malignant tumors. Am J Pathol. 2009;175:2657–67. doi: 10.2353/ajpath.2009.090202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohga N, Hida K, Hida Y, et al. Inhibitory effects of epigallocatechin-3 gallate, a polyphenol in green tea, on tumor-associated endothelial cells and endothelial progenitor cells. Cancer Sci. 2009;100:1963–70. doi: 10.1111/j.1349-7006.2009.01255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kondoh M, Ohga N, Akiyama K, et al. Hypoxia-induced reactive oxygen species cause chromosomal abnormalities in endothelial cells in the tumor microenvironment. PLoS ONE. 2013;8:e80349. doi: 10.1371/journal.pone.0080349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawamoto T, Ohga N, Akiyama K, et al. Tumor-derived microvesicles induce proangiogenic phenotype in endothelial cells via endocytosis. PLoS ONE. 2012;7:e34045. doi: 10.1371/journal.pone.0034045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurosu T, Ohga N, Hida Y, et al. HuR keeps an angiogenic switch on by stabilising mRNA of VEGF and COX-2 in tumour endothelium. Br J Cancer. 2011;104:819–29. doi: 10.1038/bjc.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsuda K, Ohga N, Hida Y, et al. Isolated tumor endothelial cells maintain specific character during long-term culture. Biochem Biophys Res Commun. 2010;394:947–54. doi: 10.1016/j.bbrc.2010.03.089. [DOI] [PubMed] [Google Scholar]
- 28.Akiyama K, Ohga N, Maishi N, et al. The F-prostaglandin receptor is a novel marker for tumor endothelial cells in renal cell carcinoma. Pathol Int. 2013;63:37–44. doi: 10.1111/pin.12031. [DOI] [PubMed] [Google Scholar]
- 29.Bussolati B, Deambrosis I, Russo S, Deregibus MC, Camussi G. Altered angiogenesis and survival in human tumor-derived endothelial cells. FASEB J. 2003;17:1159–61. doi: 10.1096/fj.02-0557fje. [DOI] [PubMed] [Google Scholar]
- 30.Hida K, Hida Y, Amin DN, et al. Tumor-associated endothelial cells with cytogenetic abnormalities. Cancer Res. 2004;64:8249–55. doi: 10.1158/0008-5472.CAN-04-1567. [DOI] [PubMed] [Google Scholar]
- 31.Ohga N, Ishikawa S, Maishi N, et al. Heterogeneity of tumor endothelial cells: comparison between tumor endothelial cells isolated from high- and low-metastatic tumors. Am J Pathol. 2012;180:1294–307. doi: 10.1016/j.ajpath.2011.11.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Suprabasin (SBSN) expression in human tumor tissues. Relative SBSN mRNA expression levels in both human colon normal and cancer tissues were analyzed by quantitative RT-PCR. *P < 0.05 versus control; two-sided Student's t-test. Clinical samples from three patients were collected.
Fig. S2. Characterization of isolated mouse tumor endothelial cells (mTEC) and mouse normal endothelial cells (mNEC). mRNA levels of CD31, CD105, VEGFR-1 (VR1), VEGFR-2 (VR2), CD11b, CD45, human HB-EGF (hHB-EGF) and GAPDH in mTEC and mNEC were evaluated by RT-PCR.
Fig. S3. SBSN expression in mouse tumor endothelial cells (mTEC) and other various tissue of mouse organs. Relative SBSN mRNA expression levels in various tissue of mouse organs besides ECs analyzed by quantitative PCR. *P < 0.01 versus control. (ND, not detected.)
Fig. S4. Effect of siSBSN on proliferation of mouse tumor endothelial cells (mTEC) transfected with control siRNA or siSBSN was analyzed using the MTS assay.
Fig. S5. (a) Effect of LY294002 treatment on mouse tumor endothelial cells (mTEC). (a) Total AKT, phosphorylated AKT (p-AKT), and beta actin protein levels in mTEC treated or not treated with LY294002 (10 or 20 μM) were determined by western blotting. (b) Total AKT, phosphorylated AKT (p-AKT), and beta actin protein levels in mTEC were compared with those of the NEC, NIH3T3 and B16F10 cell lines transfected with control siRNA or siSBSN. Approximately 20 μg of total protein was loaded into each lane for western blot analysis.
Fig. S6. Effect of ERK activation by suprabasin (SBSN) knockdown. ERK activation was determined by western blot analysis. Total ERK and phosphorylated ERK (p-ERK) protein expression levels were detected in mouse tumor endothelial cells (mTEC) transfected with control siRNA or siSBSN in melanoma tumor endothelial cells (TEC) and renal TEC. Approximately 20 μg of total protein was loaded into each lane for western blot analysis. Beta actin antibody was used as internal control.
Table S1. Clinical background of renal cell carcinoma (RCC) and colon cancer specimens. M/F, male/female; †according to 1997 tumor-node-metastasis (TNM) staging guidelines; ††according to the Fuhrman system.
Table S2. List of primers. Primer sequences for RT-PCR and quantitative PCR.


