Abstract
In an open-label, multicenter phase II study of Japanese patients with cytokine-refractory metastatic renal cell carcinoma, axitinib showed substantial antitumor activity with an acceptable safety profile. Here, we report overall survival and updated efficacy and safety results. Sixty-four Japanese patients with metastatic renal cell carcinoma following prior therapy with cytokines were treated with axitinib at a starting dose of 5 mg b.i.d. Following median treatment duration of 14.2 months, median overall survival was 37.3 months (95% CI, 28.6–49.9). The objective response rate, the primary endpoint of the study, was 51.6% (95% CI, 38.7–64.2); the median duration of response, 11.1 months (95% CI, 8.2–13.7); and the median progression-free survival was 11.0 months (95% CI, 9.2–12.0), assessed by the independent review committee. Common treatment-related all-grade adverse events were hypertension (88%), hand-foot syndrome (75%), diarrhea (66%), proteinuria (63%), fatigue (55%) and dysphonia (53%). In an exploratory analysis, median overall survival was found to be significantly longer in patients who had greater decreases in plasma levels of soluble vascular endothelial growth factor receptor-2 during the first cycle of treatment. In conclusion, the present study showed axitinib to be effective, and toxicities with long-term treatment were generally controllable with axitinib dose modification and/or standard medications in these Japanese patients. Some frequently reported adverse events warrant close monitoring and management. Changes in the plasma levels of soluble vascular endothelial growth factor receptor-2 may be used as a prognostic factor for overall survival in metastatic renal cell carcinoma following axitinib treatment. This study is registered at http://ClinicalTrial.gov (identifier NCT00569946).
Keywords: Axitinib, cytokine-refractory, Japanese, overall survival, renal cell carcinoma
Molecularly targeted therapy with agents blocking vascular endothelial growth factor (VEGF)/VEGF receptors (VEGFR)(1–6) or mammalian target of rapamycin (mTOR)(7,8) is well established as a treatment option for advanced renal cell carcinoma (RCC), and improves progression-free survival (PFS) and quality of life compared with cytokine therapy. However, a significant overall survival (OS) benefit of these agents has not been demonstrated in clinical trials,(9–15) except for first-line temsirolimus in patients with metastatic RCC (mRCC) with poor prognosis.(7) The reasons for the lack of survival benefit with molecularly targeted therapy are unclear but may include crossover of patients from control to experimental arms and/or administration of additional systemic treatment(s) post-study, which confound analysis and interpretation of OS data. Furthermore, these molecularly targeted agents have been available for <10 years and, therefore, data on their long-term efficacy and safety are limited.(16–18)
Axitinib, a potent and selective tyrosine kinase inhibitor (TKI) of VEGFR-1, 2 and 3,(19) is approved for treatment of patients with mRCC in the United States, the European Union, Japan and elsewhere. Approval has been based on the global randomized phase III AXIS trial, which showed statistically significantly longer PFS and higher objective response rate (ORR) with axitinib compared with sorafenib (PFS: 6.7 vs 4.7 months, respectively; hazard ratio [HR] 0.665; P < 0.0001 and ORR: 19% vs 9%, respectively; P = 0.0001) in previously treated patients with mRCC.(6) Although PFS remained longer (P < 0.0001) and ORR higher (P = 0.0001) with axitinib than sorafenib, there was no significant difference in OS between these two antiangiogenic TKI (20.1 vs 19.2 months; HR 0.969; P = 0.3744) in the follow-up analysis.(14)
A subgroup analysis of the AXIS trial indicated that axitinib was efficacious and well tolerated in Japanese patients with second-line mRCC, consistent with the results of the overall population. However, differences in the incidence and severity of several adverse events (AE) were noted in Japanese patients.(20) Therefore, it is critical to assess the efficacy and the long-term safety of axitinib in Japanese patients with mRCC. A phase II study of axitinib was conducted in 64 Japanese patients with cytokine-refractory mRCC. The results of the primary analysis have previously been published.(21) Here, we report the OS and final efficacy and safety data with long-term axitinib treatment from this phase II study.
Patients and Methods
Study design and patients
This open-label, non-randomized phase II study was conducted in 19 centers in Japan. The primary endpoint was independent review committee (IRC)-assessed ORR, and the secondary endpoints included investigator-assessed ORR, PFS and duration of response (both IRC-assessed and investigator-assessed), OS, safety, and changes in plasma levels of VEGF, soluble VEGFR (sVEGFR) 1, 2 and 3 and soluble stem cell factor receptor. The study protocol was approved by an institutional review board at each site, and the study was conducted in compliance with the Declaration of Helsinki, the International Conference on Harmonization Guidelines on Good Clinical Practice, and applicable local laws and regulatory requirements. Each patient provided written informed consent prior to study entry.
Patient eligibility criteria have been provided in detail.(21) In brief, patients aged 20 years or older with histologically confirmed mRCC with a clear-cell component, Eastern Cooperative Oncology Group performance status (ECOG PS) 0 or 1, prior nephrectomy, prior failure of cytokine treatment and blood pressure (BP) ≤140/90 mmHg were eligible for the study. The use of antihypertensive medications was permitted. Patients who had brain metastases, who had surgery, radiation or systemic therapy within 4 weeks of treatment initiation, or have required known potent cytochrome P450 3A4 inhibitors or inducers were excluded.
Study treatment
Axitinib was administered orally at a starting dose of 5 mg b.i.d. with food in 28-day cycles.(21) Axitinib dose could be increased to 7 mg b.i.d., and then to a maximum of 10 mg b.i.d. in patients who tolerated axitinib with no drug-related AE above grade 2 for 2 consecutive weeks unless BP >150/90 mmHg or the patient was taking antihypertensive medication. Axitinib dose could be reduced to 3 mg b.i.d., and then to 2 mg b.i.d. to manage drug-related toxicities, if necessary. Treatment was continued until progressive disease, intolerable toxicities or withdrawal of consent; however, if the physician determined that axitinib had clinical benefit (defined as sum of the diameter of measureable lesion equal to or smaller than that at baseline with no new lesion), treatment could be continued until the sum of the diameter of the measureable lesion exceeded the baseline value.
Assessments
Tumor assessments were conducted at screening, on day 1 of odd-numbered cycles starting at cycle 3, and at follow-up 28 days after the end of treatment or discontinuation. A baseline bone scan showing metastatic lesions was to be repeated every 8 weeks. Tumor responses were assessed by both the IRC and investigators according to the Response Evaluation Criteria in Solid Tumors version 1.0, and a complete response (CR) or partial response (PR) was confirmed at least 4 weeks after the initial observation.
Safety was monitored throughout the study and AE and laboratory abnormalities were graded according to Common Terminology Criteria for Adverse Events version 3.0. BP measurements were taken at each hospital/clinic visit at screening, on days 1, 8, 15 and 22 of cycle 1, on days 1 and 15 of cycles 2–4, and on day 1 of each remaining cycle. In addition, each patient monitored BP at least b.i.d. at home and was to contact their physicians when BP >150/100 mmHg or for symptoms related to elevated BP. Thyroid function tests were conducted by measuring free triiodothyronine, free thyroxine and thyroid-stimulating hormone (TSH) at screening, on days 1, 8, 15 and 22 of cycle 1, on day 1 of cycles 2 and 3, and on day 1 of subsequent odd-numbered cycles. Renal function was monitored using urinalysis at screening, on days 1, 8, 15 and 22 of cycle 1, on days 1 and 15 of cycles 2–4, and on day 1 of each remaining cycle. If urinalysis showed urinary protein ≥2+, a 24-h urine collection was performed.
Blood samples were collected on day 1 of cycles 1–7 and at the end of the study treatment to determine plasma concentrations of soluble proteins. Plasma concentrations of soluble proteins were measured using enzyme-linked immunosorbent assays (Alta Analytical Laboratory, El Dorado Hills, CA, USA).
Statistical analyses
A single-stage design required 63 patients to test the null hypothesis that the true response rate is ≤10% against the alternative hypothesis that the true response rate is ≥25%, with target α and β error rate of ≤0.05 and ≤0.10, respectively.(21) Patients who received at least one dose of axitinib were included in efficacy and safety analysis. ORR was provided with 95% confidence interval (CI) calculated based on F-distribution. PFS, duration of response, and OS were analyzed using the Kaplan–Meier method, and median values and 95% CI were summarized. In a post-hoc analysis, OS by Memorial Sloan-Kettering Cancer Center (MSKCC) risk groups and baseline ECOG PS were investigated using a Cox proportional hazard model. In an exploratory analysis, a potential association between OS and changes in diastolic BP (DBP) or plasma levels of soluble proteins was evaluated using the same method as in the post-hoc analysis.
Results
Patient baseline characteristics and treatment
A total of 64 Japanese patients were enrolled in the study between December 2007 and February 2009 and the last patient's last visit was 9 August 2013 (data cutoff date for the final analysis; Table 1). All patients had prior nephrectomy and had received one (80%) or two (20%) prior interferon-α-containing and/or interleukin-2-containing regimens.
Table 1.
Patient demographics and baseline characteristics†
| N = 64 | |
|---|---|
| Sex, n (%) | |
| Male | 44 (69) |
| Female | 20 (31) |
| Age, years, median (range) | 63 (34–80) |
| ECOG PS, n (%) | |
| 0 | 57 (89) |
| 1 | 7 (11) |
| Primary histology, n (%) | |
| Clear cell | 62 (97) |
| Papillary carcinoma | 1 (2) |
| Spindle cell | 1 (2) |
| Prior adjuvant therapy, n (%) | |
| Yes | 10 (16) |
| No | 54 (84) |
| Prior cytokine therapy for metastatic sites, n (%) | |
| Interferon | 50 (78) |
| Interleukin-2 | 3 (5) |
| Interferon/Interleukin-2 | 11 (17) |
| Duration of prior cytokine therapy, days, median (range) | 244 (2–3766) |
| MSKCC risk group,‡,§ n (%) | |
| Favorable | 10 (16) |
| Intermediate | 47 (77) |
| Poor | 4 (7) |
| Number of metastatic sites, n (%) | |
| 1 | 19 (30) |
| 2 | 18 (28) |
| 3 | 14 (22) |
| ≥4 | 13 (20) |
| Site of metastases, n (%) | |
| Lung | 53 (83) |
| Lymph node (distant) | 20 (31) |
| Bone | 12 (19) |
| Pancreas | 11 (17) |
| Kidney | 9 (14) |
| Adrenal | 8 (13) |
| Liver | 6 (9) |
| Lymph node (regional) | 6 (9) |
Adapted from Eur J Cancer, Vol 47, Tomita et al., Key predictive factors of axitinib (AG-013736)-induced proteinuria and efficacy: A phase II study in Japanese patients with cytokine-refractory metastatic renal cell carcinoma, pp. 2592–602, Copyright (2011), with permission from Elsevier.
Derived using five risk factors: lactate dehydrogenase >1.5 times the upper limit of normal, serum hemoglobin < the lower limit of normal, corrected serum calcium >10 mg/dL, ECOG PS 1, and the time from initial diagnosis to axitinib treatment <1 year. MSKCC risk groups were defined as favorable (0 risk factor), intermediate (1 or 2 risk factors) or poor (≥3 risk factors).
Unknown for 3 patients. ECOG PS, Eastern Cooperative Oncology Group performance status; MSKCC, Memorial Sloan-Kettering Cancer Center.
As of the data cutoff date, 59 of 64 patients discontinued the study treatment due to objective progression or relapse (n = 42), treatment-related AE (n = 16), or global deterioration of health status (n = 1). Five patients continued treatment (switching to commercially available axitinib upon its approval in Japan) and were followed for OS. All 59 patients who discontinued the axitinib study subsequently received other systemic treatments, such as sorafenib, sunitinib or everolimus (Table 2).
Table 2.
Follow-up systemic treatments
| N = 64 | |
|---|---|
| Number of treatments, n (%) | |
| Any subsequent treatment | 59 (92) |
| 1 subsequent treatment | 29 (45) |
| 2 subsequent treatments | 18 (28) |
| 3 subsequent treatments | 5 (8) |
| 4 subsequent treatments | 2 (3) |
| 5–10 subsequent treatments | 5 (8) |
| Type of medication†, n (%) | |
| Sorafenib | 29 (45) |
| Sunitinib | 26 (41) |
| Everolimus | 21 (33) |
| Axitinib | 10 (16) |
| Temsirolimus | 5 (8) |
| Interferon-α | 5 (8) |
Includes those administered to >5% of patients.
A total of 19 patients received >24 cycles of axitinib treatment and median duration of treatment was 14.2 months (range, 0.4–56.1). The median total daily dose of axitinib administered to patients was 6.6 mg (range, 1.6–16.4). The axitinib dose was uptitrated to 7 mg b.i.d. in 5 patients and 10 mg b.i.d. in 1 patient, whereas 46 patients had their dose reduced below 5 mg b.i.d..
Efficacy
Although there was no CR, 33 patients treated with axitinib achieved PR and an additional 28 patients had stable disease ≥8 weeks. The final IRC-assessed ORR was 51.6% (95% CI, 38.7–64.2) (Table 3) and the median duration of response was 11.1 months (95% CI, 8.2–13.7). The investigator-assessed ORR (56.3% [95% CI, 43.3–68.6]) and median duration of response (12.8 months [95% CI, 7.7–17.5]) were generally in agreement with those determined by the IRC. Median PFS per the IRC assessment was 11.0 months (95% CI, 9.2–12.0) (Fig. 1a). Forty-eight patients had objective progression and 16 patients were censored due to treatment discontinuation (n = 8) or administration of new anti-cancer treatment (n = 6) prior to tumor progression, or lack of on-study disease assessments (n = 2). Median PFS per the investigator assessment (12.0 months [95% CI, 9.2–14.8]) was similar to that assessed by the IRC.
Table 3.
Summary of IRC-assessed and investigator-assessed tumor response
| IRC-assessed N = 64 | Investigator-assessed N = 64 | |||
|---|---|---|---|---|
| n | % | n | % | |
| Best response by RECIST | ||||
| Complete response | 0 | 0 | 0 | 0 |
| Partial response | 33 | 51.6 | 36 | 56.3 |
| Stable disease† | 28 | 43.8 | 25 | 39.1 |
| Progressive disease | 1 | 1.6 | 1 | 1.6 |
| Indeterminate‡ | 2 | 3.1 | 2 | 3.1 |
| Objective response rate | 33 | 51.6 | 36 | 56.3 |
| 95% CI§ | — | 38.7–64.2 | — | 43.3–68.6 |
Stable disease ≥8 weeks.
No tumor assessment after dosing due to adverse event-related discontinuation.
Using exact method based on binomial distribution. CI, confidence interval; IRC, independent review committee; RECIST, Response Evaluation Criteria in Solid Tumors, —; not applicable.
Fig. 1.
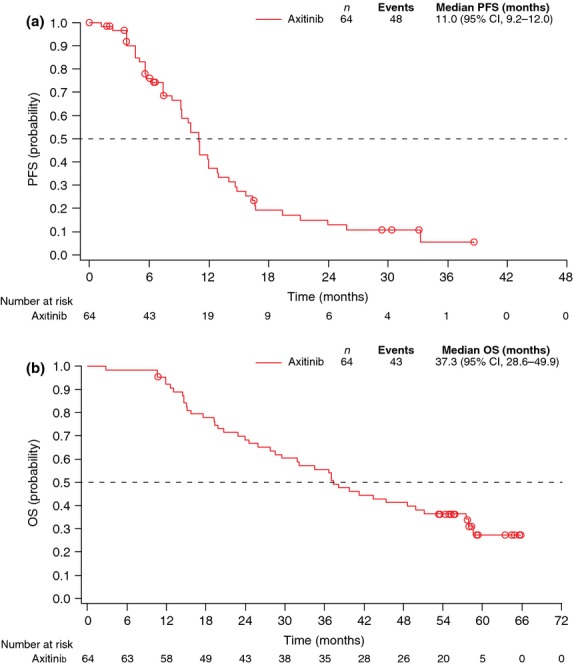
Kaplan–Meier estimates of (a) independent review committee-assessed progression-free survival and (b) overall survival. CI, confidence interval; OS, overall survival; PFS, progression-free survival.
At the data cutoff date, 43 patients had died due to disease progression and 21 were censored (20 alive and 1 lost to follow up). Median OS was 37.3 months (95% CI, 28.6–49.9; Fig. 1b). When stratified by baseline MSKCC risk factors, median OS was 33.8 months (95% CI, 14.6–45.3) for the favorable group, 41.3 months (95% CI, 31.8–57.9) for the intermediate group and 17.4 months (95% CI, 14.4 to not estimable) for the poor group (Fig. 2a). The HR for the intermediate νersus the favorable risk group was 0.723 (95% CI, 0.330–1.583; P = 0.3365) in favor of the intermediate risk group, and that for the poor νersus the favorable risk group was 1.320 (95% CI, 0.347–5.016; P = 0.8670) in favor of the favorable risk group. When stratified by baseline ECOG PS, median OS was 41.3 months (95% CI, 31.8–57.9) for PS 0 and 19.4 months (95% CI, 2.8–37.0) for PS 1 (HR: PS 1 vs 0, 3.402 [95% CI, 1.479–7.828; P = 0.0022]; Fig. 2b).
Fig. 2.
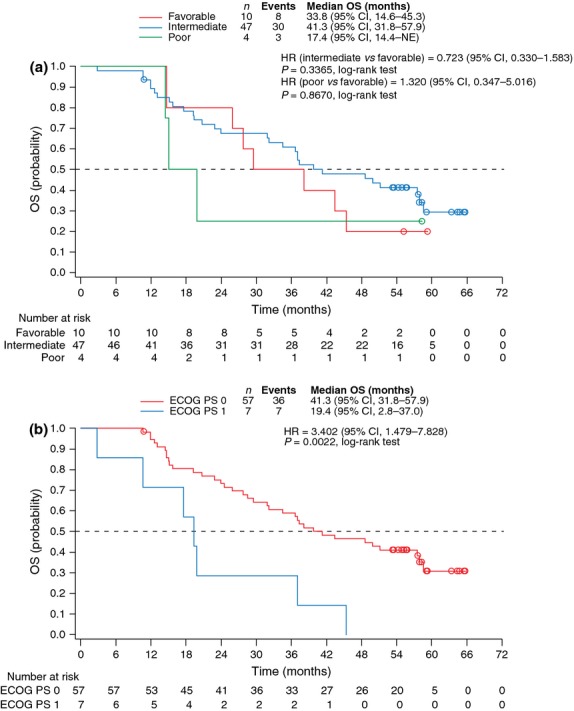
Kaplan–Meier estimates of overall survival by (a) MSKCC risk group and (b) baseline ECOG PS. CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; MSKCC, Memorial Sloan-Kettering Cancer Center; NE, not estimable; OS, overall survival.
Safety
Common all-grade treatment-related AE reported by more than 50% of axitinib-treated Japanese patients were hypertension, hand-foot syndrome, diarrhea, proteinuria, fatigue and dysphonia (Table 4). Treatment-related grade ≥3 AE experienced by >5% of patients included hypertension, hand-foot syndrome, proteinuria, fatigue and malaise (Table 4). A total of 6 patients experienced treatment-related grade 4 AE (anemia, hypertension, hyperthyroidism, myocardial infarction [MI], cerebral infarction and acute MI [n = 1 each]). A total of 16 patients discontinued the study due to treatment-related AE: proteinuria (n = 9) and polycythemia, malaise, MI, subarachnoid hemorrhage, anxiety, weight decrease and hyperthyroidism (n = 1 each). Common treatment-related AE that led to temporary dose interruptions or reductions included hypertension (n = 35), hand-foot syndrome and proteinuria (n = 20 each), diarrhea (n = 19), fatigue (n = 11) and anorexia (n = 10).
Table 4.
Treatment-related adverse events, and laboratory abnormalities reported by >10% of patients
| Adverse event/Laboratory abnormalities, n (%) |
N = 64 |
|
|---|---|---|
| All grade | Grade 3/4† | |
| Hypertension | 56 (88) | 47 (73) |
| Hand-foot syndrome | 48 (75) | 14 (22) |
| Diarrhea | 42 (66) | 3 (5) |
| Proteinuria‡ | 40 (63) | 6 (9) |
| Fatigue | 35 (55) | 4 (6) |
| Dysphonia | 34 (53) | 0 |
| Hypothyroidism | 31 (48) | 0 |
| Anorexia | 26 (41) | 3 (5) |
| Increased blood TSH | 21 (33) | 0 |
| Decreased weight | 20 (31) | 3 (5) |
| Nausea | 18 (28) | 1 (2) |
| Epistaxis | 16 (25) | 0 |
| Headache | 16 (25) | 0 |
| Increased ALT | 15 (23) | 2 (3) |
| Increased AST | 15 (23) | 1 (2) |
| Stomatitis | 15 (23) | 0 |
| Arthralgia | 13 (20) | 2 (3) |
| Rash | 13 (20) | 0 |
| Increased ALP | 12 (19) | 0 |
| Dysgeusia | 12 (19) | 0 |
| Vomiting | 12 (19) | 0 |
| Constipation | 10 (16) | 0 |
| Chest pain | 9 (14) | 0 |
| Malaise | 9 (14) | 4 (6) |
| Abdominal pain | 8 (13) | 0 |
| Cough | 8 (13) | 0 |
| Periodontitis | 8 (13) | 1 (2) |
| Abdominal pain upper | 7 (11) | 0 |
| Back pain | 7 (11) | 0 |
| Increased LDH | 7 (11) | 0 |
| Abnormal hepatic functional | 7 (11) | 0 |
| Oropharyngeal pain | 7 (11) | 0 |
| Decreased platelet count | 7 (11) | 1 (2) |
No grade 5 adverse event was reported.
Includes proteinuria, protein urine, and protein urine present. ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; LDH, lactate dehydrogenase; TSH, thyroid stimulating hormone.
After one cycle of axitinib treatment, 29 patients (of 63 with BP measurements) had DBP ≥90 mmHg, 4 had ≥100 mmHg and 1 had ≥105 mmHg. During the study, the number of patients who were given concomitant antihypertensive medications increased to 60 (94%) from 28 (44%) at study entry. Common antihypertensive medications included amlodipine besilate, candesartan cilexetil and doxazosin mesilate. Although 14 patients had abnormal baseline TSH levels (11 with >5 UIU/mL and 3 with <0.5 UIU/mL) at screening, none was taking thyroid medication prior to axitinib treatment. During the study, 54 patients had TSH levels increased by more than 1.2 times above the upper limit of normal, whereas 17 patients had TSH levels decreased to 0.8 times below the lower limit of normal. Fifty percent of patients received concomitant thyroid hormone replacement therapy with levothyroxine. By dipstick analysis, over 50% of patients had ≥2+ shift in urine protein.
Exploratory pharmacodynamic analyses
Patients were grouped into two categories according to whether or not they had observed maximum DBP ≥90 mmHg during the first cycle of axitinib treatment. Median OS in patients who had maximum DBP ≥90 mmHg (n = 48) was 41.3 months (95% CI, 28.6 to not estimable) compared with 30.8 months (95% CI, 15.1–43.4) in those who had DBP <90 mmHg (n = 16) (HR: DBP <90 vs ≥90 mmHg, 1.864 [95% CI, 0.978–3.553]; P = 0.0542; Fig. 3a). A potential association between OS and change in sVEGFR-2 levels from baseline to cycle 2 day 1 was also investigated. The median OS in patients who had percent change in sVEGFR-2 <median of −33.5% (greater decrease) (n = 31) was 47.0 months (95% CI, 29.5 to not estimable), which was significantly longer than the 34.6 months (95% CI, 15.7–49.9) in those who had ≥median percent change (lesser decrease) (n = 32) (HR: sVEGFR-2 ≥median vs <median % change, 1.994 [95% CI, 1.061–3.748]; P = 0.0289; Fig. 3b).
Fig. 3.
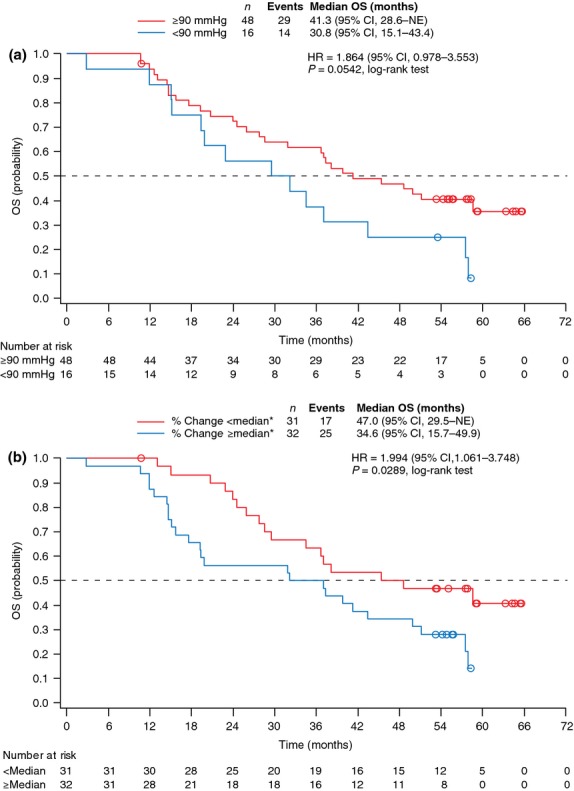
Kaplan–Meier estimates of overall survival by (a) maximum diastolic blood pressure from initiation of treatment to cycle 2 day 1 and (b) percent change in sVEGFR-2 from baseline to cycle 2 day 1. *Median % change = −33.5. CI, confidence interval; HR, hazard ratio; NE, not estimable; OS, overall survival; sVEGFR, soluble vascular endothelial growth factor receptor.
Discussion
In this final analysis of the phase II study of axitinib conducted in Japanese patients with cytokine-refractory mRCC, IRC-assessed ORR remained just over 50% with median PFS of 11.0 months, confirming the results of the primary analysis that axitinib has substantial antitumor activity in Japanese patients with previously treated mRCC.(21) In addition, median OS was estimated at 37.3 months, and toxicities with long-term axitinib treatment (median treatment duration of 14.2 months) were generally manageable in Japanese patients. Although any cross-study comparison must be interpreted with caution, ORR and median PFS observed in this single-arm, open-label phase II study were comparable to those observed in axitinib-treated Japanese patients with cytokine-refractory mRCC enrolled in the phase III AXIS trial (65.0% and 12.1 months, respectively, assessed by the IRC),(20) providing further support for axitinib as an effective second-line treatment option for mRCC in Japanese patients.
Overall survival is impacted not only by the study treatment but also by several factors including patient baseline characteristics as well as any treatment(s) patients may receive post-study. In the current study, Japanese patients were heavily treated with other systemic treatments after they discontinued; all 59 patients who discontinued the axitinib study received at least one follow-up treatment and approximately 50% received two or more treatments. Other VEGFR inhibitors were used more frequently than mTOR inhibitors as post-axitinib treatment. Following the AXIS trial, just over 50% of patients who discontinued axitinib on study were treated with any subsequent systemic treatment, and mTOR inhibitors were administered slightly more often than VEGFR inhibitors (39% vs 33%, respectively).(14) It is conceivable that aggressive post-study systemic treatment may have contributed, at least in part, to the longer OS achieved in this study compared with median OS of 29.4 months in the overall population previously treated with cytokines in the AXIS trial.(14) The median OS of 37.3 months achieved in this study with axitinib is numerically longer than 32.5 months (95% CI, 19.8-not reached) for sunitinib(22) or 25.3 months (95% CI, 19.0–32.0) for sorafenib(23) reported in phase II studies of these VEGFR inhibitors in Japanese patients with mRCC. Although baseline patient characteristics are seemingly comparable, no information on post-study treatment is provided in either study.
Baseline ECOG PS 0 was associated with longer OS in Japanese patients with mRCC, in agreement with the results of the post-hoc analyses from the AXIS trial.(14) Whereas the AXIS trial analyses additionally identified baseline MSKCC risk group as a prognostic factor for survival, the difference in OS between the favorable and poor risk group did not reach statistical significance in our study, which may be explained, at least in part, by the much smaller number of patients in this study than in the AXIS trial (favorable vs poor: 10 vs 4 patients in this study; 201 vs 238 patients in the AXIS trial). The median OS for the intermediate risk group (41.3 months; n = 47) was longer than for the favorable risk group (33.8 months; n = 10). It should be pointed out that when investigating a potential association between baseline performance status or risk factors and OS, any changes in the status or factors during the study are not being taken into account and a possible impact of any such changes would likely be magnified with a smaller number of patients analyzed.
In an exploratory analysis, we evaluated a potential association between OS and changes in DBP or plasma level of sVEGFR-2. Because BP increases have been reported to occur early after starting treatment with axitinib or other VEGF-targeted therapies,(24–26) maximum DBP observed during the first cycle was used for the analysis. In addition, a maximum increase in DBP during the first cycle has less potential to be influenced by treatment with antihypertensive agents in response to elevated BP than in subsequent cycles. Median OS was longer in patients who experienced DBP ≥90 mmHg than in those who did not (P = 0.0542). Hypertension is a known AE associated with agents that block VEGF or VEGFR, including axitinib.(1–6) Landmark analyses from the AXIS trial showed that patients with DBP ≥90 mmHg within the first 8 and 12 weeks of randomization had longer OS compared with those who did not experience elevated BP during the same period of time for both axitinib and sorafenib.(14) Furthermore, pharmacokinetic-pharmacodynamic analysis of three phase II studies of axitinib in mRCC, including the current study, indicated that DBP was only weakly correlated with plasma exposure, and it was an independent predictor for OS, PFS and higher probability of achieving PR.(27) These findings taken together suggest that axitinib-related elevated BP/hypertension may be used as an early marker for identifying patients who benefit most from axitinib treatment. Decreased plasma levels of soluble (extracellular domain) VEGFR-2 and increased levels of VEGF have also been observed following administration of VEGFR TKI,(28–30) although the results of analyses evaluating the relationship between these plasma proteins and efficacy endpoints have been mixed. In our analysis, patients who had greater percent decrease than median (−33.5%) in sVEGFR-2 had longer OS than those who had less than median decrease (P = 0.0289), which is consistent with better PFS and ORR observed with greater reduction in sVEGFR-2 reported in the primary publication.(21) Neither percent change in sVEGFR-1, sVEGFR-3 or VEGF levels nor baseline levels of sVEGFR-2 were strongly correlated with OS (data not shown). The current findings support the use of changes in sVEGFR-2 as a biomarker for survival in patients with mRCC following axitinib treatment.
The safety profile of axitinib after longer duration of treatment in this study did not show any unexpected AE or increased incidence of individual AE compared with the original report,(21) which is in agreement with negligible plasma accumulation of axitinib following multiple dosing.(26,31,32) Although 44% of patients were already taking antihypertensive medications prior to study entry, over 50% of patients developed hypertension after one cycle of axitinib treatment, which was the main reason for axitinib dose reductions or temporary dose interruptions. However, none of the patients discontinued axitinib treatment due to hypertension because they were closely monitored for BP, and elevated BP was generally controlled with dose modifications and/or additional or new antihypertensive medications. Hand-foot syndrome, diarrhea, proteinuria and fatigue were other common AE leading to dose modifications, but only proteinuria led to axitinib treatment discontinuation. The safety profile of axitinib in Japanese patients in this study was similar to that in patients in the Western study of cytokine-refractory mRCC,(33) except Japanese patients had higher incidence of proteinuria and hand-foot syndrome (63% vs 8% and 75% vs 8%, respectively). In addition, 48% of Japanese patients developed hypothyroidism, one half of whom were given thyroid hormone replacement therapy. Higher incidence rates for some AE observed in this study might account for the higher axitinib dose reduction (72% vs 29%, respectively) and lower median total daily dose (6.6 vs 8.83 mg, respectively) in Japanese patients than Western patients. Previous subgroup analyses of the AXIS trial indicated hypothyroidism in 44% of axitinib-treated Japanese patients compared with 19% in the overall population, confirming higher incidence of hypothyroidism reported in Japanese patients.(20) Abnormalities of thyroid function associated with sunitinib(34) and sorafenib(35) treatment in Japanese patients with mRCC have also been reported.
In conclusion, the study showed median OS exceeding 3 years with axitinib use in Japanese patients with cytokine-refractory mRCC, and toxicities with long-term treatment with axitinib were generally controllable with axitinib dose modification and/or standard medications in this population. Hypertension, hand-foot syndrome, proteinuria and hypothyroidism were reported frequently in axitinib-treated Japanese patients, warranting close monitoring and management of these toxicities. Changes in the plasma levels of sVEGFR-2 may be used as a prognostic factor for OS in mRCC following axitinib treatment.
Acknowledgments
Japan Axitinib Phase II Study Group: the following investigators and investigational sites also participated in this study: H. Fujimoto, National Cancer Center Hospital, Department of Urology, Tokyo, Japan; H. Nakazawa, Tokyo Women's Medical University Medical Center East, Department of Urology, Tokyo, Japan; N. Matsubara, National Cancer Center Hospital East, Department of Oncology/Hematology, Chiba, Japan; T. Fujioka, Iwate Medical University School of Medicine, Department of Urology, Iwate, Japan; M. Niwakawa, Shizuoka Cancer Center, Department of Urology, Shizuoka, Japan; J. Miyazaki, University of Tsukuba, Department of Urology, Ibaraki, Japan; T. Nakamura, Kyoto Prefectural University of Medicine, Department of Urology, Kyoto, Japan; T. Shuin, Kochi University, Kochi Medical School, Department of Urology, Kochi, Japan; Y. Hasegawa, National Kyushu Cancer Center, Department of Urology, Fukuoka, Japan; N. Tsuchiya, Akita University School of Medicine, Department of Urology, Akita, Japan; S. Takahashi, Nihon University School of Medicine Itabashi Hospital, Department of Urology, Tokyo, Japan; N. Nonomura, Osaka University Graduate School of Medicine, Department of Urology, Osaka, Japan; and K. Nishiyama, Kagoshima University Graduate School of Medical and Dental Sciences, Department of Urology, Kagoshima, Japan. This study was sponsored by Pfizer Japan Inc. Medical writing support was provided by Mariko Nagashima of Engage Scientific Solutions (Southport, CT, USA), and was funded by Pfizer Inc.
Disclosure Statement
M. Eto received honoraria from Pfizer, Bayer, Novartis and GlaxoSmithKline, and research funding from Pfizer and Novartis. H. Uemura received honoraria from Pfizer, Bayer, Novartis and GlaxoSmithKline, research funding from Pfizer and Novartis, and fees for promotional materials from Pfizer, Bayer and Novartis. Y. Tomita receivied honoraria and research funding from Pfizer and Novartis. H. Kanayama received honoraria from Pfizer, Bayer, Novartis and GlaxoSmithKline, and fees for promotional materials from Pfizer. N. Shinohara received honoraria from Pfizer, Bayer and Novartis. Y. Kamei, Y. Fujii, and Y. Umeyama are employees of Pfizer Japan Inc. S. Ozono received honoraria from Pfizer, Novartis and GlaxoSmithKline, research funding from Pfizer, Bayer, Novartis and GlaxoSmithKline, and fees for promotional materials from Pfizer. S. Naito received honoraria from Pfizer, Bayer, Novartis and GlaxoSmithKline, research funding from Novartis, and fees for promotional materials from Bayer and GlaxoSmithKline. H. Akaza received honoraria from Pfizer, Bayer and GlaxoSmithKline.
References
- 1.Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet. 2007;370:2103–11. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 2.Rini BI, Halabi S, Rosenberg JE, et al. Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB 90206. J Clin Oncol. 2008;26:5422–8. doi: 10.1200/JCO.2008.16.9847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–34. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 4.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–24. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 5.Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061–8. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 6.Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378:1931–9. doi: 10.1016/S0140-6736(11)61613-9. [DOI] [PubMed] [Google Scholar]
- 7.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–81. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 8.Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–56. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 9.Escudier B, Bellmunt J, Negrier S, et al. Phase III trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma (AVOREN): final analysis of overall survival. J Clin Oncol. 2010;28:2144–50. doi: 10.1200/JCO.2009.26.7849. [DOI] [PubMed] [Google Scholar]
- 10.Rini BI, Halabi S, Rosenberg JE, et al. Phase III trial of bevacizumab plus interferon alfa versus interferon alfa monotherapy in patients with metastatic renal cell carcinoma: final results of CALGB 90206. J Clin Oncol. 2010;28:2137–43. doi: 10.1200/JCO.2009.26.5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Escudier B, Eisen T, Stadler WM, et al. Sorafenib for treatment of renal cell carcinoma: final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial. J Clin Oncol. 2009;27:3312–8. doi: 10.1200/JCO.2008.19.5511. [DOI] [PubMed] [Google Scholar]
- 12.Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:3584–90. doi: 10.1200/JCO.2008.20.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sternberg CN, Hawkins RE, Wagstaff J, et al. A randomised, double-blind phase III study of pazopanib in patients with advanced and/or metastatic renal cell carcinoma: final overall survival results and safety update. Eur J Cancer. 2013;49:1287–96. doi: 10.1016/j.ejca.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 14.Motzer RJ, Escudier B, Tomczak P, et al. Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol. 2013;14:552–62. doi: 10.1016/S1470-2045(13)70093-7. [DOI] [PubMed] [Google Scholar]
- 15.Motzer RJ, Escudier B, Oudard S, et al. Phase 3 trial of everolimus for metastatic renal cell carcinoma: final results and analysis of prognostic factors. Cancer. 2010;116:4256–65. doi: 10.1002/cncr.25219. [DOI] [PubMed] [Google Scholar]
- 16.Hutson TE, Bellmunt J, Porta C, et al. Long-term safety of sorafenib in advanced renal cell carcinoma: follow-up of patients from phase III TARGET. Eur J Cancer. 2010;46:2432–40. doi: 10.1016/j.ejca.2010.06.121. [DOI] [PubMed] [Google Scholar]
- 17.Molina AM, Jia X, Feldman DR, et al. Long-term response to sunitinib therapy for metastatic renal cell carcinoma. Clin Genitourin Cancer. 2013;11:297–302. doi: 10.1016/j.clgc.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rini BI, de La Motte Rouge T, Harzstark AL, et al. Five-year survival in patients with cytokine-refractory metastatic renal cell carcinoma treated with axitinib. Clin Genitourin Cancer. 2013;11:107–14. doi: 10.1016/j.clgc.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Akaza H, Fukuyama T. Axitinib for the treatment of advanced renal cell carcinoma. Expert Opin Pharmacother. 2014;15:283–97. doi: 10.1517/14656566.2014.868436. [DOI] [PubMed] [Google Scholar]
- 20.Ueda T, Uemura H, Tomita Y, et al. Efficacy and safety of axitinib versus sorafenib in metastatic renal cell carcinoma: subgroup analysis of Japanese patients from the global randomized Phase 3 AXIS trial. Jpn J Clin Oncol. 2013;43:616–28. doi: 10.1093/jjco/hyt054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomita Y, Uemura H, Fujimoto H, et al. Key predictive factors of axitinib (AG-013736)-induced proteinuria and efficacy: a phase II study in Japanese patients with cytokine-refractory metastatic renal cell Carcinoma. Eur J Cancer. 2011;47:2592–602. doi: 10.1016/j.ejca.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 22.Tomita Y, Shinohara N, Yuasa T, et al. Overall survival and updated results from a phase II study of sunitinib in Japanese patients with metastatic renal cell carcinoma. Jpn J Clin Oncol. 2010;40:1166–72. doi: 10.1093/jjco/hyq146. [DOI] [PubMed] [Google Scholar]
- 23.Naito S, Tsukamoto T, Murai M, Fukino K, Akaza H. Overall survival and good tolerability of long-term use of sorafenib after cytokine treatment: final results of a phase II trial of sorafenib in Japanese patients with metastatic renal cell carcinoma. BJU Int. 2011;108:1813–9. doi: 10.1111/j.1464-410X.2011.10281.x. [DOI] [PubMed] [Google Scholar]
- 24.Maitland ML, Kasza KE, Karrison T, et al. Ambulatory monitoring detects sorafenib-induced blood pressure elevations on the first day of treatment. Clin Cancer Res. 2009;15:6250–7. doi: 10.1158/1078-0432.CCR-09-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Azizi M, Chedid A, Oudard S. Home blood-pressure monitoring in patients receiving sunitinib. N Engl J Med. 2008;358:95–7. doi: 10.1056/NEJMc072330. [DOI] [PubMed] [Google Scholar]
- 26.Mukohara T, Nakajima H, Mukai H, et al. Effect of axitinib (AG-013736) on fatigue, thyroid-stimulating hormone, and biomarkers: a phase I study in Japanese patients. Cancer Sci. 2010;101:963–8. doi: 10.1111/j.1349-7006.2009.01465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rini BI, Garrett M, Poland B, et al. Axitinib in metastatic renal cell carcinoma: results of a pharmacokinetic and pharmacodynamic analysis. J Clin Pharmacol. 2013;53:491–504. doi: 10.1002/jcph.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deprimo SE, Bello CL, Smeraglia J, et al. Circulating protein biomarkers of pharmacodynamic activity of sunitinib in patients with metastatic renal cell carcinoma: modulation of VEGF and VEGF-related proteins. J Transl Med. 2007;5:32. doi: 10.1186/1479-5876-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pena C, Lathia C, Shan M, Escudier B, Bukowski RM. Biomarkers predicting outcome in patients with advanced renal cell carcinoma: Results from Sorafenib Phase III Treatment Approaches in Renal Cancer Global Evaluation Trial. Clin Cancer Res. 2010;16:4853–63. doi: 10.1158/1078-0432.CCR-09-3343. [DOI] [PubMed] [Google Scholar]
- 30.Hutson TE, Davis ID, Machiels JH, et al. Biomarker analysis and final efficacy and safety results of a phase II renal cell carcinoma trial with pazopanib (GW786034), a multi-kinase angiogenesis inhibitor. J Clin Oncol. 2008;26(Suppl):5046. abstract. [Google Scholar]
- 31.Rugo HS, Herbst RS, Liu G, et al. Phase I trial of the oral antiangiogenesis agent AG-013736 in patients with advanced solid tumors: pharmacokinetic and clinical results. J Clin Oncol. 2005;23:5474–83. doi: 10.1200/JCO.2005.04.192. [DOI] [PubMed] [Google Scholar]
- 32.Fujiwara Y, Kiyota N, Chayahara N, et al. Management of axitinib (AG-013736)-induced fatigue and thyroid dysfunction, and predictive biomarkers of axitinib exposure: results from phase I studies in Japanese patients. Invest New Drugs. 2012;30:1055–64. doi: 10.1007/s10637-011-9637-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rixe O, Bukowski RM, Michaelson MD, et al. Axitinib treatment in patients with cytokine-refractory metastatic renal-cell cancer: a phase II study. Lancet Oncol. 2007;8:975–84. doi: 10.1016/S1470-2045(07)70285-1. [DOI] [PubMed] [Google Scholar]
- 34.Sato S, Muraishi K, Tani J, et al. Clinical characteristics of thyroid abnormalities induced by sunitinib treatment in Japanese patients with renal cell carcinoma. Endocr J. 2010;57:873–80. doi: 10.1507/endocrj.k10e-130. [DOI] [PubMed] [Google Scholar]
- 35.Miyake H, Kurahashi T, Yamanaka K, et al. Abnormalities of thyroid function in Japanese patients with metastatic renal cell carcinoma treated with sorafenib: a prospective evaluation. Urol Oncol. 2010;28:515–9. doi: 10.1016/j.urolonc.2009.08.011. [DOI] [PubMed] [Google Scholar]


