Abstract
Temporal lobe epilepsy (TLE) is a disorder of the central nervous system in which hippocampus is mostly involved and causes memory impairment. Kindling is a model of inducing epilepsy which is created through pentylenetetrazol (PTZ) administration. This study examines the role of the aqueous extract of Boswellia on the learning and development of brain (formation of dendritic branches and axons) of the PTZ-induced kindled rats. The study is conducted on sixty-four male rats divided into 8 groups. Kindling seizures are induced by three injections of 25 mg/kg of PTZ every 15 min. The aqueous extracts (0, 0.1, 0.5, 1 g/kg, i.p) are administrated to all animals for three consecutive days. Passive avoidance learning of animals is examined using shuttle box apparatus and step-through latency (STL) method. Rats are anesthetized and their brains are fixed by transcardial perfusion method and are analyzed by morphometric methods after applying Golgi and Cresyl violet staining methods. PTZ-induced kindling indicates a significant decrease in the number of pyramidal neurons and dendritic spines in hippocampal region cornu ammonis (CA1). The STL of the kindled rats is significantly reduced compared with control ones. Also, Boswellia extract dramatically increased the number of neuronal processes in CA1 region and improves passive-avoidance learning ability in both control and PTZ-kindled animals in 1 g/kg dose administration of Boswellia extract, especially at high doses can eliminate adverse effects of seizures on cognitive function in hippocampal area CA1 in rats.
Keywords: PTZ-kindling, Boswellia serrata, Hippocampus, CA1
INTRODUCTION
Temporal lobe epilepsy (TLE) is one of the most common diseases of central nervous system in the world which is characterized by recurrent spontaneous seizures due to neuronal hyperactivity in the brain (1). The most important signs of epileptic patients are memory and learning dysfunctions (2). TLE is the most common type of epilepsy in adults. In most cases, the involving area is the medial temporal structure in the brain, especially hippocampus (3). Hippocampal structures have indicated a specific pattern of cell damage and death in CA1 region in a large group of patients with TLE (4). Hippocampal sclerosis is the most common tissue damage in TLE (5). Cornu ammonis (CA1) region belongs to hippocampus and plays a vital role in converting short-term memory to long-term memory (6). A periodic systemic injection of convulsive drugs, such as pentylenetetrazol (PTZ) induces seizures in animals (7). PTZ-induced kindling is an accepted animal model for studying epilepsy and its consequences on memory (8). Kindling, at cell surface, increases the N-methyl-D-aspartate (NMDA) receptors-dependent ion process and influences the inhibition induced by gamma amino butyric acid (GABA) (9). The seizure resulting from kindling causes atrophy, damage, and decrease of neurons in different areas of hippocampus as well as spatial memory damage (10). These days, pharmacotherapy with psychoactive drugs are available specially in diseases such as epilepsy and dementia, however they are not effective in all cases and exert numerous side effects, especially upon long-term administration (11). Herbal medicine is commonly used for treating the diseases such as amnesia as well as reinforcing memory. One of the most common herbs used for improving memory performance is frankincense. The resin of this plant is known by various names like olibanum and frankincense obtained from Burseracea tree of genus Boswellia which grows in regions such as Ethiopia, India and Saudi Arabia (12). In Ayurvedic medicine, Indian frankincense (Boswellia Serrata) has been used for hundreds of years for treating arthritis, healing wounds and strengthening female hormone system (13). Frankincense was mentioned as an effective resin in Iran's traditional medicine and by Islamic physicians like Ave Sina, al-Razi, and others (14). It seems that Boswellia can positively affect the development of brain and probably proper formation of dendritic trees and axons and sets up a proper relationship between them (15). These effects can be attributed to the active compound, boswellic acid, which increases in-vitro polymerization of microtubule protein (MTP) in hippocampal neurons, which in turn is followed by acceleration of axonal sprouting and dendritic branching (16). The present study was conducted to investigate the effects of Boswellia extract on learning and hippocampal deficit in kindled rats.
MATERIALS AND METHODS
Experimental animals and groups
Sixty-four Wistar male rats with the weight range of 220-250 g were purchased from Pasteur institute (Tehran-Iran). The animals were kept in the temperature of 24 ± 2° C under controlled environmental conditions, 12/12 h light/dark cycle and free access to water and food. All experiments were carried out according to the guidelines of German protection of animal act (Deutsches tiers chutzges etz, BGBI 1998 pant I no 30, S.1109 ff). The rats were randomly divided in eight groups (1- saline-saline, 2- ptz-saline, 3- saline-extract 0.1 g/kg, 4- saline-extract 0.5 g/kg, 5- saline-extract 1 g/kg, 6- ptz-extract 0.1 g/kg, 7- ptz-extract 0.5 g/kg, 8- ptz-extract 1 g/kg) (n=8).
Preparation of extract
Boswellia serrata was purchased from a traditional medicine center and was identified and authenticated by a botanist. 100 g of the dry powder of frankincense resin was soaked in 1000 ml boiled water. This solution was gently heated for one h and centrifuged to obtain a clear smell. Twenty-four g of the dry extract was obtained from every 100 g of powder. This extract was dissolved in normal saline and injected intraperitonealy (i.p.) to the animals (17).
Kindling method
Kindling seizures were induced by repetitive i.p. injections of PTZ (25 mg/kg, 1 ml/kg) every 15 min, but the total dose did not exceed 75 mg/kg (three injections) (18). Control animals received normal saline instead. Immediately after injection, the incidence of the seizures activity of rats was observed in an isolated Plexiglas box for 45 min. The severity of the seizures was evaluated using five-score scale (19). 0: no change in behavior, 1: contraction of the face and hands’ muscles, 2: diffusion of contractile wave around the body, 3: myoclonic jerks and standing on two feet, 4: tonic-clonic attacks and falling on the side, 5: tonic-clonic attacks and falling on the back.
Only the animals which reached stages 4/5 were selected for the experiments. LD50 (2 g/kg) has been reported for Boswellia extract in mice and rats (20). The first extract injection was done in the first day 45 min after the PTZ or normal saline administration. The extract was administered to each group (n=8) in specified doses (0, 0.1, 0.5 or 1 g/kg, 1 ml/kg) in the second and third days as well.
Shuttle box
Twenty-four h later, the passive-avoidance learning ability of animals were evaluated using shuttle-box apparatus. The apparatus consisted of two separate chambers (20 × 30 × 20 cm3) separated by a guillotine door through which the animal can pass when it is open. The walls and floor of one of the chambers were white (light chamber) while those of the other one was black (dark chamber). The both chambers’ floor was covered by parallel metal bars through which electric stimulation with desired voltage and time could be delivered to animals’ feet using the stimulator attached to them. Passive-avoidance learning was evaluated in three stages based on a routine method. In summary, the passive avoidance task is a fear-aggravated test used to evaluate learning and memory in rodent models of CNS disorders. In this test, subjects learn to avoid an environment in which an aversive stimulus (such as a foot-shock) was previously delivered (21).
Tissue preparation via transcardial perfusion
At first, the animals were intraperitoneally anesthetized with ketamine (200 μl) and diazepam (50 μl) with insulin syringe (the injection volume was 1 ml/kg per animal). The cannula connected to normal saline solution was inserted into aorta by making an incision in the left ventricle. The descending aorta was closed and after washing the brain, the solution was removed through the incision made in the right atrium. Formalin 4% and buffer phosphate 6% were injected into brain via cannula and the brain was fixed in 20 min (22).
Histological methods
Having removed the brains, the right hemispheres were stained by Golgi staining method to determine the number of neuronal processes in CA1 region. In summary, samples were fixed in formaldehyde solution and were transferred in the first solution (50 ml of 2% potassium dichromate solution, 10 ml of 37% formalin solution and 5 ml of concentrated acetic acid solution), were washed with distilled water, and were shifted to the second solution (1 g of 75% silver nitrate was dissolved in 133 ml distilled water). Subsequently, tissue processing, including dehydration, clearing, and embedding, was performed. Microscopic sections (35 μm) were prepared and analyzed morphologically (23).
Cresyl violet staining method
After making 5 μm incisions by microtome and performing tissue processing, the left hemispheres were stained using Cresyl violet staining method by a progressive dehydration protocol procedure to count the number of neurons. Brain slices were progressively dehydrated in 70% alcohol (for 10 min), 95% alcohol (with a few drops of 10% acetic acid; for 2–3 min), and finally 100% alcohol (for 10 min). Slices were cleared in xylene for 5 min before being covered with DPX and a coverslip and were stained with cresyl violet (24).
Morphometrical method
In the slides stained by Golgi staining method, the fully stained neurons with cell bodies in the middle part of the tissue sections, distant from the surrounding stained neurons, were included in the study. The dendritic tree of pyramidal neurons was illustrated by camera lucida with 750× magnification and the dendritic exclusion order from the cell body was used for counting the dendritic sections (25). Further, the Sholl method was utilized to assess the concentration of dendritic branches (26). In the slides stained by Cresyl violet method, the round cells without peak nose were considered as live cells. The slides were imaged by Motic microscope and cells were counted by Image J software 1.45.
Statistical analysis
All the quantitative data were presented as mean ± standard deviation. One-way analysis of variance (ANOVA) and LSD post-hoc tests were performed to determine the statistical significance between different groups using SPSS software package 16.0. Significance was accepted at the level of P<0.05.
RESULTS
The shuttle-box apparatus results showed that retention latencies of kindled animals were significantly reduced compared to the control group (p<0.05) (Fig. 1).
Fig. 1.
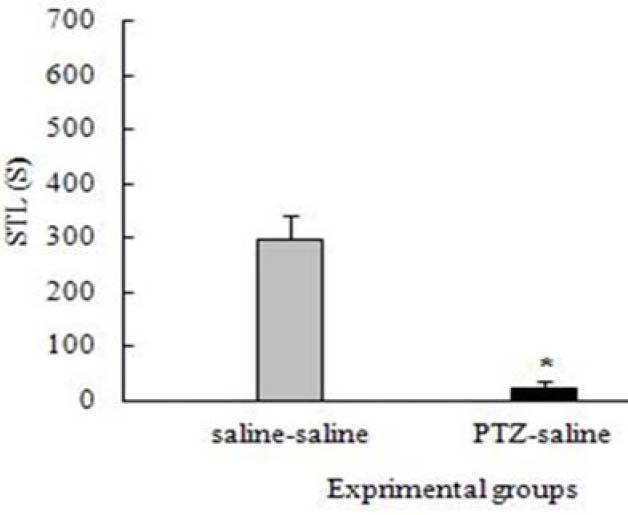
Effect of pentylenetetrazol-induced kindling on the step-through latency (STL). *p<0.05.
When the effect of Boswellia extract on the learning ability of the saline-received (control) animals was tested, an increasing trend of step-hrough latency (STL) was found out which was significant in the group received 0.5 g/kg extract (p<0.05) (Fig. 2). The results revealed that the retention latencies of the kindled animals received Boswellia extract (0.1, 0.5, 1 g/kg) were significantly increased compared to the control group (p<0.05) (Fig. 3). Analyzing PTZ-induced kindling indicated a great decrease in the number of pyramidal neurons and dendritic spines in hippocampal region CA1 compared to control group (p<00.01) (Figs. 4 and 5). Boswellia extract significantly increased the number of neurons in CA1 region at doses of 0.5 and 1 g/kg in comparison with kindled (control) group (p<0.05) (Fig. 6). The number of neuronal dendritic spines revealed a great increase in the kindled rats (1 g/kg), due to the effect of Boswellia extract, compared to the control group (p<0.05). In other groups, however, this increase was not significant (p<0.05) (Fig. 7). Also, administration of Boswellia extract caused an increase in the number of neurons at doses of 0.5 and 1 g/kg in the healthy unkindled rats compared to control (saline) group (p<0.05). However, this increase was not remarkable in other groups (p<0.05) (Fig. 8).
Fig. 2.
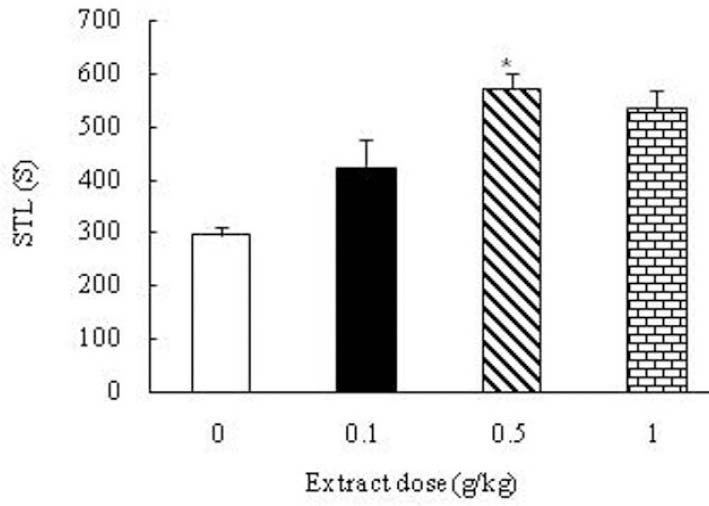
Effect of the Boswellia extract (0, 0.1, 0.5, 1 g/kg) on the learning ability of the saline received (control) animals. Administration of the extract increased step-through latency in the group received 0.5 g/kg of the extract compared to the control group. *p<0.05.
Fig. 3.
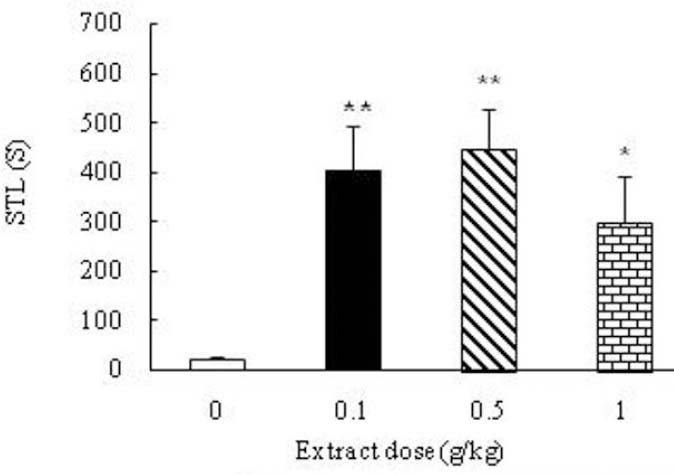
Effect of Boswellia extract on the passive- avoidance learning deficits of the pentylenetetrazol- kindled rats. Administration of the extract significantly increased retention latencies of the kindled animals. *p<0.05, **p<0.01.
Fig. 4.
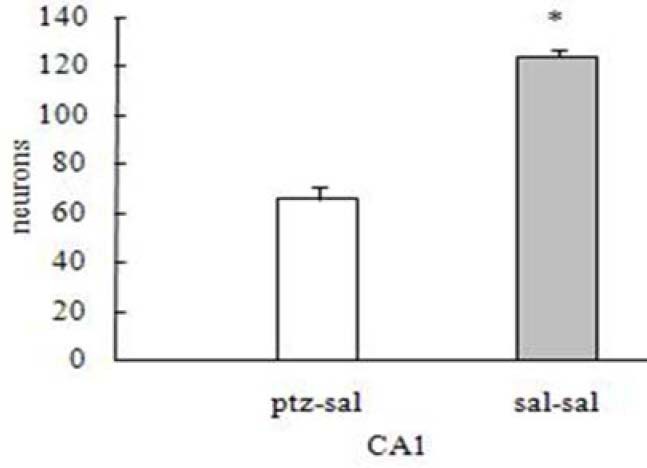
Effect of pentylenetetrazol-induced kindling on the number of pyramidal neurons compared to the control group. *p<0.05
Fig. 5.
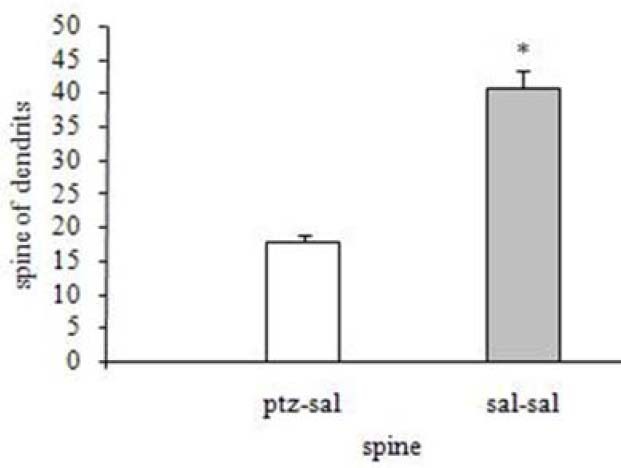
Effect of pentylenetetrazol-induced kindling on the number of dendritic spines in hippocampal region CA1 compared to the control group. *p<0.05
Fig. 6.
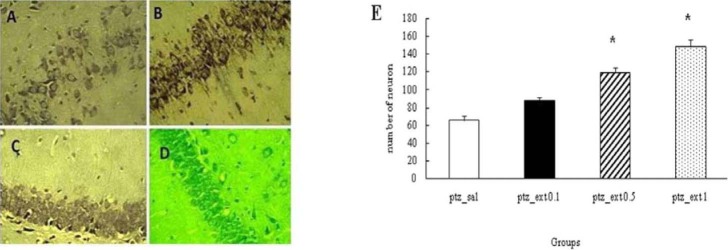
Effect of different doses of Boswellia extract on the number of neurons in CA1 region in the kindled rats. * P<0.05. A;pentylenetetrazol-saline, B;pentylenetetrazol-extract 0.1 g/kg, C;pentylenetetrazol-extract 0.5 g/kg, D;pentylenetetrazol-extract 1 g/kg (magnification 100X)
Fig. 7.
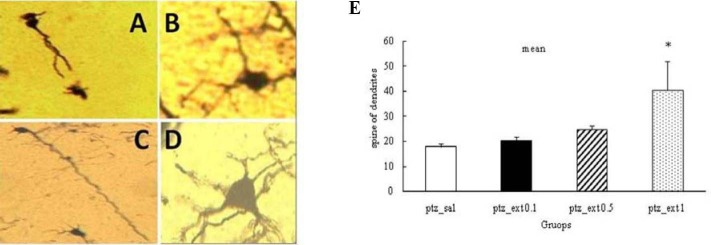
Effect of different doses of Boswellia extract on the number of dendritic spines in CA1 region in the kindled rats. *P<0.05. A; pentylenetetrazol-saline, B; pentylenetetrazol-extract 0.1 g/kg, C; pentylenetetrazol-extract 0.5 g/kg, D; pentylenetetrazol-extract 1 g/kg (magnification 100X)
Fig. 8.
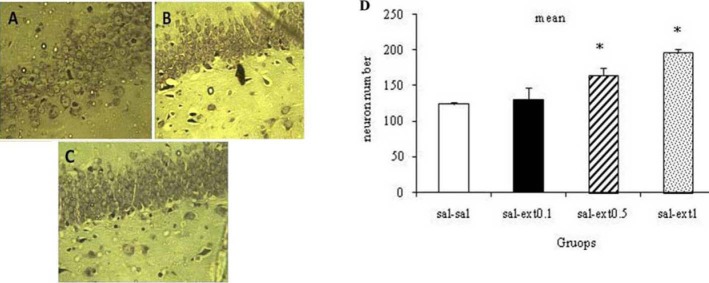
Effect of different doses of Boswellia extract on the number of neurons in CA1 region in the healthy rats. * P<0.05. A; saline-saline, B; saline-extract 0.1 g/kg, C; saline-extract 0.5 g/kg,. D; saline-extract 1 g/kg (magnification 100X).
Further, Boswellia extract increased the dendritic spines of neurons in the healthy unkindled rats in comparison with saline (control) group, which was only noticeable in 1 g/kg group (p<0.05) (Fig. 9).
Fig. 9.
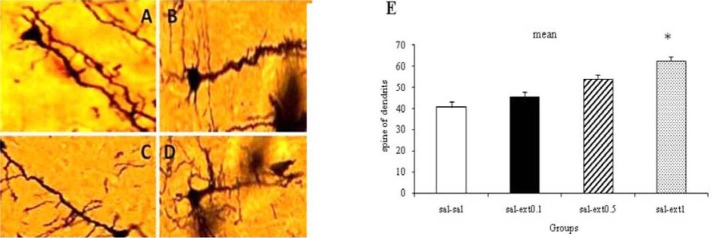
Effect of different doses of Boswellia extract on the number of dendritic spines in CA1 region in the healthy rats * P< 0.05. A; saline-saline, B; saline-extract 0.1 g/kg, C; saline-extract 0.5 g/kg, D; saline-extract 1 g/kg (magnification 100X).
DISCUSSION
Memory defect in neurodegenerative diseases like epilepsy is always considered a challenge, because amnesia and learning difficulties are the most common symptoms of cognitive disorders that cause depression in the epileptic patient. One of the findings of this study is the neurodegenerative effects of PTZ on CA1 region in the rat's brain. It seems that PTZ induces cellular damage in TLE, related to increased expression of some markers involved in cell cycle and apoptosis such as Cyclin B1 (which can interfere with neuronal death) and Bax protein (which plays a major role in cell apoptosis) (27). At the cellular level, PTZ interacts with the NMDA receptor and/or GABAergic system activity. GABA is intimately involved in the regulation of synaptic inhibition in the adult brain. GABA receptor antagonist has been used to induce epileptic seizures in the experimental animals (28). PTZ, as a GABA receptor antagonist, could reduce the GABA- mediated inward chloride current in the adult brain (29). In the study conducted by Thom and coworkers, no evidence was found for the decrease of neurons in the autopsy of the epileptic patients, which is contrary to the results of the present study (30).
Our results also clearly demonstrate that the PTZ-induced seizure has deleterious effects on the learning ability as indicated by passive-avoidance test. A decrease in the learning ability has been reported in clinical studies on memory disorders in epileptic patients (31). According to the current findings, administration of Boswellia extract remarkably improves learning deficits in kindled animals. Frankincense is known as a potent anti-inflammatory agent. In various studies, epilepsy is introduced as a neurodegenerative disease, which can happen following the events that induce inflammatory responses in the central nervous system (32). Calcium is a major stimulus for releasing neurotransmitters, so it plays an essential role in the synaptic facilitation. It has been shown that the extracts of different species of Boswellia cause displacement of calcium.
This plays a role in the synaptic enhancement in hippocampus (33). Also, it can be assumed that Boswellia extracts and various kinds of boswellic acids improve spatial memory through affecting the metabolism of arachidonic acid (34). In the study carried out by Mahmoudi and coworkers, it was reported that different doses of hydroalcoholic extract of Boswellia papyrifera enhanced spatial memory, which is in line with the findings of the present study. The results of the present study are indicative of hippocampal neurogenesis following Boswellia extract administration. The findings indicate that administration of Boswellia extract can exert protective effects on the brain neurons in kindled rats. Thus, it can be assumed that Boswellia is a protective factor against neuronal damages during seizure. It seems that Boswellia decreases expression of Cyclin B1 and Bax protein (involved in cell cycle and apoptosis). Also, potent anti-inflammatory effects are found in Boswellia (27).
Moreover, the findings of this study show an increase in the number of neurons and dendritic spines in hippocampal region CA1 as a consequence of administration of different doses of Boswellia in unkindled rats. Although the precise biological mechanism behind the Boswellia-induced neuritic enhancement is unclear the psychopharmacological and sedative action of Boswellia extract may help to explain enhancement of CA1 dendritic arborization which agrees with the findings reported by Hosseini-sharifabad and colleagues (34). Although the precise mechanism of the increased size of the hippocampus neurons in young rats by maternal consumption of frankincense is unknown but increasing the size of the cell bodies of neurons can be considered as a marker for increasing of neuronal metabolism that have positive effects on hippocampal activity which is a responsible organ for memory.
CONCLUSION
Based on the findings of this study and other relevant studies, it can be argued that the administration of Boswellia extract increases the learning ability and regenerative effects in epileptic animals. However, further studies are needed to identify the advantages and possible complications of this issue.
ACKNOWLEDGMENTS
We sincerely and gratefully thank the Kermanshah University of Medical Sciences for financial support of this project (No. 80152).
REFERENCES
- 1.Engel J. A proposed diagnostic scheme for people with epileptic seizures and with epilepsy: Report of the ILAE task force on classification and terminology. Epilepsia. 2001;42:796–803. doi: 10.1046/j.1528-1157.2001.10401.x. [DOI] [PubMed] [Google Scholar]
- 2.Kang H, Hu Q, Xiao-yan L, Zhi-guang L, Zeng Z, Jian-lin L, et al. A follow-up study on newer anti-epileptic drugs us add-on and monotherapy for partial epilepsy in china. Chin Med J. 2012;125:646–651. [PubMed] [Google Scholar]
- 3.Brodie MJ, Elder AT, Kwan P. Epilepsy in later life. Lancet neurology. 2009;8:1019–1023. doi: 10.1016/S1474-4422(09)70240-6. [DOI] [PubMed] [Google Scholar]
- 4.Babb TL. Axonal growth and neo pathogenesis in human and experimental hippocampal Epilepsy. Adv Neurol. 1997;72:45–51. [PubMed] [Google Scholar]
- 5.Chang BS, Lowenstein DH. Epilepsy. N Engl J Med. 2003;349:1257–1266. doi: 10.1056/NEJMra022308. [DOI] [PubMed] [Google Scholar]
- 6.Portavella M, Vargas JP, Torres B, Salas C. The effect of telencephalic pallial lesions on spatial, temporal and emotional learning in goldfish. J Neurol Neurosurg Psychiatry. 2001;71:397–399. doi: 10.1016/s0361-9230(01)00699-2. [DOI] [PubMed] [Google Scholar]
- 7.Assouline G, Barkaie E, Gutnick MJ. Cysteamine suppresses kindled seizures in pentylenetetrazol-kindled rats . Eur J phormacol. 1984;106:649–652. doi: 10.1016/0014-2999(84)90073-6. [DOI] [PubMed] [Google Scholar]
- 8.Chen Z, Li Z, Sakurai E, Lazed-Mobarakeh J, Ohtsu H, Watanabe T, et al. Chemical kindling induced by pentylentetrazol in histamine H1 receptor gene knockout mice (H1Ko) histidine decarboxylase– deficient mice (HDC (-/-1)) and mast cell–deficient W/W (v) mice. Brain Res. 2003;968:160–162. doi: 10.1016/s0006-8993(03)02229-7. [DOI] [PubMed] [Google Scholar]
- 9.Barkai E, Grossman Y, Gutnick MJ. Long term change in neocortical activity after chemical kindling with systemic pentylen tetrazole: An in vitro study. J Neurophysiol. 1994;72:24–35. doi: 10.1152/jn.1994.72.1.72. [DOI] [PubMed] [Google Scholar]
- 10.Szyndler J, Piechal A, Blecharz- kline K, Skorzewska A. Effect of kindled seizures on rat behavior in water Morris maze test and amino acid concentrations in brain structures . Pharmacological Report. 2006;58:75–82. [PubMed] [Google Scholar]
- 11.Thakur VD, Neugias A. Neuropharmachological profile of Eclipta alba (Lima).Hassk. J Ethnopharmacol. 2005;102:23–31. doi: 10.1016/j.jep.2005.05.037. [DOI] [PubMed] [Google Scholar]
- 12.Archier P, viellescazes C. Characterization of various geographical origin incense based on chemical criteria. Analusis. 2000;28:233–237. [Google Scholar]
- 13.Qurishi Y, Hamid A, Zargar MA, Singh SK, Saxena AK. Potential role of natural molecules in health and disease: Importance of boswellic acid. J Med Plants Res. 2010;4:2778–2785. [Google Scholar]
- 14.Winkling M, Sarikaya S, Rahmanian A, Jodicke A, Boker DK. Boswellic acids inhibit glioma growth: a new treatment options. J Neuro-oncology. 2000;46:97–105. doi: 10.1023/a:1006387010528. [DOI] [PubMed] [Google Scholar]
- 15.Yassin NA, El-Shenawy SM, Mahdy KA, Gouda NA, Marrie AE, Farrag AR. Effect of Boswellia serrata on Alzheimer's disease induced in rats. J Arab Soc Med Res. 2013;8:1–11. [Google Scholar]
- 16.Karima O, Riazi G, Yousefi R, Mosavi Movahedi A. The enhancement effect of beta boswellic acid on hippocampal neuritis outgrowth and branching (an in vitro study) Neurological Science. 2010;31:315–320. doi: 10.1007/s10072-010-0220-x. [DOI] [PubMed] [Google Scholar]
- 17.Raad NH, Mun’im RA, Shakier SM, Khudhair AM, Moayad SH, Kadum YA. Antibacterial activity of aqueous and alcoholic extracts of Capsella Bursa against selected pathogenic bacteria. Amer J BioSci. 2013;1:6–10. [Google Scholar]
- 18.Klioueva LA, Luijtelaar EV, Chepurnova NE, Chepurnova SA. PTZ- induced seizures in rats: effects of age and strain. Physiology & Behavior. 2001;72:421–426. doi: 10.1016/s0031-9384(00)00425-x. [DOI] [PubMed] [Google Scholar]
- 19.Racine RJ. Modification of seizure activity by electrical stimulation Ll Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- 20.Ammon HPT, Make T, Singh GB, Shafayhi H. Inhibitionof leukotriene B4 formation in rat peritoneal neutrophils by an ethanolic extract of the gum resin exudate of boswellia. Olan Ned. 1991;57:253–257. doi: 10.1055/s-2006-960074. [DOI] [PubMed] [Google Scholar]
- 21.Alaei H, Moatar F, Tory L. Effect of the abstract of olibanum on learning and memory. J Qazvin Univ Med Sci. 1999;11:21–28. [Google Scholar]
- 22.Paul CA, Beltz B, Breger Sweeneg J. Perfusion of brain tissues with fixative . Cold Spring Harbor Protocols. 2008;5:110–120. [Google Scholar]
- 23.Chan K-K, Lowe JS. Techniques in neuropathology. In: Bancroft JD, Gamble M, editors. Theory and practice of histological techniquesm. 5th edn. London: Churchill Livingstone; 2002. pp. 397–398. [Google Scholar]
- 24.Pilati N, Barker M, Panteleimonitis S, Donga R, Hamann M. Rapid method combining Golgi and Nissl staining to study neuronal morphology and cyto architecture. J Histochem Cytochem. 2008;56:539–550. doi: 10.1369/jhc.2008.950246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sousa N, Lukoyonav NV, Madieira MD, Almedia OF, Barbosa MM. Reorganization of the morphology of hippocompal neuritis and synapses after stress- induced damage correlates with behavioral improvement. Neuroscience. 2000;97:253–266. doi: 10.1016/s0306-4522(00)00050-6. [DOI] [PubMed] [Google Scholar]
- 26.Sholl PA. Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat. 1953;87:387–406. [PMC free article] [PubMed] [Google Scholar]
- 27.Nagy Z, Esiri MM. Neuronal cyclin B1 expression in the hippocampus in temporal lobe epilepsy. Exp Neurol. 1998;150:240–247. doi: 10.1006/exnr.1997.6753. [DOI] [PubMed] [Google Scholar]
- 28.Rogawski MA, Loscher W. The neurobiology of antiepileptic drugs. Nat Rev Neurosci. 2004;5:553–564. doi: 10.1038/nrn1430. [DOI] [PubMed] [Google Scholar]
- 29.Huang RQ, Bell-Horner Cl, Dibas MI, Covey DF, Drewe JA, Dillon GH. Pentylentetrazone–induced inhibition of recombinant gama-amino butyric acid type A (GABA (A)) receptors: mechanism and site of action. J Pharmacol Exp Ther. 2001;298:986–995. [PubMed] [Google Scholar]
- 30.Thom M, Zhou J, Martinia L. Quantitive postmortem study of the hippocampus in chronic epilepsy: seizure don’t inevitably cause neuronal loss. Brain. 2005;128:1344–1345. doi: 10.1093/brain/awh475. [DOI] [PubMed] [Google Scholar]
- 31.Tavakoli M, Neshat Doost H, Molavi H, Barekatein M, Kormi Nouri R, Mehravi J. Evaluation of memory in refractory temporal lobe epilepsy. J Res Behav Sci. 2011;9:63–69. [Google Scholar]
- 32.Guyton AC, Hall JE. 12th ed. USA: 2011. Text Book of Medical Physiology; pp. 751–752. [Google Scholar]
- 33.Miao L, Peng C, Berthold B, Shengjian D, Dake H, Cuiping R, et al. A boswellic acid-containing extract attenuates hepatic granuloma in C57BL/6 mice infected with Schistosoma japonicum. Parasitology Research. 2013;112:1105–1111. doi: 10.1007/s00436-012-3237-7. [DOI] [PubMed] [Google Scholar]
- 34.Hossein-sharifabad M, Esfandiary E. The effect of maternal Administration of boswellia gum Resin (Frankincense) during lactation or stereological of rat hippocampus. J Isfahan Med Sch. 2012;29:165–172. [Google Scholar]


