Abstract
Background:
Oral lichenoid lesions or reactions (OLLs/OLRs) are clinical and histological contemporaries of the classical oral lichen planus (OLP) that have generated a lot of debate in literature. In contrast to the idiopathic nature of OLP, OLLs are often associated with a known identifiable inciting factor. A superficial examination of these lesions clinically and histologically often reveals many similarities with OLP, but recent data indicate that distinguishable features do exist and form the basis of most classifications.
Aims and Objectives:
This paper attempts to collate available data in English literature on OLLs, highlight distinguishing features clinically and histologically and reflect on the malignant transformation potential and treatment modalities of the condition.
Materials and Methods:
A comprehensive search of medical and dental databases including PubMed, Ovid, Cochrane, Pubget, Researchgate, and non-medical search engines were utilized for the review. The search words included “oral lichen planus”, “oral lichenoid lesions”, “oral drug reactions”, “lichenoid dysplasia”, and “adverse effects of dental materials”.
Review Results:
OLLs seem to grossly underrated and most cases were clubbed as OLP. Definite clinical and histological features were uncovered to establish the identity of this lesion. Associations with dental restorative materials, drugs, and medications have been conclusively proven in the etiology of this condition. Specific markers are being utilized to diagnose the condition and monitor its progress.
Conclusion:
Substantial differentiating features were uncovered to delineate OLLs as a separate entity with definite etiology, pathogenesis, and a high malignant transformation rate compared with OLP.
Keywords: Drug association, literature review.malignant transformation, oral lichenoid lesions, oral lichen planus, oral lichenoid reactions
What was known?
The occurrence of lesions resembling oral lichen planus clinically and histologically in response to inciting factors, for example, drugs and dental restorative materials. The difficulties in diagnosis and differentiation between the two entities especially with regards to treatment modalities.
Introduction
Oral Lichenoid Reactions or Lesions (OLRs/OLLs) are clinical and histological contemporaries of Oral Lichen Planus (OLP) often indistinguishable in manifestations. The benchmark of differentiation between the two groups is the association of the former with known inciting factors, which when identified and eliminated, often cause a regression of the lesion. This may not always be so and the differentiation then becomes more difficult and academic. It is well established in literature that the differences between the two groups are almost nonexistent and the lesions may be considered subtle variations of the same disease entity, oral lichenoid conditions. With increasing case reports, controlled cohort studies and random cohort studies, a clear picture is emerging of the manifestations of OLLs and the need for it to be recognized as a separate entity. This is almost endorsed by the World Health Organization (WHO) classification and guidelines on OLP and OLRs where the latter is diagnosis by exclusion and not inclusion.
This review attempts to collate information in world literature on the characteristics of the two lesions and identifies parameters that can be used by the clinician to differentiate between the two to enhance treatment success.
Materials and Methods
A comprehensive search of medical and dental databases including PubMed, Ovid, Cochrane, Pubget, Researchgate, and nonmedical search engines were utilized for the review. The search words included “oral lichen planus”, “oral lichenoid lesions”, “oral drug reactions”, “lichenoid dysplasia”, and “adverse effects of dental materials”.
History
Drug interactions resulting in oral lesions clinically similar to OLP were first mentioned in the literature in 1971 by Almeyda and Levantine.[1] The review by these authors lists a recorded case in 1929, but the details are sketchy and unconfirmed. The use of antimalarials, quinacrine, and metacrine during World War II resulted in many reports of development of lichenoid reactions. With increasing use of dental restorative materials, gold being the forerunner, reports of OLLs flourished.[2] Interestingly, till date almost all dental restorative materials in use with the exception of high-end alloys like titanium, palladium, and zirconium, have been implicated in the etiology of OLLs. Comprehensive reviews of OLLs have been presented in literature listing associated factors, especially dental restorative materials and drugs and medications. This paper attempts to collate and highlight the associations, differentiating points clinically and histopathologically and the malignant transformation potential of OLLs.
Nomenclature
It was interestingly seen that lesions resembling lichen planus clinically and histologically, but associated with an inciting factor, were variably termed as “oral lichenoid lesions”, “oral lichenoid reactions”, “lichenoid reactions”, and “oral drug-induced lichenoid reactions (LDE)”. In certain instances the terms “oral lichenoid disease (OLD)” was used to club the lesions of OLP and OLLs/OLRs.[3] The term “oral lichenoid lesions” seems to be most suited in description of the occurrence and this text will refer to the lesions similarly.
Associated factors with OLRs
The associated factors with OLRs may broadly be divided into four groups. Table 1 lists the possible agents in each of the groups.
Table 1.
Factors involved in the pathogenesis of oral lichenoid lesions (OLLs)
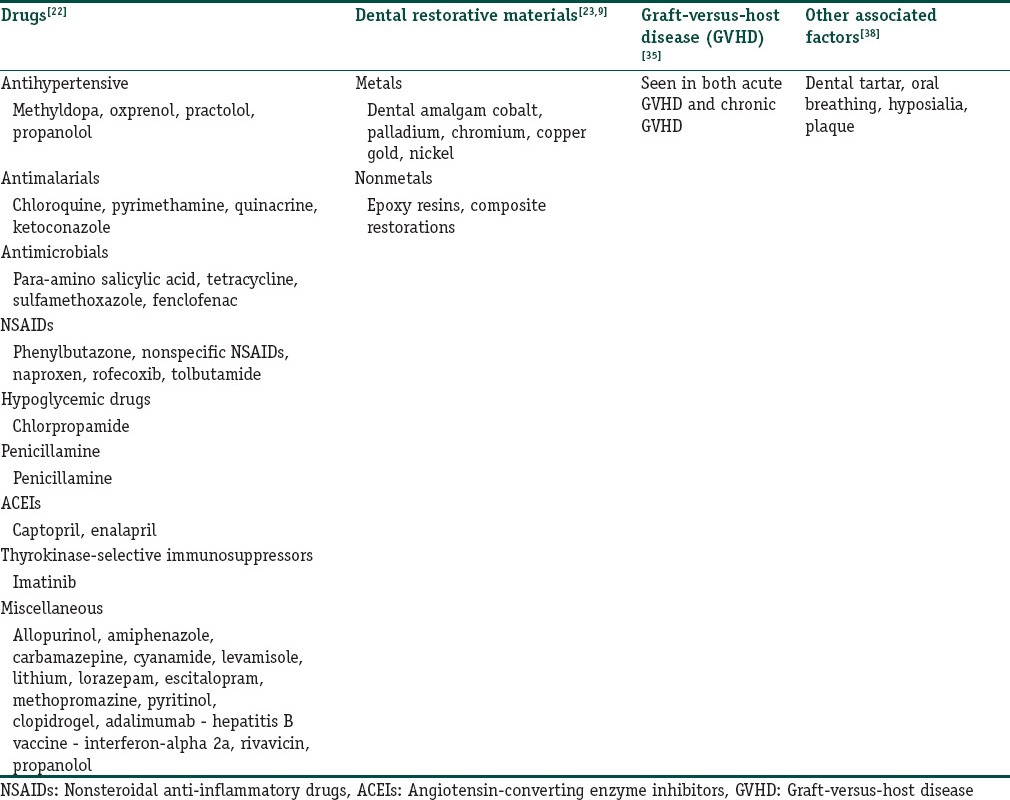
Dental restorative materials (modified from original description)
Drugs and medications
Graft-versus-host disease (GVHD)
Other factors
Dental restorative materials
The biocompatibility of dental materials is dependent on their biodegradation and release of elements that may or may not incite an adverse reaction.[4] Two main mechanisms have been postulated for the occurrence of these adverse reactions.
-
Electrochemical reactions
The interaction between dental materials especially dental amalgam and the oral fluids result in structural degradation of the materials. Release of byproducts of these degradations sets off the adverse reaction. The most severe corrosion of amalgam restorations takes place in localized corrosion cells, such as pits and crevices.[5,6,7]
-
Mechanical force degradation
Masicatory forces induce considerable wear and tear on dental materials. Abrasions and fractures of structural components of dental materials in an electrolytic oral fluid environment release byproducts inciting reactions of oral mucosal tissues.[8] There are significant synergisms among electrochemical and mechanical forces.
The adverse reactions on the oral mucosal tissues can be either due to the toxic or irritative nature of the material or allergic.
Lesions caused due to toxic or irritant nature of the dental material are a form of local inflammation induced by primary contact with chemicals and are not mediated by lymphocytes. A chronic toxic reaction may be established due to repeated or constant influence of toxic agents in low concentrations over long periods of time. Such reactions are most frequently localized to the contact zone with the toxic agent and may be seen in areas of the oral mucosa in direct contact with restorations.[9] Experimental studies have demonstrated the pronounced cytotoxic effect of dental materials on cell cultures of oral cells.[10]
Allergic contact lesions represent a lymphocyte-mediated delayed type of hypersensitivity reaction that requires previous sensitization to the same chemical. In a study, Massone et al., (1991)[11] found that nickel, cobalt, and potassium dichromate were the three most common sensitizers; concomitant positive reactions were present at significant levels.
Therefore, the etiology of OLLs may represent the oral manifestation of a chronic irritation in some patients or be the clinical result of a delayed hypersensitivity reaction in others.
A thorough clinical examination of the oral mucosa at the site of the lesion and epicutaneous patch testing should assist in resolving this conflict.
The literature reports association of majority of common materials used in dental restorations. Interestingly high-end alloys like titanium, zirconium, and palladium have not been implicated in the development of OLLs.
Role of dental amalgam
Dental amalgam has been the most implicated restorative material in the induction of OLLs. It has been well-documented in literature that biological events like dissolution, evaporation, corrosion, or other form of degradation causes a release of mercury from amalgam alloys. The released mercury is reportedly taken up by the oral soft tissues causing toxic or allergic effects in sensitized individuals that present as lichenoid reactions.[12,13] The complex environment at the surface of restorations is comprised mainly of saliva whose composition varies over time in each individual. The corrosion resistance of alloys in this environment results, in part, from the formation of a protective oxide film. Under certain conditions the stability of this protected film can be damaged and constituent elements in the alloy are released.
Several authors have reported clearing of the lesions after removal of amalgam restorations in such patients.[14,15,16] The substitution of amalgam by another material in these studies was based upon a positive patch test to mercury[17,18] and/or the anatomical relationship with the amalgam restoration.[19,20]
The clinical value of epicutaneous patch tests to materials used in dentistry is disputable and largely discarded in recent times. This follows reports of regression of oral mucosa lesions after removal of amalgam, regardless of the results of the patch test. Also it was seen that not all patients with topographical association to dental materials elicited positivity in the patch tests.[21]
Drugs and medications
Drugs and medications used locally or systemically have been implicated in the discovery of OLLs. As stated earlier in the text, one of the first reported cases of OLLs, as they are accepted today, was a drug-induced lesion. The list of drugs associated with OLLs development is vast and increasing by the week. The most commonly implicated agents are nonsteroidal anti-inflammatory drugs and angiotensin-converting enzyme inhibitors.[22,23,24] Systemic medications like antimalarials[25,26] and antihypertensive drugs[27,28] are also implicated. Diuretics, oral hypoglycemic agents, gold salts, penicillamine, and beta blockers are also reportedly associated with development of OLLs.
In many cases these drugs are used in combination, thus, suggesting the possible existence of synergic effects between them. The popular “Grinspan syndrome”, in which OLP is related to diabetes mellitus and arterial hypertension, is now recognized to be simply an example of OLR induced by the drugs simultaneously used to treat the latter two diseases.[29]
Interestingly, drugs that are used to treat intractable, recurrent, or refractory cases of OLP like dapsone have also been implicated in the development of OLLs. Thus, it is believed that the prevalence of OLLs attributable to drugs and medications is increasing.[30]
The development of OLLs in individuals using drugs and medications is by no means definitive. Though most cases have retrospective associations and a cause-and-effect relationship is presumed by withdrawal of the offending drug and remission of the lesion, prospective studies tell a different story. In an interesting cohort-controlled study, Hirota et al.,[31] analyzed the relationship between drug intake and development of OLP. The authors concluded that use of systemic medication does not lead to a significant increase in the incidence of OLP lesions. In their opinion, lichenoid drug reactions were likely to occur only in a very low percentage of patients.
The pathogenesis of drug-induced OLLs is still unknown. It is presumed that different routes of antigen presentation may be a primary factor.[32] Penicillamine is known to change surface antigen and the sulfhydryl groups of captopril change enzyme systems.[33] These may precipitate an immune response to epithelial antigens leading to the development of OLLs. In a comprehensive review on drug reactions on the oral mucosal tissues, Scully and Bagan[23] raised certain interesting points. They query the biopharmacological pathways that cause the same drug brings about different clinical manifestations. They also query the reason behind different chemical structures coinciding in the clinical expression of their side-effects. Another interesting and pertinent point raised by the authors is the supposedly contra behavior of some drugs belonging to the same family (such as antimalarials) wherein both produce a lichenoid reaction and at the same time find some use in the treatment of OLP.[34] The unraveling of these complexities need more research and till then it would be safe to assume that the exact pathogenesis of drug-induced OLLs will still be a subject of conjecture.
GVHD
GVHD is the most common complication of allogeneic hematopoietic stem cell transplantation (HSCT). In HSCT, the patient's bone marrow is destroyed with chemotherapy and/or radiation and replaced by donor hematopoietic stem cells. In allogeneic HSCT, the donor is usually a close family member or occasionally someone outside the family who has been found to be a “match”.
GVHD occurs when transplanted donor's immune cells (graft) react to patient's tissues (host) and tries to destroy them. Recent studies have shown that host-derived, rather than donor-derived, antigen presenting cells (APCs) initiate GVHD.[35] As a consequence of this action, the patient's organs impair their ability to function, and increase the patient's susceptibility to infection.
GVHD is generally divided into two syndromes: Acute and chronic. Acute GVHD (aGVHD), is that occurring within 3 months after transplantation, and chronic GVHD (cGVHD), usually develops after the 3rd month post-HSCT. Patients may develop one, both or neither reaction.
aGVHD and cGVHD differ in their symptoms, clinical signs, and time of onset. aGVHD primarily involves the skin, liver, oral mucosa, and gastrointestinal tract. In contrast, cGVHD presents a far more varied clinical picture including liver dysfunction, pulmonary fibrosis, sclerodermatous skin changes, oral and gastrointestinal mucosa changes, and a reduced production of tears and saliva. The skin is the main organ involved in cGVHD and manifests as a lichen planus-like eruption or scleroderma.[36]
Oral involvement occurs in 33–75% of patients with aGVHD and up to 80% of patients with cGVHD.[37] Lichen planus-like lesions are the most distinctive oral change of cGVHD: Hyperkeratotic striae, patches, plaques, and papules may affect oral and perioral tissues and severe atrophy and ulcerations, resembling erosive lichen planus, can be noted in patients with severe extensive cGVHD. The ulcerations are often covered with a heavy pseudomembranous clot that is grayish white to yellowish. In addition, mucosal surfaces appear erythematous and atrophic, aspects attributed to a relative loss of keratinization or loss of surface structures as filiform papaillae or gingival stippling.
Other associated factors
A few case reports have mentioned the occurrence of OLLs in patients with no evidence of association with any of the above mentioned factors. In these individuals factors like dental tartar and plaque accumulation have been cited. There is an interesting case report of OLL in a patient with a triad of dental tartar, hyposialia, and mouth breathing. The patient had no other associated factors. It was opined by the authors that accumulation of pathogens in the tartar and lack of salivary antibody protection due to hyposialia probably resulted in the OLL.[38] Similar reports have supported the occurrence of this phenomenon, and hence, the recognition of this feature as a probable factor in the etiology of OLLs.[39]
Clinical and Histological Features of OLLs
OLLs share the clinical features of OLP and presentations as plaques or erosive patches with presence of Wickham's striae are seen. OLLs are known to occur in all clinical varieties of presentation seen in OLP like reticular, atrophic, erosive, bullous, and keratotic.
However, there are certain distinctive features that OLLs exhibit that differentiate them from OLP. OLLs are usually unilateral, have a topographical association with a dental restorative material and a causative association with a drug or medication, if it is the inciting factor, and rarely occur in sites like tongue and palate [Figures 1 and 2]. The causal effect can be confirmed by withdrawal of the suspected drug, if medically feasible, and observation of the regression of the lesion. Occasionally, when dental material association is suspected then epicutaneous patch tests and replacement of the material may bring about the desired result.
Figure 1.
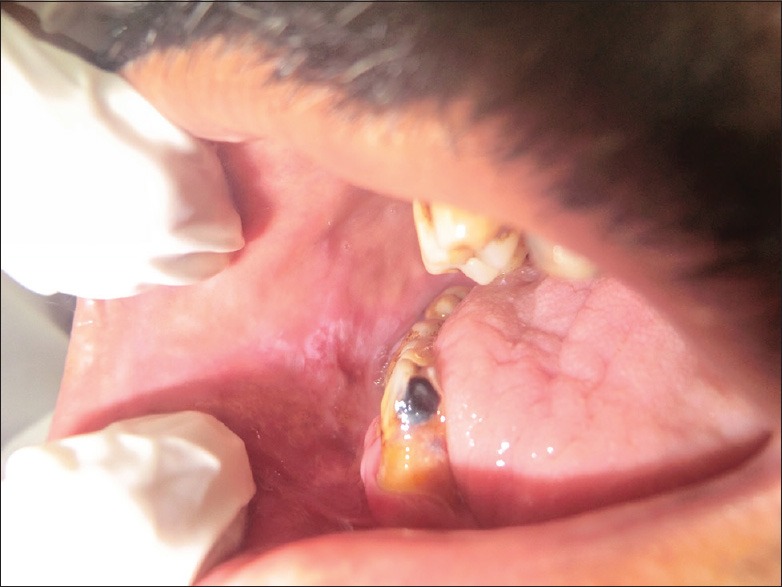
Clinical presentation of the lesion next to an old amalgam restoration (Courtesy: Dr Lavanya Sundaram)
Figure 2.
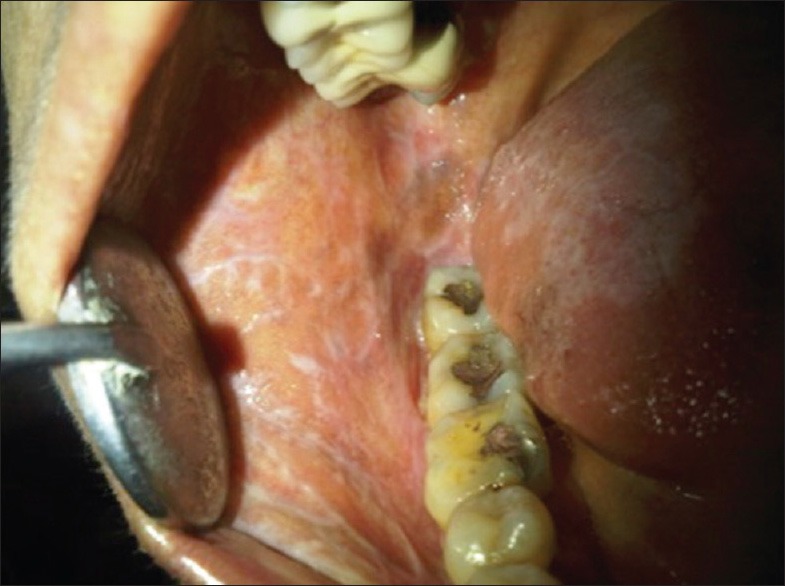
A more classical oral lichen planus (OLP) like presentation in another case with proximity to amalgam restorations
Table 2 outlines the clinical and histological differences in the two conditions.
Table 2.
Differentiating features between oral lichen planus (OLP) and oral lichenoid lesions
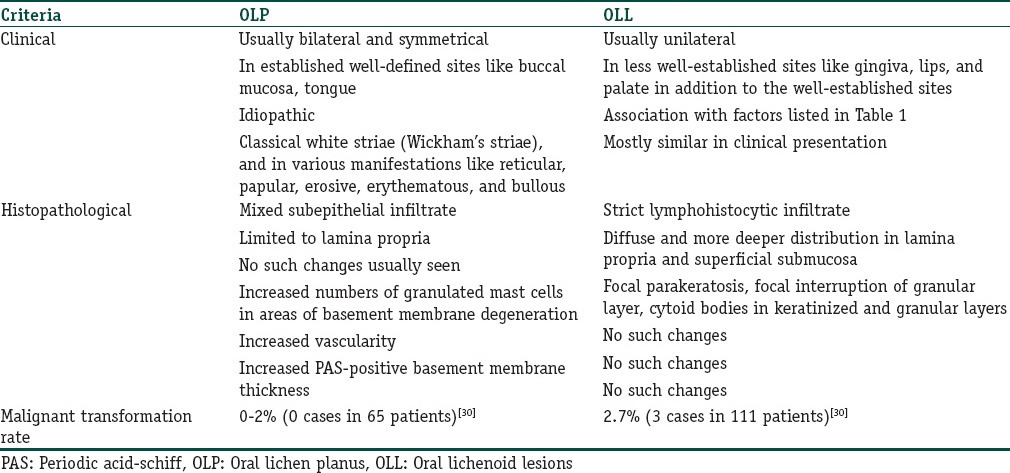
The histology of OLP is characterized by a dense subepithelial lymphohistiocytic infiltrate, increased numbers of intraepithelial lymphocytes, and degeneration of basal keratinocytes. Degenerating basal keratinocytes form colloid (civatte, hyaline, and cytoid) bodies that appear as homogenous eosinophilic globules.[40]
The histological features of OLLs differ in several aspects. While the subepithelial infiltrate in OLP is limited to the lamina propria, it is more diffuse and penetrating in OLLs [Figure 3]. The nature of the infiltrate is also lymphohistiocytic compared with the mixed variety of OLP.[41] There is a tendency for perivascular congregration of the inflammatory cells, as seen in many allergic reactions, in OLLs [Figures 4 and 5]. Epithelial changes include focal parakeratosis, focal interruption of the granular layer, and presence of cytoid bodies in the granular and keratinized layers.[42] These features are uncommon in OLP. Degranulating mast cells form an integral feature of the histological presentation and etiopathogenesis of OLP. Mast cell presence in OLL is more subdued. Various other minor features like increased vascularity and increased positivity of periodic acid-schiff (PAS) material in the basement membrane of OLP are not usually found in OLLs.[43]
Figure 3.
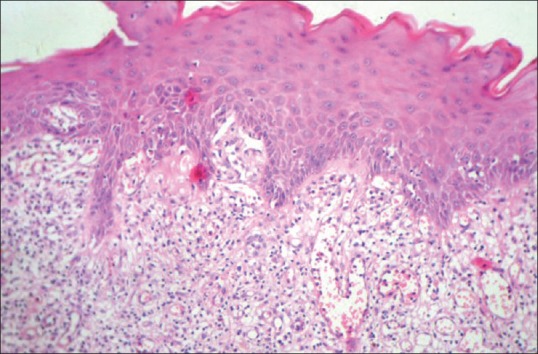
Histopathological image of biopsy from case 2 showing diffuse proliferation of lymphocytic cells with increased vascularity and perivascular concentration of inflammatory cells (hematoxylin and eosin (H and E, ×10))
Figure 4.
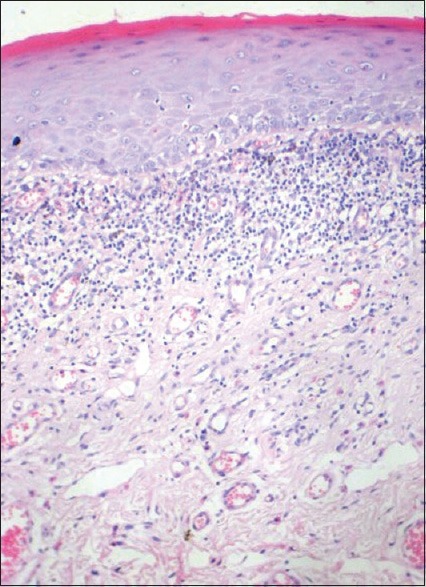
Histopathological image of biopsy from case 1 showing a more OLP like presentation (H and E, ×10)
Figure 5.
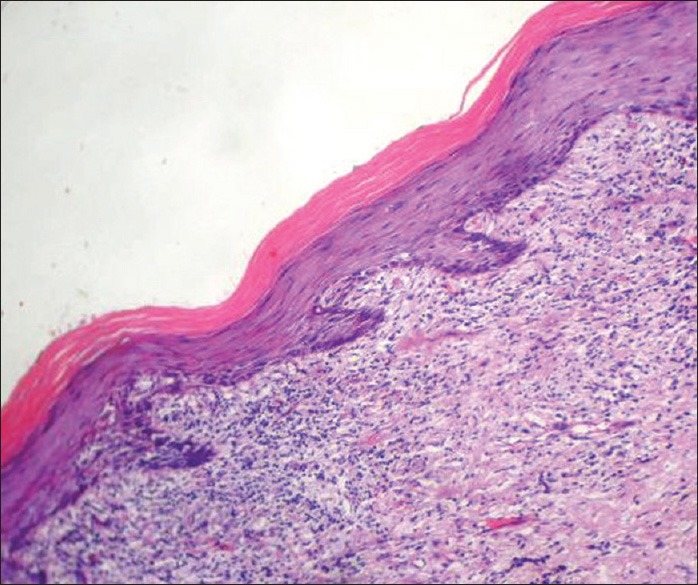
Histopathological image of classical OLP showing compact band of subepithelial lymphocytic inflammatory infiltrate with hyperkeratosis and saw-tooth shaped rete ridges (H and E, ×10)
Markers of OLLs
The literature does not reveal any specific or definitive marker associated with OLLs that would aid in its diagnosis. Immunofluorescency, immunohistochemistry, and genetic studies have been employed in the detection of various markers. Table 3 presents an overview of markers utilized in OLLs and OLP gleaned from the literature.
Table 3.
Investigations/parameters assessed in OLLs
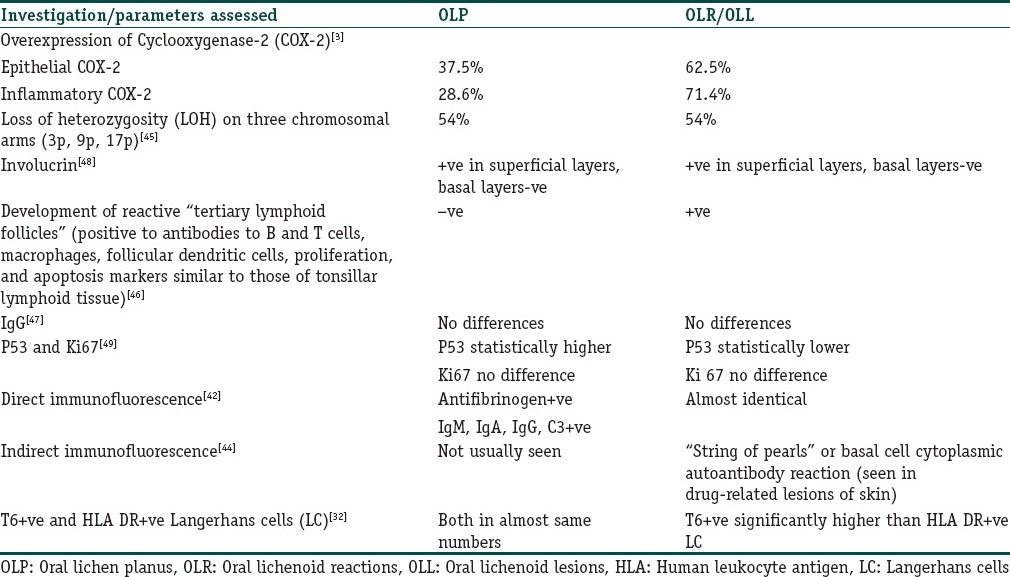
An interesting observation by McQueen and Behan (1982)[44] was of the expression of basal cell cytoplasmic autoantibodies in patients with drug induced lichenoid reactions of the skin. The expression was described as a “string of pearls” in the basal cell layers of the epithelium by indirect immunofluorescent techniques. Subsequent confirmation in OLLs has not been too encouraging [Figure 6].
Figure 6.
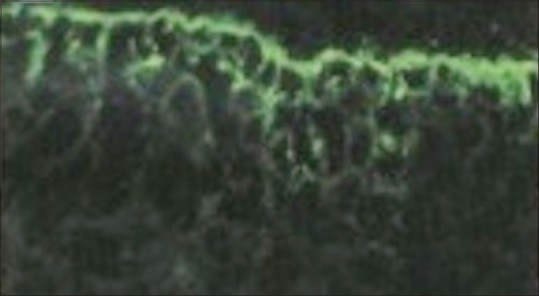
Classical immunofluorescent presentation of oral lichenoid lesions (OLL)
Overexpression of epithelial and inflammatory cyclooxygenase (COX)-2 cytokines in OLLs as compared with OLP has been reported and suggested as a pathogenetic pathway of the lesions. Genetic studies have shown no obvious differences between OLLs and OLP.[45]
Interestingly, development of tertiary lymphoid follicles expressing markers similar to those found in tonsillar lymphoid tissue have been exclusively detected in OLLs.[46] These were absent in OLP lesions included in the study. The authors have used this finding to reinforce an allergic pathway for the pathogenesis of OLLs.
Immunohistochemical studies of IgG,[47] involucrin,[48] p53, and Ki-67[49] expressions have failed to reveal any substantial differences between OLLs and OLP. An increase in p53 in OLP was detected but was attributed to the nature of the inflammatory infiltrate of the lesions.
McCartan and Lamey (1997)[32] detected increased numbers of Langerhans cells expressing T6 receptors as compared with human leukocyte antigen (HLA)-DR receptors in OLLs than OLP. The inclusion of a small number of cases and lack of further confirmation of the observation makes it difficult to draw firm inferences.
Classifications
Based on our literature review, three types of classifications for OLLs were present.
The WHO classification (van der Meij et al., 2007)[30] is an all-encompassing classification listing which features of OLP and OLLs, the diagnosis of the latter based on exclusion rather than exclusion. It is rather a descriptive list than a practically oriented one and serves its purpose in strict inclusion of cases for studies on the condition.
van der Waal (2009)[50] has proposed a causal classification based on the etiological factors of the condition. It is; in our opinion; practical, restrictive, and reflective of the clinical aspects of the condition. The drawbacks of this classification, of course, are the non-inclusion of differentiating histological criteria. This is understandable, in view of lack of conformity amongst various case studies and the overlap with OLP. The only change, in our opinion, in this listing, would be replacement of the term “amalgam associated” with “dental restorative material associated” which would make the clinical realities more encompassing.
Issa et al., (2005)[9] have proposed a rather tedious and impractical listing based on site and association. Interestingly, the concept of topographical association has been alluded to in both the classifications by van der Waal (2009)[50] and Issa et al., (2005).[9] Both the authors suggest that intimate topographical association of OLLs with amalgam and other materials would presume a cause-and-effect relationship and should be used for nomenclature of the lesion. Van der Meij (2009)[30] have also suggested a separate category for such type of lesions alluding to them as lichenoid contact reactions (LCRs) and have pointed out its absence of notification in the WHO monograph.
In view of case reports of OLLs occurring in areas topographically not associated with materials, the negativity of patch tests, the lack of immediate regression upon withdrawal or replacement of the offending material, such a subclassification would be premature. More reports in the literature need to be analyzed before a separate class of lesions as those mentioned above is included.
Table 4 presents an overview of the three classifications discussed above.
Table 4.
Classifications of oral lichenoid reactions/lesions (OLRs/OLLS)

Malignant Transformation
The malignant transformation rate of OLP is variably listed as 0.5-2% in various studies.[9] While no large scale studies exist on the prediction of malignant transformation of OLLs, the study of van der Meij et al.,[30] stands out as a benchmark being the only controlled study of its kind. Interestingly, in their study the authors have reported a malignant transformation rate of 2.1% for OLLs as compared with 0.5% for OLP.
Four out of 192 patients (2.1%), two men and two women, developed a squamous cell carcinoma of the oral mucosa during follow-up. All malignant transformations occurred in the OLL group. The malignant transformation of the OLL group, based on a mean follow-up of 53.8 months, was calculated at 0.71% per year. The length of follow-up before malignant transformation ranged from 11–70 months (mean, 40 months). This is significant and statistically sustainable in their cohort selection and raises disturbing questions on the prognosis of this seemingly innocuous lesion.
In a study by Mignogna et al., (2001)[51] meticulous examination of OLP patients at least thrice annually was advocated to improve the prognosis of patients undergoing malignant transformation. In another study, the same authors[52] concluded on their data of four cases that OLP-related squamous cell carcinoma amongst 502 cases of OLP may have a worse prognosis because of increased metastatic potential. All four tumors were initially stage I tumors with a mean thickness of 1.75 mm. Recent studies indicate a tumor thickness over 4 mm as predictive of nodal metastasis,[53,54] but all four patients in the Mignogna study developed lymphnodal metastasis within 6 months of follow-up. In view of the above van der Meij et al.,[30] advise to monitor only the subgroup of OLL patients twice a year for early detection of possible malignant transformation.
Management and Treatment
The management and treatment of OLLs follows the empirical route adopted for OLP.
Table 5 outlines the most common treatment modalities employed for OLLs and OLP.
Table 5.
Therapy for oral lichenoid lesions

Steroids form the mainstay in symptomatic lesions.
In cases of topographical association with dental materials, removal, and replacement with alternatives may have a role. Patch tests are usually nonconfirmatory, but it is advisable to utilize them, if available and feasible, to underline a cause-and-effect relationship.
In cases of drug associations, withdrawal and replacement may need medical consultation. It has been frequently observed that in spite of withdrawal, lesions do no show immediate remission. This has been postulated to the sensitization of the oral mucosal tissues by the offending drug molecule, which persists as an independent factor even after withdrawal.[23]
The treatment of OLLs associated with GVHD follows the immunosuppressant route adopted for the main medical condition.
Maintenance of proper oral hygiene; removal of accumulated plaque, tartar, and films on surfaces of restoration; and use of prophylactic mouth rinses may go a long way in removal of the inciting factors.
It cannot be overemphasized that in view of the high malignant transformation rates for OLLs, regular follow-ups and biopsies need to be taken to monitor the progress of the lesion.
Conclusions
OLLs need to be recognized as a definite entity occurring due to association of dental restorative materials, drugs, and medications and as a reaction to cGVHD. Though they share clinical and histological similarities to OLP sufficient data has been presented in literature for their differentiation. It is essential that oral clinicians and dermatologists recognize this fact, especially since their treatment is much more issue based compared to the empirical therapy of OLP. In view of recent studies highlighting their malignant potential strict guidelines need to be followed in their management to avoid or detect early the malignant changes.
What is new?
The inclusion of several inciting factors, especially dental restorative materials in the etiology of oral lichenoid reactions. The classification systems now established and adhered to and the awareness of the fact that treatment is targeted to removal of inciting factor, unlike lichen planus. The malignant transformation potential of both the entities is discussed to impress on the need for clarity and targeted therapy.
Footnotes
Source of support: Nil
Conflict of Interest: Nil.
References
- 1.Almeyda J, Levantine A. Drug reactions. XVI. Lichenoid drug eruptions. Br J Dermatol. 1971;85:604–7. doi: 10.1111/j.1365-2133.1971.tb14100.x. [DOI] [PubMed] [Google Scholar]
- 2.Penneys NS, Ackerman AB, Gottlieb NL. Gold dermatitis. A clinical and histopathological study. Arch Dermatol. 1974;109:372–6. doi: 10.1001/archderm.109.3.372. [DOI] [PubMed] [Google Scholar]
- 3.Cortés-Ramírez DA, Rodríguez-Tojo MJ, Gainza-Cirauqui ML, Martínez-Conde R, Aguirre-Urizar JM. Overexpression of cylcooxygenase-2 as a biomarker in different subtypes of the oral lichenoid disease. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110:738–43. doi: 10.1016/j.tripleo.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Wataha JC, Malcolm CT, Hanks CT. Correlation between cytotoxicity and the elements released by dental casting alloys. Int J Prosthodont. 1995;8:9–14. [PubMed] [Google Scholar]
- 5.Marek M. Interactions between dental amalgams and the oral environment. Adv Dent Res. 1992;6:100–9. doi: 10.1177/08959374920060010101. [DOI] [PubMed] [Google Scholar]
- 6.Marek M. Dissolution of mercury from dental amalgam at different pH values. J Dent Res. 1997;76:1308–15. doi: 10.1177/00220345970760061101. [DOI] [PubMed] [Google Scholar]
- 7.Moberg LE, Johansson C. Release of corrosion products from amalgam in phosphate containing solutions. Scand J Dent Res. 1991;99:431–9. doi: 10.1111/j.1600-0722.1991.tb01051.x. [DOI] [PubMed] [Google Scholar]
- 8.Gil FJ, Espias A, Sanchez LA, Planell JA. Comparison of the abrasive wear resistance between amalgams, hybrid composite material and different dental cements. Int Dent J. 1999;49:337–42. doi: 10.1111/j.1875-595x.1999.tb00534.x. [DOI] [PubMed] [Google Scholar]
- 9.Issa Y, Duxbury AJ, Macfarlane TV, Brunton PA. Oral lichenoid lesions related to dental restorative materials. Brit Dent J. 2005;198:361–6. doi: 10.1038/sj.bdj.4812176. [DOI] [PubMed] [Google Scholar]
- 10.Issa Y, Watts DC, Duxbury AJ, Brunton PA, Watson MB, Waters CM. Mercuric chloride: Toxicity and apoptosis in a human oligodendroglial cell line MO3.13. Biomaterials. 2003;24:981–7. doi: 10.1016/s0142-9612(02)00436-2. [DOI] [PubMed] [Google Scholar]
- 11.Massone L, Anonide A, Borghi S, Isola V. Positive patch test reactions to nickel, cobalt, and potassium dichromate in a series of 576 patients. Cutis. 1991;47:119–22. [PubMed] [Google Scholar]
- 12.Bolewska J, Hansen HJ, Holmstrup P, Pindborg JJ, Stangerup M. Oral mucosal lesions related to silver amalgam restorations. Oral Surg Oral Med Oral Pathol. 1990;70:55–8. doi: 10.1016/0030-4220(90)90178-u. [DOI] [PubMed] [Google Scholar]
- 13.Bolewska J, Holmstrup P, Moller-Madsen B, Kenrad B, Danscher G. Amalgam associated mercury accumulations in normal oral mucosa, oral mucosal lesions of lichen planus and contact lesions associated with amalgam. J Oral Pathol Med. 1990;19:39–42. doi: 10.1111/j.1600-0714.1990.tb00779.x. [DOI] [PubMed] [Google Scholar]
- 14.Laine J, Kalimo K, Happonen RP. Contact allergy to dental restorative materials in patients with oral lichenoid lesions. Contact Dermatitis. 1997;36:141–6. doi: 10.1111/j.1600-0536.1997.tb00396.x. [DOI] [PubMed] [Google Scholar]
- 15.Laine J, Konttinen YT, Beliaev N, Happonen RP. Immunocompetent cells in amalgam associated oral lichenoid contact lesions. J Oral Pathol Med. 1999;28:117–21. doi: 10.1111/j.1600-0714.1999.tb02008.x. [DOI] [PubMed] [Google Scholar]
- 16.Koch P, Bahmer FA. Oral lesions and symptoms related to metals used in dental restorations: A clinical, allergological, and histologic study. J Am Acad Dermatol. 1999;41:422–30. doi: 10.1016/s0190-9622(99)70116-7. [DOI] [PubMed] [Google Scholar]
- 17.James J, Ferguson MM, Forsyth A, Tulloch N, Lamey PJ. Oral lichenoid reactions related to mercury sensitivity. Br J Oral Maxillofac Surg. 1987;25:474–80. doi: 10.1016/0266-4356(87)90139-2. [DOI] [PubMed] [Google Scholar]
- 18.Skoglund A, Egelrud T. Hypersensitivity reactions to dental materials in patients with lichenoid oral mucosal lesions and in patients with burning mouth syndrome. Scand J Dent Res. 1991;99:320–8. doi: 10.1111/j.1600-0722.1991.tb01035.x. [DOI] [PubMed] [Google Scholar]
- 19.Henriksson E, Mattsson U, Hakansson J. Healing of lichenoid reactions following removal of amalgam. A clinical follow-up. J Clin Periodontol. 1995;22:287–94. doi: 10.1111/j.1600-051x.1995.tb00150.x. [DOI] [PubMed] [Google Scholar]
- 20.Bratel J, Hakeberg M, Jontell M. Effect of replacement of dental amalgam on oral lichenoid reactions. J Dent. 1996;24:41–5. doi: 10.1016/0300-5712(95)00004-6. [DOI] [PubMed] [Google Scholar]
- 21.Skoglund A. Value of epicutaneous patch testing in patients with oral, mucosal lesions of lichenoid character. Scand J Dent Res. 1994;102:216–22. doi: 10.1111/j.1600-0722.1994.tb01183.x. [DOI] [PubMed] [Google Scholar]
- 22.Serrano-Sánchez P, Bagán JV, Jiménez-Soriano, Sarrión G. Drug-induced oral lichenoid reactions. A literature review. J Clin Exp Dent. 2010;2:e71–5. [Google Scholar]
- 23.Scully C, Bagan JV. Adverse drug reactions in the orofacial region. Crit Rev Oral Biol Med. 2004;15:221–39. doi: 10.1177/154411130401500405. [DOI] [PubMed] [Google Scholar]
- 24.Bagán JV, Thongprasom K, Scully C. Adverse oral reactions associated with the COX-2 inhibitor rofecoxib. Oral Dis. 2004;10:401–3. doi: 10.1111/j.1601-0825.2004.01024.x. [DOI] [PubMed] [Google Scholar]
- 25.Cutler TP. Lichen planus caused by pyrimethamine. Clin Exp Dermatol. 1980;5:253–6. doi: 10.1111/j.1365-2230.1980.tb01697.x. [DOI] [PubMed] [Google Scholar]
- 26.Zain RB. Oral lichenoid reactions during prophylaxis with sulphadoxine-pyrimethamine combination. Southeast Asian J Trop Med Public Health. 1989;20:253–6. [PubMed] [Google Scholar]
- 27.Lakshmi C, Srinivas CR, Ramachandran B, Pillai SB, Nirmala V. Perforating lichenoid reaction to amlodipine. Indian J Dermatol. 2008;53:98–9. doi: 10.4103/0019-5154.41659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Upadhyayai JB, Nangia AK, Mukhija RD, Misra M, Mohan L, Singh KK. Cutaneous reactions due to antihypertensive drugs. Indian J Dermatol. 2006;51:189–91. [Google Scholar]
- 29.Aljabre SH. Grinspan's syndrome. J Am Acad Dermatol. 1994;30:671. doi: 10.1016/s0190-9622(09)80125-4. [DOI] [PubMed] [Google Scholar]
- 30.Van der Meij EH, Mast H, van der Waal I. The possible premalignant character of oral lichen planus and oral lichenoid lesions: A prospective five-year follow-up study of 192 patients. Oral Oncol. 2007;43:742–8. doi: 10.1016/j.oraloncology.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 31.Hirota SK, Moreno RA, dos Santos CH, Seo J, Migliari DA. Analysis of a possible association between oral lichen planus and drug intake. A controlled study. Med Oral Patol Oral Cir Bucal. 2011;16:e750–6. doi: 10.4317/medoral.17095. [DOI] [PubMed] [Google Scholar]
- 32.McCartan BE, Lamey PJ. Expression of CD1 and HLA-DR by Langerhan cells (LC) in oral drug induced lichenoid reactions (LDE) and idiopathic oral lichen palnus (LP) J Oral Pathol Med. 1997;26:176–80. doi: 10.1111/j.1600-0714.1997.tb00454.x. [DOI] [PubMed] [Google Scholar]
- 33.Powell FC, Rogers RS, 3rd, Dickson ER. Primary biliary cirrhosis and lichen planus. J Am Acad Dermatol. 1983;9:540–5. doi: 10.1016/s0190-9622(83)70166-0. [DOI] [PubMed] [Google Scholar]
- 34.Eisen D. Hydroxychloroquine sulfate (Plaquenil) improves oral lichen planus: An open trial. J Am Acad Dermatol. 1993;28:609–12. doi: 10.1016/0190-9622(93)70082-5. [DOI] [PubMed] [Google Scholar]
- 35.Shlomchik WD, Couzens MS, Tang CB, McNiff J, Robert ME, Liu J, et al. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science. 1999;285:412–5. doi: 10.1126/science.285.5426.412. [DOI] [PubMed] [Google Scholar]
- 36.Kuechle K. Graft versus host disease. e-Medicine (2001) [Last accessed on May 2001]. Available from: http://www.emedicine.com/derm/topic478.htm .
- 37.Schubert MM, Sullivan KM. Recognition, incidence, and management of oral graft-versus-host disease. NCI Monogr. 1990;9:135–43. [PubMed] [Google Scholar]
- 38.Bäckman K, Jontell M. Microbial-associated oral lichenoid reactions. Oral Dis. 2007;13:402–6. doi: 10.1111/j.1601-0825.2006.01312.x. [DOI] [PubMed] [Google Scholar]
- 39.Holmstrup P. Reactions of the oral mucosa related to silver amalgam: A review. J Oral Pathol Med. 1991;20:1–7. doi: 10.1111/j.1600-0714.1991.tb00878.x. [DOI] [PubMed] [Google Scholar]
- 40.Sugerman PB, Savage NW, Walsh LJ, Zhao ZZ, Zhou XJ, Khan A, et al. The Pathogenesis of oral lichen planus. Crit Rev Oral Biol Med. 2002;13:350–65. doi: 10.1177/154411130201300405. [DOI] [PubMed] [Google Scholar]
- 41.Thornhill MH, Sankar V, Xu XJ, Barrett AW, High AS, Odell EW, et al. The role of histopathological characteristics in distinguishing amalgam-associated oral lichenoid reactions and oral lichen planus. J Oral Pathol Med. 2006;35:233–40. doi: 10.1111/j.1600-0714.2006.00406.x. [DOI] [PubMed] [Google Scholar]
- 42.Van den Haute V, Antoine JL, Lachapelle JM. Histopathological discriminant criteria between lichenoid drug eruption and idiopathic lichen planus: Retrospective study on selected samples. Dermatologica. 1989;179:10–3. doi: 10.1159/000248091. [DOI] [PubMed] [Google Scholar]
- 43.Juneja M, Mahajan S, Rao NN, George T, Boaz K. Histochemical analysis of pathological alterations in oral lichen planus and oral lichenoid lesions. J Oral Sci. 2006;48:185–93. doi: 10.2334/josnusd.48.185. [DOI] [PubMed] [Google Scholar]
- 44.McQueen A, Behan WM. Immunofluorescence microscopy. The “string of Pearls” phenomenon: An immunofluorescent serological finding in patients with adverse drug reactions. Am J Dermatopathol. 1982;4:155–9. doi: 10.1097/00000372-198204000-00010. [DOI] [PubMed] [Google Scholar]
- 45.Zhang L, Cheng X, Li Y, Poh C, Zeng T, Priddy R, et al. High frequency of allelic loss in dysplastic lichenoid lesions. Lab Invest. 2000;80:233–7. doi: 10.1038/labinvest.3780026. [DOI] [PubMed] [Google Scholar]
- 46.Larsson A, Warfvinge G. Immunohistochemistry of ‘tertiary lymphoid follicles’ in oral amalgam-associated lichenoid lesions. Oral Dis. 1998;4:187–93. doi: 10.1111/j.1601-0825.1998.tb00277.x. [DOI] [PubMed] [Google Scholar]
- 47.Ghalayani P, Razavi SM, Gholami D. Comparative study of number and distribution of IgG cells in oral lichen planus and oral lichenoid lesions. Dent Res J. 2009;6:1–5. [PMC free article] [PubMed] [Google Scholar]
- 48.McCullough MJ, Radden BG. Involucrin expression in some oral lichenoid lesions. J Oral Pathol Med. 1992;21:367–9. doi: 10.1111/j.1600-0714.1992.tb01367.x. [DOI] [PubMed] [Google Scholar]
- 49.Acay RR, Felizzola CR, de Araujo N, de Sousa SO. Evaluation of proliferative potential in oral lichen planus and oral lichenoid lesions using immunohistochemical expression of p53 and Ki67. Oral Oncol. 2006;42:475–80. doi: 10.1016/j.oraloncology.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 50.van der Waal I. Oral lichen planus and oral lichenoid lesions; a critical appraisal with emphasis on the diagnostic aspects. Med Oral Patol Oral Cir Bucal. 2009;14:E310–4. [PubMed] [Google Scholar]
- 51.Mignogna MD, Lo Muzio L, Lo Russo L, Fedele S, Ruoppo E, Bucci E. Clinical guidelines in early detection of oral squamous cell carcinoma arising in oral lichen planus: A 5-year experience. Oral Oncol. 2001;37:262–7. doi: 10.1016/s1368-8375(00)00096-8. [DOI] [PubMed] [Google Scholar]
- 52.Mignogna MD, Lo Muzio L, Lo Russo L, Fedele S, Ruoppo E, Bucci E. Metastases in small thickness oral squamous cell carcinoma arising in oral lichen planus. Med Oncol. 2001;18:159. doi: 10.1385/MO:18:2:159. [DOI] [PubMed] [Google Scholar]
- 53.Hayashi T, Ito J, Taira S, Katsura K. The relationship of primary tumor thickness in carcinoma of the tongue to subsequent lymph node metastasis. Dentomaxillofac Radiol. 2001;30:242–5. doi: 10.1038/sj/dmfr/4600615. [DOI] [PubMed] [Google Scholar]
- 54.Yuen AP, Lam KY, Wei WI, Lam KY, Ho CM, Chow TL, et al. A comparison of the prognostic significance of tumor diameter, length, width, thickness, area, volume and clinicopathological features of oral tongue carcinoma. Am J Surg. 2000;180:139–43. doi: 10.1016/s0002-9610(00)00433-5. [DOI] [PubMed] [Google Scholar]


