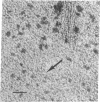
Abstract
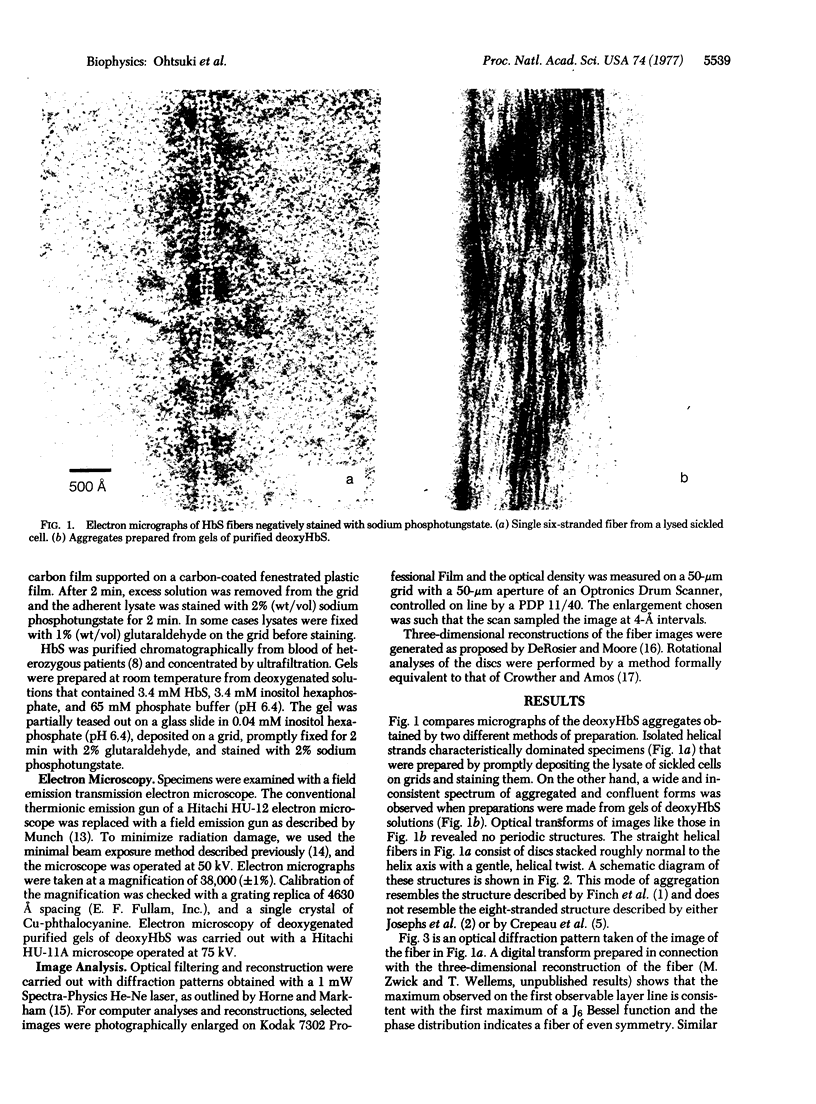
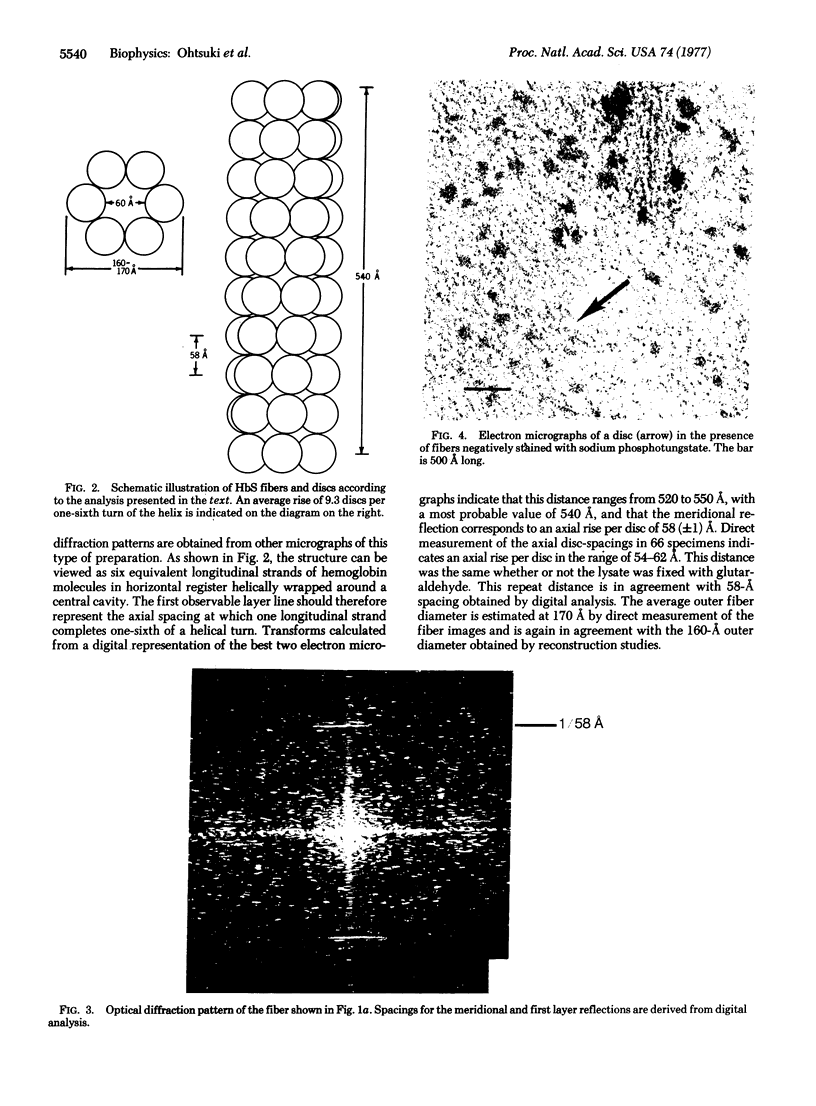
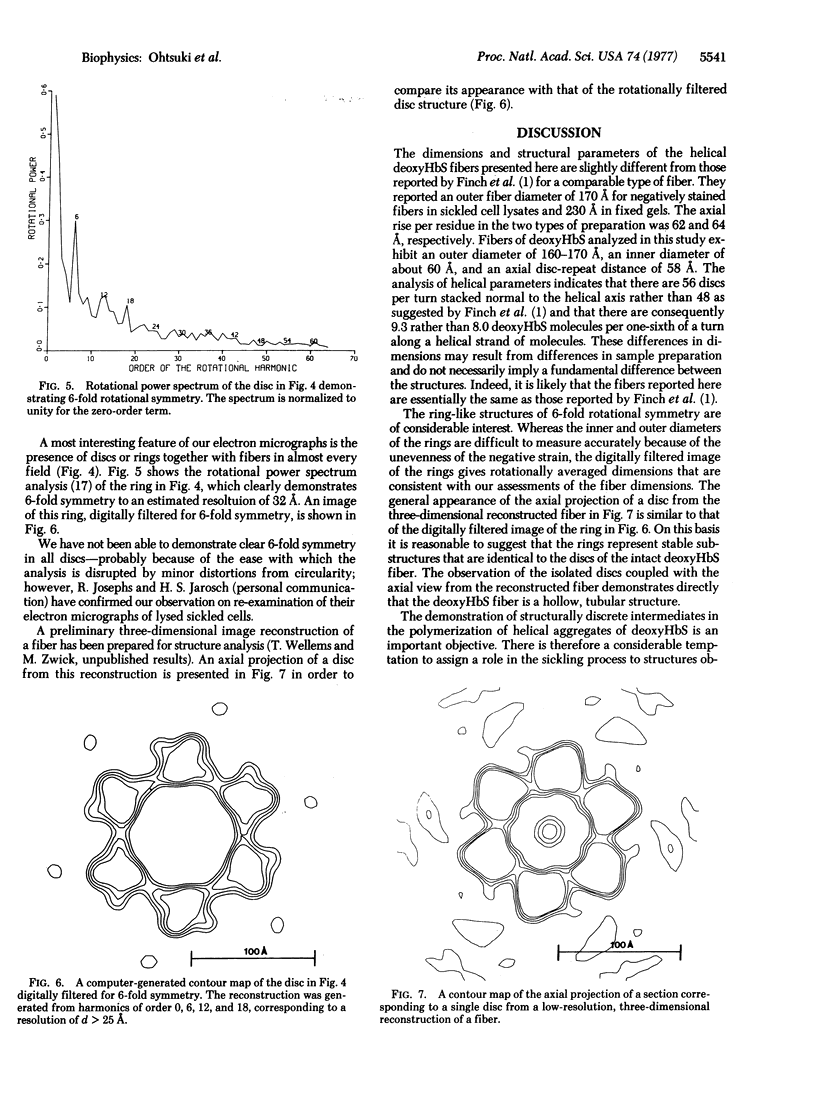
Aggregated forms of deoxyhemoglobin S were examined with a field emission transmission electron microscope. Images of isolated helical fibers were obtained from sickled cell lysates stained directly on the electron microscope grid. Optical and digital analyses of the electron micrographs showed that the fibers are similar to those characterized by J. T. Finch, M. F. Perutz, J. F. Bertles, and J. Döbler [(1973) Proc. Natl. Acad. Sci. USA 70, 718-722] in that they consist of stacked discs each composed of six hemoglobin molecules. The fibers exhibit an outer diameter of 160-170 Å and an inner diameter of about 60 Å with an axial spacing of 58 Å per disc. The fiber can be described as a helix consisting of 56 discs per helical turn. We observed discs of six hemoglobin molecules, which may be stable substructural components of the fibers. They were observed in preparations of hemoglobin fibers and exhibited 6-fold symmetry by power spectrum analysis. A reconstructed image of a disc digitally filtered for 6-fold symmetry has a maximum external diameter of ∼170 Å and a central hole of 60 Å diameter and is similar to the axial projection of a single disc from a low-resolution, three-dimensional reconstructed model of a fiber.
Keywords: deoxyhemoglobin S, image reconstruction, mutant hemoglobins, optical diffraction, sickle cell anemia
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertles J. F., Döbler J. Reversible and irreversible sickling: a distinction by electron microscopy. Blood. 1969 Jun;33(6):884–898. [PubMed] [Google Scholar]
- Bookchin R. M., Nagel R. L. Ligand-induced conformational dependence of hemoglobin in sickling interactios. J Mol Biol. 1971 Sep 14;60(2):263–270. doi: 10.1016/0022-2836(71)90292-0. [DOI] [PubMed] [Google Scholar]
- Crepeau R. H., Dykes G., Edelstein S. J. Structure of the fibers of sickle cell hemoglobin in the presence of 2,3-diphosphoglycerate. Biochem Biophys Res Commun. 1977 Mar 21;75(2):496–502. doi: 10.1016/0006-291x(77)91069-5. [DOI] [PubMed] [Google Scholar]
- Crowther R. A., Amos L. A. Harmonic analysis of electron microscope images with rotational symmetry. J Mol Biol. 1971 Aug 28;60(1):123–130. doi: 10.1016/0022-2836(71)90452-9. [DOI] [PubMed] [Google Scholar]
- DeRosier D. J., Moore P. B. Reconstruction of three-dimensional images from electron micrographs of structures with helical symmetry. J Mol Biol. 1970 Sep 14;52(2):355–369. doi: 10.1016/0022-2836(70)90036-7. [DOI] [PubMed] [Google Scholar]
- Finch J. T., Perutz M. F., Bertles J. F., Döbler J. Structure of sickled erythrocytes and of sickle-cell hemoglobin fibers. Proc Natl Acad Sci U S A. 1973 Mar;70(3):718–722. doi: 10.1073/pnas.70.3.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofrichter J., Hendricker D. G., Eaton W. A. Structure of hemoglobin S fibers: optical determination of the molecular orientation in sickled erythrocytes. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3604–3608. doi: 10.1073/pnas.70.12.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofrichter J., Ross P. D., Eaton W. A. Kinetics and mechanism of deoxyhemoglobin S gelation: a new approach to understanding sickle cell disease. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4864–4868. doi: 10.1073/pnas.71.12.4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INGRAM V. M. Gene mutations in human haemoglobin: the chemical difference between normal and sickle cell haemoglobin. Nature. 1957 Aug 17;180(4581):326–328. doi: 10.1038/180326a0. [DOI] [PubMed] [Google Scholar]
- Josephs R., Jarosch H. S., Edelstein S. J. Polymorphism of sickle cell hemoglobin fibers. J Mol Biol. 1976 Apr 15;102(3):409–426. doi: 10.1016/0022-2836(76)90324-7. [DOI] [PubMed] [Google Scholar]
- Levinthal C., Wodak S. J., Kahn P., Dadivanian A. K. Hemoglobin interaction in sickle cell fibers. I: Theoretical approaches to the molecular contacts. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1330–1334. doi: 10.1073/pnas.72.4.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magdoff-Fairchild B., Swerdlow P. H., Bertles J. F. Intermolecular organization of deoxygenated sickle haemoglobin determined by x-ray diffraction. Nature. 1972 Sep 22;239(5369):217–219. doi: 10.1038/239217a0. [DOI] [PubMed] [Google Scholar]
- Moffat K., Gibson Q. H. The rates of polymerization and depolymerization of sickle cell hemoglobin. Biochem Biophys Res Commun. 1974 Nov 6;61(1):237–242. doi: 10.1016/0006-291x(74)90558-0. [DOI] [PubMed] [Google Scholar]
- Ohtsuki M., Zeitler E. Minimal beam exposure with a field emission source. Ultramicroscopy. 1975 Dec;1(2):163–165. doi: 10.1016/s0304-3991(75)80021-0. [DOI] [PubMed] [Google Scholar]
- Ross P. D., Hofrichter J., Eaton W. A. Calorimetric and optical characterization of sickle cell hemoglobin gelation. J Mol Biol. 1975 Aug 5;96(2):239–253. doi: 10.1016/0022-2836(75)90345-9. [DOI] [PubMed] [Google Scholar]
- White J. G. Ultrastructural features of erythrocyte and hemoglobin sickling. Arch Intern Med. 1974 Apr;133(4):545–562. [PubMed] [Google Scholar]
- Wishner B. C., Ward K. B., Lattman E. E., Love W. E. Crystal structure of sickle-cell deoxyhemoglobin at 5 A resolution. J Mol Biol. 1975 Oct 15;98(1):179–194. doi: 10.1016/s0022-2836(75)80108-2. [DOI] [PubMed] [Google Scholar]