Abstract
Tragopogon buphthalmoides (DC.) Boiss, is widely used as a food additive with some imputed health effects in folk medicine of western Iran. Unfortunately, despite the prevalent medicinal uses of the plant, there are no reports on the toxic effects of T. buphthalmoides aerial parts. The present study evaluated the potential toxicity of dried hydroethanolic extract of the species in wistar rats. Also, we investigated antioxidant activity and total phenolic content (TPC) of the extract. In the acute study, single doses of extract were administered orally, and the rats were then monitored for 14 days. In the subchronic toxicity study, the sample was administered to the rats for 45 days. In the antioxidant capacity assays dried extract showed moderate to weak antioxidant activities. Also, the sample showed relatively notable TPC. The results of acute study indicated that the LD50 of T. buphthalmoides is higher than 2500 mg/kg. Biochemical analysis showed some significant changes including creatinine, glucose and triglyceride levels. Moreover, some significant abnormality of lung, kidney and liver organs was observed. Based on the results of this study, adverse effect level (AEL) of dried hydroethanolic extract of T. buphthalmoides considered to be less than 175 mg/kg/day for the male and female rats.
Keywords: Tragopogon buphthalmoides, Antioxidant capacity, Acute toxicity, Subchronic toxicity
INTRODUCTION
Tragopogon buphthalmoides (DC.) Boiss. (Synonym: T. persicus Boiss), which is also named as “Sheng e Iran” and “Sheng e cheshmgaavi”, belongs to the family Asteraceae, tribe Cichorieae (1,2). It is a perennial herb of 5-50 cm height, with linear-lanceolate leaves and yellow flowers that distributed in many countries such as Syria, Turkey and Iran (1). The Genus Tragopogon, commonly known as “salsify” or “goat's beard”, represents 25 species in the flora of Iran (2). Previous phytochemical investigations on T. porrifolius, T. orientalis and T. pretensis led to the identification of dihydro isocoumarins, stilbenoids, olean saponins, triterpene saponins, bibenzyls and flavonoids (3,4,5,6,7,8). Some biological studies showed diaphoretic, antitussive, anti acetylcholine esterase and antioxidant properties for plants of this genus (9,10,11). However, studies on phytochemical and biological profile of T. buphthalmoides have not been carried out, in order to confirm its assumed beneficial properties in folk medicine of western Iran. The present study was undertaken to elucidate the safety profile of T. buphthalmoides via evaluation of acute and subchronic toxicity of hydroethanolic extract in male and female Wistar rats. Additionally, total phenolic content (TPC) and the antioxidant activity of the extract were investigated by different methods.
MATERIALS AND METHODS
Chemicals
β-Carotene and 1,1-diphenyl-2-picryl-hydrazyl (DPPH) were purchased from Sigma- Aldrich. Linoleic acid, gallic acid, ferrous chloride, sodium carbonate, dimethyl sulfoxide (DMSO), chloroform, Tween® 40, Folin-Ciocalteu's phenol reagent and ethanol were purchased from Merck, ferrozine iron reagent from Acros Organics.
Plant material
Aerial parts of Tragopogon buphthalmoides (DC.) Boiss. were collected from Noorabad (Lorestan province, Iran) in May 2012. The plant was identified by Dr Sayyed Ahamad Emami, School of Pharmacy, Mashhad University of Medical Sciences (MUMS). A voucher specimen (No. 12595) of the plant is deposited in the herbarium of School of Pharmacy at MUMS.
Preparation of the extract
The dried powdered aerial parts (238 g) of Tragopogon buphthalmoides (DC.) Boiss. were extracted with ethanol -water (7:3 v/v) (sequential maceration with ca. 3×4 L of the solvent). The extract was filtrated with filter paper and the solvent was removed using rotary evaporator under reduced pressure and at a temperature below 45 °C to yield 69.16 g of dried extract.
Animals
Four-week-old Wistar rats of both sexes were purchased from Kermanshah University of Medical Sciences (Iran). Rats were divided in 4 groups of 5 animals each and acclimatized for two weeks before starting the experiment. Environmental conditions were maintained at 23 ± 2°C and a relative humidity of 40 ± 10% with a 12 h light/dark cycle and allowed free access to food and water. All rats were allowed an 8-day acclimation period before initiation of acute or subchronic dosing, randomly assigned to a control or treatment group, and fasted for 3 h before dosing. Study was conducted in accordance with the internationally accepted principles for laboratory animal use and care as found in the US guidelines (NIH Publication no. 85–23, revised in 1985).
Acute toxicity study
At the onset of dosing, the males and the females weighed 194 ± 3 and 148 ± 9 g respectively. The extract was administered to Wistar rats as a single oral dose of 2.5 g/kg body weight via a gavage. The control rats received tap water by the gavage with the same volume. Observations were made and recorded systematically at 1, 2, 4 and 6 h after administration of the extract, and daily thereafter for a total of 14 days. Animals were observed for body weight, general behavioral changes, and mortality for a period of 14 days post treatment. All of the animals were sacrificed on day 15 and the relative organ weight (weight of organ as proportion to the total body weight of each rat) was calculated.
Subchronic toxicity study
Group assignment and the treatments
Dosing was initiated when the rats were five weeks old, when the males and females weighed 155 ± 3 g and 131 ± 2 g, respectively. The animals were divided into four groups (five rats per sex per group). The first group was given 1 ml distillated water and used as the control. The second, third, and fourth groups received single doses of 175, 300, and 700 mg/kg of T. buphtalmoides by gavages daily for 45 days. All of the solutions were prepared just prior to dosing and were kept chilled and tightly capped. Animals were observed for signs of abnormalities during the treatment period. Besides, the body weights of animals were recorded at the end of the first day and at weekly intervals throughout the course of the study.
Measurement of haematological and biochemical parameters
After 45 days, 12 h-fasted animals were anesthetized with ether and their blood was collected with and without anticoagulant (ethylene diaminetetraacetate, EDTA) by retro-orbital puncture (12) using capillary tubes for haematological and biochemical studies, respectively. The hematological evaluations included erythrocyte (RBC), total and differential leukocyte (WBC), hematocrit (Hct), hemoglobin (Hb), platelet count, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC) and retinoculocyte. Blood samples for biochemical analyses were centrifuged at 3,000 × g for 5 min, and the plasma was collected and analyzed for glucose, creatinine, urea, albumin, globulinecholesterol, triglycerides, aAspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydro-genase LDH, phosphorus, K+, Na+ and total protein using a COBAS Mira S chemistry analyzer (Roche Diagnostic Systems, West Sussex, England).
Necropsy
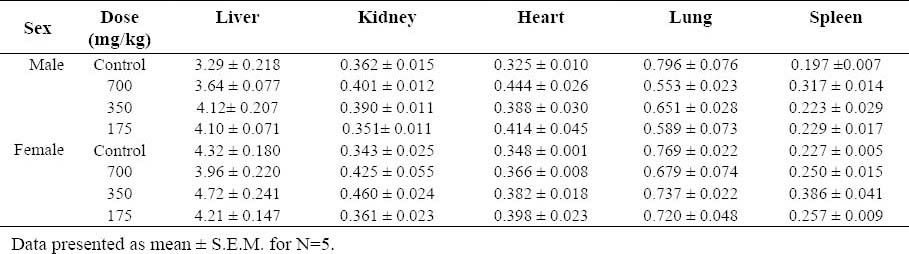
Following blood collection, the rats were sacrificed by decapitation, and the organs identified in Table 1 were removed. Then, organ weight per 100 g body weight (relative weight) was calculated based on the fasted animal's body weight. All above tissues preserved in 10% neutral phosphate-buffered formalin were weighed and examined macroscopically. Histopathological examination was conducted by using routine paraffin embedding technique. Sections of 5 mm thickness were stained with hematoxylin and eosin (H&E) and observed under light microscope for histomorphological alterations.
Table 1.
Relative organ weight at termination of the treatment (g % body weight)
Antioxidant assays and determination of total phenolic content
Folin–Ciocalteu method (13,14) was used to determine the TPC. TPC was calculated on the basis of the calibration curve of gallic acid and expressed as gallic acid equivalents (GAE), in milligrams per gram of the sample.
The (DPPH) activity was assayed using the method of Hatano et al. (15). The chelating activity of dried extract for ferrous ions Fe2+ was determined by the ferrous iron–ferrozine complex method (FIC) (16). β-carotene bleaching method (BCB) (17) was used to specify the radical scavenging activity of the sample as well.
Statistical analysis
Data were analyzed using statistical methods and values were presented as the mean with the standard errors (S.E.M). One-way analysis of variance (ANOVA) followed by Tukey's test was used to compare the differences between means separately for each sex. A probability value of <0.05 was considered statistically significant. Because no treatment-related animal deaths were observed, the LD50 values were not measured.
RESULTS
Amodiaquine acute toxicity
The obtained result in the study of acute toxicity indicated that the high dose of T. buphthalmoides did not produce any symptoms of toxicity. In the 14-days toxicity study, daily gavage administration of the extract at dose levels of 2500 mg/kg did not result in the treatment-related deaths.
Normal body weight gains were observed in the males and females of all of the dose groups (Fig. 1). No abnormal gross findings were observed in any of the animals. Moreover, treatment-related changes in organ weights were not observed (data are not shown). The oral acute toxicity of total extract (LD50) was, therefore, considered to be unclassified; doses up to 2500 mg/kg did not induce death or toxic symptoms.
Fig. 1.
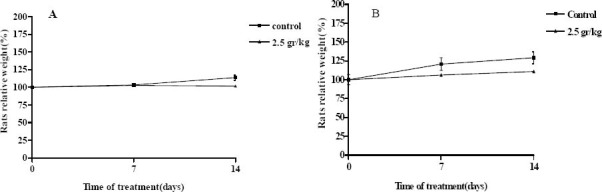
Changes in females (A) and males (B) rat body weight with duration of acute treatment. Each point represents mean ±SEM. N=5.
Subchronic toxicity
Evaluation of animal body weight and organ weights
One animal was died on the 28th day of the treatment. As shown in Fig. 2, both control and animals treated with T. buphtalmoides presented constant increase in body weight. However female rats treated with highest dose of extract showed significant increase in weight gain beginning on 28th day and continuing throughout the treatment as compared to the control. On the day 42 of the study compared to the baseline value, the control rats gained 34.42 (1.4%), while female rats treated with the 700 mg/kg gained 73.36 (2.16%). Macroscopic analysis of target organs of the treated animals (liver, heart, lung, kidneys and spleen) did not show significant changes in color and texture when compared with the control group. There were no significant differences in the organ weights between the control and the treatment groups (Table 1).
Fig. 2.
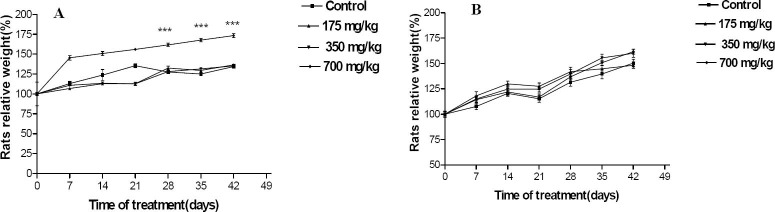
Changes in females (A) and males (B) rat body weight with duration of subchronic treatment. Each point represents mean ± SEM. N=5.***p<0.05.
Biochemical and hematological parameters
Animals exposed to the T. buphtalmoides did not present any difference in the hematological parameters evaluated, compared to control (Table 2). Plasma biochemical data at termination of the study are presented in Table 3.
Table 2.
Hematological parameters of Wistar rats after 45 days of the treatment with Tragopogon buphtalmoides
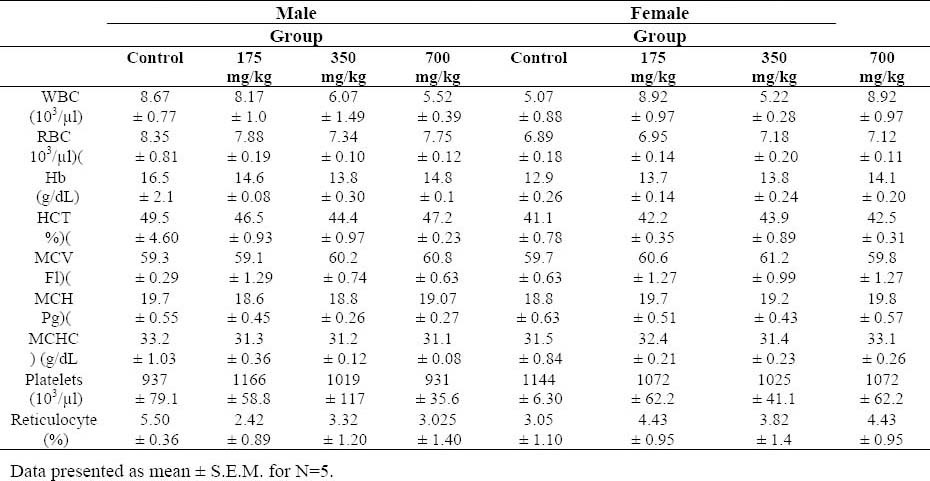
Table 3.
Biochemical parameters of Wistar rats after 45 days treatment with tragopogon buphtalmoides extract.
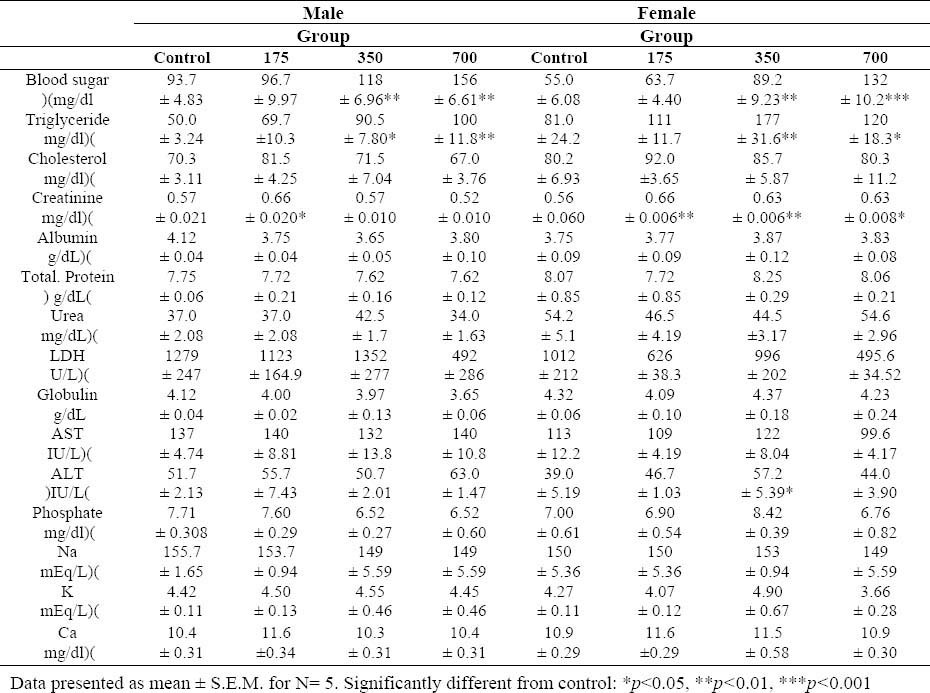
Glucose and triglycerides increased significantly in the highest and middle dose groups in both sexes. Moreover, it was observed an increase in SGPT of the female rats treated with T. buphtalmoides (350 mg/kg). All the other analyzed biochemical parameters were normal (Table 3).
Histopathology
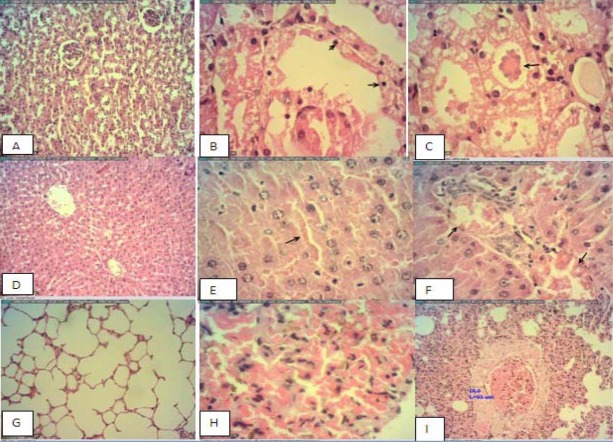
The results of microscopic examination of control and the treatment animals showed hyperemia, congestion of alveolar capillaries, alveolar wall thickness in lung and congestion of sinusoidal and portal vein and accumulation of glycogen in the liver (Fig. 3). The changes in the kidney included congestion, hemorrhage and degeneration of tubules, and protein cast within tubules in the cortex. These changes were observed in the treatments but not the control animals. No histological findings in the spleen or heart could be attributed to the T. buphtalmoides.
Fig. 3.
Selected microphotographs of kidney, liver and lung hematoxylin and eosin of male rats that received the highest dose of tragopogon buphtalmoides. A; Negative control of kiney, B;Showing tubular degeneration, C; Showing protein cast in the tubules, D; Negative control liver, showing normal appearance with narrow sinusoids, E; Showing sinusoidal congestion, F; Vascular congestion, G; Showing normal appearance of lung, H; Showing increased arteriolar wall diameter I showing severe hyperaemia.
Antioxidant assays and determination of total phenolic content
Dried extract showed moderate to weak antioxidant activity in BCB, DPPH and FIC assays with the EC50 values of 43.404 ± 8.407, 65.228 ± 1.413 and 987.038 ± 45.140 μg/ml, respectively. The sample exhibited relatively notable TPC with the value of 40.667 ± 1.477 mg GAE/g.
DISCUSSION
The need to evaluate the toxicity profile of hydroalcoholic extract of T. buphtalmoides was encouraged by the increasing knowledge and interest in medicinal plants and their preparations commonly known as herbal medicine. In general, the first toxicity test performed on a compound is acute toxicity, determined from the administration of a single exposure.
The main objective of acute toxicity testing is to provide an approximation of the intrinsic toxicity of the substances often expressed as median lethal dose (LD50) (18). It is indicated that substances which present LD50 greater than 2000 mg/kg when administered orally can be categorized practically non-toxic (18). Therefore, it can be suggested that T. buphtalmoides is devoid of acute oral toxicity.
Evaluation of oral toxicity through a repeated dose 45-day experiment has been applied in many herbal safety assessment studies (19,20).
In subchronic study analyzing of blood parameters showed that T. buphtalmoides does not interfere with the formation of erythrocytes and leukocytes nor does it cause microcytosis or macrocytosis in rats. Hence, there were no toxicologically significant alterations in the hematological parameters. Blood sugar and triglycerides were observed to increase with increasing dose in female and male rats. Abnormal rise in FBS and triglycerides indicated significant metabolic effects of this extract in long term administration (21).
Liver function was evaluated by means of serum proteins, SGOT and SGPT. These markers usually help to detect chronic liver disease (22). In the current study, an increase in SGPT of female rats treated with T. buphtalmoides (350 mg/kg) was observed. Also the histopathological observations showed vascular and sinusoidal congestion and also an increased glycogen storage in the liver. Hepatic glycogen storage is regarded as a normal metabolic function but excessively intracellular accumulation can be related to hepatotoxicity or disturbance of carbohydrate metabolism (23). Kidney function was assessed by means of serum urea, creatinine, potassium and sodium. Serum creatinine is present after the chemical creatine is broken down by the body in order to make energy (24). The kidney is normally able to filter out large amount of creatinine. Hence, high blood creatinine is a reliable indicator of a negative impact on kidney function or impaired glomerular filtration (25).
In the current study there was a statistically significant difference in serum creatinine in treated animals. It must be noted that the relationship between creatinine and the actual GFR is hyperbolic, not linear. Hence initial elevation of serum concentrations of these substances indicates a large decrease in renal performance (26). Additionally, the histo-pathological analysis of kidneys revealed some abnormalities in this organ. Therefore, these results indicated that T. buphtalmoides has negative impacts on the kidneys. Moreover, congestion of alveolar capillaries and alveolar wall thickness in lung of rats (700 mg/kg) indicated that T. buphtalmoides may have some effects on this organ. The histopathological evaluation of other organs did not explain changes that could establish the dose–response-dependent relationship to treatment with the T. buphtalmoides hydroalcoholic extract. The difference between male and female body weight indicates that female is more sensitive than male to subchronic toxicity effect of T. buphtalmoides because gender is one of the factors that could influences the response (19). The principle goals of the subchronic study are to establish a “no observed adverse effect level” NOAEL and to further identify or characterize the specific organ or organs that affected by test compound after repeated exposure (26).
The NOAEL is defined as the highest exposure level at which there is not any statistically or biologically significant increase in the frequency or severity of adverse effects between exposed and control groups. Determination of NOAEL has numerous regulatory implications. For example it used to calculate reference dose (RfD) by environmental protection agency EPA (18). On the basis of current study it can be concluded that liver and kidneys are possible target organs of T. buphtalmoides. Also it was indicated that NOAEL of the T. buphtalmoides is less than 175 mg/kg for male and female rats.
CONCLUSION
In conclusion, a number of significant clinical and pathological changes were associated with the subchronic oral administration of T. buphtalmoides hydro-alcoholic extract to Wistar rats. Based on the results of this study it can be concluded that the risk of oral toxicity to mammals is not insignificant. Additionally, in the antioxidant capacity assays dried extract showed moderate to weak antioxidant activities.
ACKNOWLEDGMENTS
The authors appreciate the Research Council of Kermanshah University of Medical Sciences for the financial supports. This work was performed in partial fulfillment of the requirement for Pharm.D. of A. Khan Mohammadi.
REFERENCES
- 1.Davis P. Vol. 5. Edinburgh: University press; 1975. Flora of Turkey; p. 664. 5. [Google Scholar]
- 2.Mozaffarian V. Tehran: Farhange Moaser; 2007. A Dictionary of Iranian Plants Names; p. 552. [Google Scholar]
- 3.Kroschewsky JR, Mabry TJ, Markham KR, Alston RE. Flavonoids from the genus Tragopogon compositae. Phytochemistry. 1969;8:1495–1498. [Google Scholar]
- 4.Miyase T, Kohsaka H, Ueno A. Tragopogonosides A-I, oleanane saponins from Tragopogon pratensis. Phytochemistry. 1992;31:2087–2091. [Google Scholar]
- 5.Sareedenchai V, Ganzera M, Ellmerer EP, Lohwasser U, Zidorn C. Phenolic compounds from Tragopogon porrifolius L. Biochem Sys Eco. 2009;37:234–236. [Google Scholar]
- 6.Zidorn C, Lohwasser U, Pschorr S, Salvenmoser D, Ongania KH, Ellmerer EP, et al. Bibenzyls and dihydroisocoumarins from white salsify Tragopogon porrifolius subsp. porrifolius. Phytochemistry. 2005;66:1691–1697. doi: 10.1016/j.phytochem.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Zidorn C, Grass S, Ellmerer EP, Ongania KH, Stuppner H. Stilbenoids from Tragopogon orientalis. Phytochemistry. 2006;67:2182–2188. doi: 10.1016/j.phytochem.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 8.Zidorn C, Petersen BO, Sareedenchai V, Ellmerer EP, Duus J. Tragoponol, a dimeric dihydroisocoumarin from Tragopogon porrifolius L. Tetrahedron Lett. 2010;51:1390–1393. [Google Scholar]
- 9.Gholamhoseinian A, Moradi MN, Sharifi far F. Screening the methanol extracts of some Iranian plants for acetylcholinesterase inhibitory activity. Res Pharm Sci. 2009;4:105–112. [PMC free article] [PubMed] [Google Scholar]
- 10.Mavi A, Lawrence G, Kordali S, Yildirim A. Inhibition of iron-fructose-phosphate-induced lipid peroxidation in lectthin liposome and linoleic acid emulsion systems by some edible plants. J Food Biochem. 2011;35:833–844. [Google Scholar]
- 11.Wegiera M, Smolarz HD, Druch MJ, Korczak M, Kopro K. Cytotoxic effect of some medicinal plants from Asteraceae family on J-45.01 Leukemic cell line pilot study. Acta Pol Pharm. 2012;69:263–8. [PubMed] [Google Scholar]
- 12.Wolford ST, Schroer RA, Gohs FX, Gallo PP, Brodeck M, Falk HB, et al. Reference range data base for serum chemistry and hematology values in laboratory animals. J Toxicol Environ Health. 1986;18:161–188. doi: 10.1080/15287398609530859. [DOI] [PubMed] [Google Scholar]
- 13.Lee JH, Renita M, Fioritto RJ, Martin SST, Schwartz SJ, Vodovotz Y. Isoflavone characterization and antioxidant activity of Ohio soybeans. J Agr Food Chem. 2004;52:2647–2651. doi: 10.1021/jf035426m. [DOI] [PubMed] [Google Scholar]
- 14.Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteau reagent. Methods Enzymol. 1999;299:152–178. [Google Scholar]
- 15.Hatano T, Edamatsu R, Mori A, Fujita Y, Yasuhara T, Yoshida T, et al. Effects of the interaction of tannins with co-existing substances. VI. Effects of tannins and related polyphenols on superoxide anion radical, and on 1,1- diphenyl-pierylhydrazyl radical. Chem Pharm Bull. 1989;37:2016–2021. [Google Scholar]
- 16.Dinis TCP, Madeira VMC, Almeida LM. Action of phenolic derivatives acetaminophen, salicylate, and 5-aminosalicylate as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys. 1994;315:161–169. doi: 10.1006/abbi.1994.1485. [DOI] [PubMed] [Google Scholar]
- 17.Miraliakbari H, Shahidi F. Antioxidant activity of minor components of tree nut oils. Food Chem. 2008;111:421–427. doi: 10.1016/j.foodchem.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 18.Curtis D. Principle of toxicology. In: Eaton D, Gilbert S, editors. Casaretts Doull's Toxicology. Mc Graw Hill; 2007. pp. 7–14. [Google Scholar]
- 19.Mirghazanfari SM, Hosseinzadeh L, Shokoohinia Y, Aslany M, Kamali-Nejad M. Acute and subchronic toxicological evaluation of Echinophora platyloba DC Apiaceae total extract in Wistar rats. CLINICS. 2012;675:497–502. doi: 10.6061/clinics/2012(05)15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rasekh HR, Hosseinzadeh L, Mehri S, Kamli-Nejad M, Aslani M, Tanbakoosazan F. Safety assessment of ocimum basilicum hydroalcoholic extract in wistar rats: acute and subchronic toxicity studies. Iran J Basic Med Sci. 2012;15:645–53. [PMC free article] [PubMed] [Google Scholar]
- 21.Giugliano D, Ceriello A, Esposito K. Glucose metabolism and hyperglycemia. Am J Clin Nutr. 2008;87:217S–22S. doi: 10.1093/ajcn/87.1.217S. [DOI] [PubMed] [Google Scholar]
- 22.Nyblom H, Berggren U, Balldin J, Olsson R. “High AST/ALT ratio may indicate advanced alcoholic liver disease rather than heavy drinking”. Alcohol Alcohol. 2004;39:336–339. doi: 10.1093/alcalc/agh074. [DOI] [PubMed] [Google Scholar]
- 23.Rogers V, Wickstorm M, Liber K, Mackinon M. Acute and subchronic mammalian toxicity of naphtenic acids from oil sands tailing. Toxicol Sci. 2002;66:347–355. doi: 10.1093/toxsci/66.2.347. [DOI] [PubMed] [Google Scholar]
- 24.Tortora GJ, Derrickson B. John Wiley & Sons; 2009. Principles of Anatomy and Physiology. [Google Scholar]
- 25.Hor SY, Ahmad M, Farsi E, Yam M, Hashim M, Pin C. Safety assessment of methanol extract of red dragon fruit Hylocereuspolyrhizus: Acute and subchronic toxicity studies. Reg Toxicol Pharmacol. 2012;63:106–114. doi: 10.1016/j.yrtph.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 26.Hoffman N, Howland L, Goldfrank F. 8th ed. McGraw Hill; 2007. Goldfrank's manual of toxicological emergencies; pp. 237–240. [Google Scholar]