Abstract
Background:
Systemic sclerosis (SS) is a chronic, multisystem collagen vascular disorder of undefined etiology, whose prognosis and overall survival is determined by visceral especially the lung involvement.
Aim:
To evaluate the pulmonary involvement in SS by imaging methods.
Materials and Methods:
Clinical examination, pulmonary function tests, chest X-ray and high resolution computed tomography (HRCT) scans were carried out in a series of 25 patients prospectively over a period of 3 years (2009-2011AD).
Results:
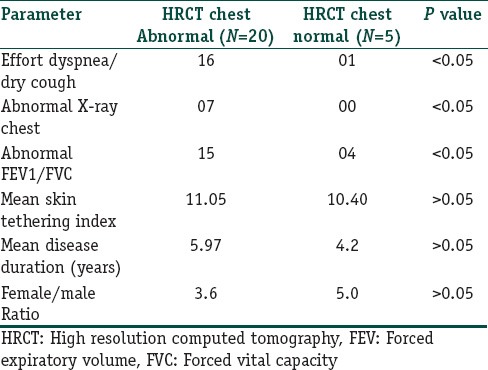
Of the total 25 patients of the study, the group with abnormal HRCT chest (n = 20), 16 had clinical symptoms of respiratory involvement, only 7 had an abnormal chest X-ray and 15 had abnormal forced expiratory volume/forced vital capacity (FEV1/FVC) spirometric parameter. While the group with normal HRCT chest (n = 5), 1 had clinical symptoms of respiratory involvement and 4 had abnormal FEV1/FVC spirometric parameter. The differences in these parameters between the two groups were statistically significant, while the differences for mean skin tethering index, mean disease duration and female/male sex ratio were statistically meaningless. Most common HRCT finding observed in the study was ground glass opacities (GGO) (9/20). Only 4 of total 9 patients who had only GGO in HRCT were symptomatic for respiratory involvement as compared to 100% (11/11) in the group who had HRCT findings other than or in addition to GGO.
Conclusion:
The HRCT outscores Chest X-ray in detecting early lung involvement in SS patients more so early in the course of the disease thereby underscoring its importance in identifying SS patients who will be potential candidates for early institution of therapy that might reverse/limit pulmonary involvement by the disease.
Keywords: High resolution computed tomography, interstitial lung disease, systemic sclerosis
What was known?
Pulmonary involvement in systemic sclerosis is very well established and is better detected by high resolution computed tomography (HRCT) than conventional X-ray technique.
Introduction
Multisystem deposition of excessive collagen, a pathophysiological hallmark of systemic sclerosis (SS) is attributed to a poorly understood phenomenon of immune dysregulation involving fibroblasts and endothelial cells. With the advances in anti-hypertensive therapies and renal replacement therapies particularly renal transplantation, pulmonary involvement has taken-over as the leading cause of morbidity and mortality in SS patients.[1] Two thirds of patients suffering from SS present with pulmonary disease with effort dyspnoea being the most usual respiratory symptom, many times associated with dry cough.[2]
Until unraveling of molecular mechanisms for exuberant collagen deposition by fibroblasts will open up the disease for a therapeutic approach aimed at its basic pathophysiology, organ-system oriented therapeutics will continue to play a central role for curbing the morbidity and mortality associated with the disease. Pulmonary artery hypertension and interstitial lung disease (ILD) are the two main syndromes associated with SS.[3] Efforts at identifying surrogate markers, clinical and/or radiological, for the two syndromes at a stage where institution of currently available medical therapy will limit or reverse the pulmonary involvement of the disease represent the forefront of the research.
Materials and Methods
Twenty five patients of SS were studied prospectively for a period of 3 years (2009-2011) in dermatology department of a state medical college. Evaluation included a complete clinical examination, skin tethering index (modified Rodnan Skin Score), spirometric pulmonary function tests, chest-X-rays postero-anterior views and HRCT of the chest. The HRCT was performed in helical equipment, sequential mode, with 1 mm thick slices at 10 mm intervals and reconstruction with hard filter, with pulmonary parenchyma and mediastinum windows, without contrast administration with the patient in a dorsal decubitus. The study review was carried out by a radiologist. Patients with other associated collagen vascular disorders such as rheumatoid arthritis, systemic lupus erythematosus and dermatomyositis/polymyositis were not included in the study.
Results
Of the total 25 patients of the study, the group with abnormal HRCT chest (n = 20), 16 had clinical symptoms of respiratory involvement, only 7 had an abnormal chest X-ray and 15 had abnormal forced expiratory volume/forced vital capacity (FEV1/FVC) spirometric parameter. While the group with normal HRCT chest (n = 5), 1 had clinical symptoms of respiratory involvement and 4 had abnormal FEV1/FVC spirometric parameter. The differences in these parameters between the two groups were statistically significant (P < 0.05). While the differences of the parameters like mean skin tethering index, Mean disease duration and Female/male sex ratio were statistically meaningless (P > 0.05). Most common HRCT finding observed in the study was ground glass opacities (GGO) (9/20). Among those who had only GOO in HRCT only 44% (4/9) were symptomatic for respiratory involvement as compared to 100% (11/11) in the group who had HRCT findings other than or in addition to GGO.
Table 1 Compare various parameters between those with abnormal HRCT and normal HRCT while Table 2 displays study parameters in individual patients of the study.
Table 1.
Various study parameters as observed in individual patients of the study
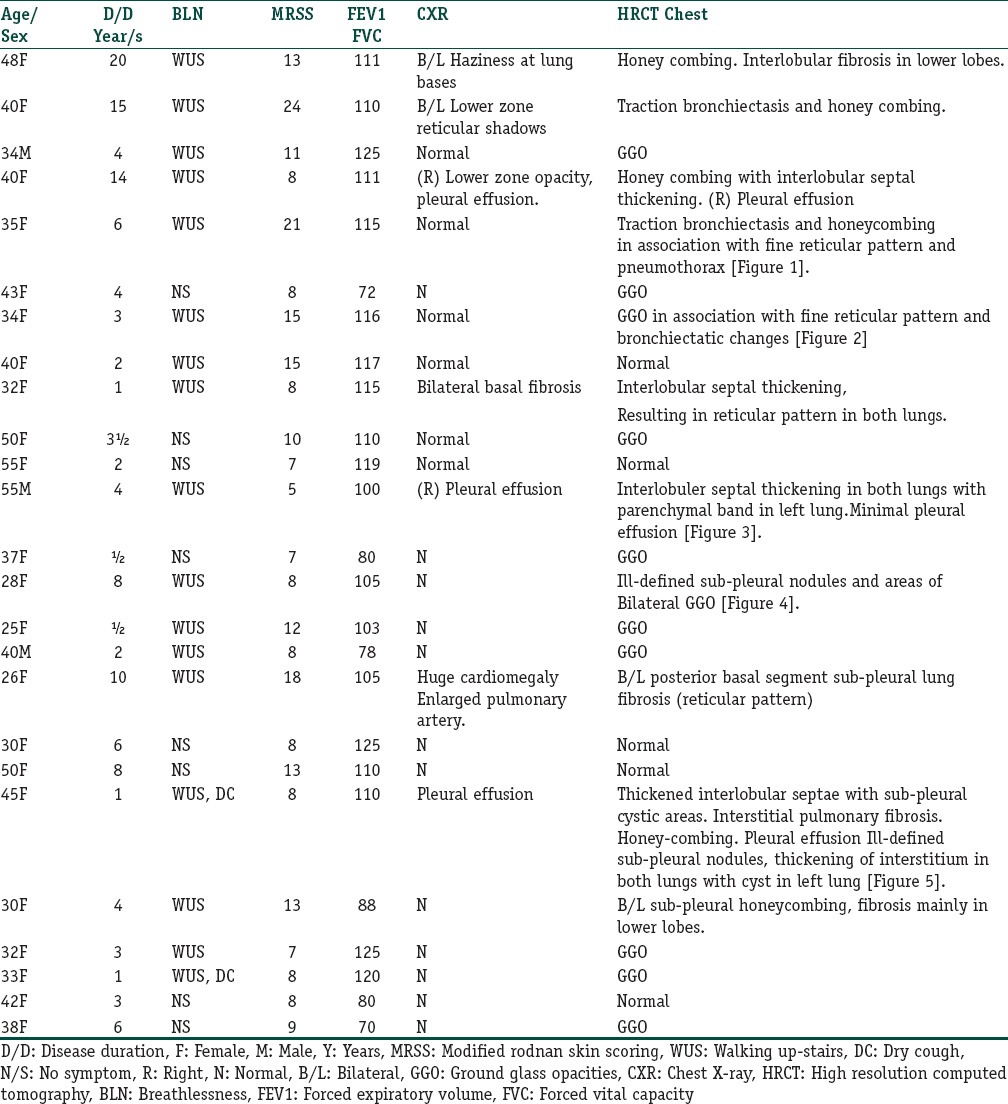
Table 2.
Comparison of parameters between those with abnormal and normal HRCT chest
Discussion
SS is a multisystem disease of excessive collagen deposition with fibroblast and the vascular endothelial cell at the crux of its patho-physiological mechanisms. Involvement of organs responsible for more critical functions such as the lungs and the kidney contribute to the mortality while other organs such as esophagus and skin are associated with the morbidity of this disease. Remarkable advances in antihypertensive drug therapy and renal transplantation has led to a decrease in renal associated mortality and pulmonary system related complications are now surfacing as the leading cause of death in SS. So, understandably, patterns of pulmonary involvement both clinical and radiological can surrogate the early pulmonary involvement.
Clinical symptoms, spirometric studies, bronchoalveolar lavage analysis and radiological (both X-ray and conventional and HRCT scan) studies have been used for assessing the pulmonary involvement in patients with SS. The objectivity of the modern medicine, subjectivity of the breathing maneuvers required for spirometry associated with a broad normal range of a particular parametric value instead of a normal absolute one and the lack of sensitivity of an X-ray sets aside such kind of procedures for pulmonary assessment in SS patients.
Most studies show that up to two thirds of patients suffering from SS present with pulmonary disease with effort dyspnoea being the most usual respiratory symptom, many times associated with dry cough.[4] We found pulmonary involvement in 80% (20/25) of cases on HRCT as against 16% (4/25) on chest X-ray. Among patients with documentation of pulmonary involvement on HRCT, predominant patterns of involvement included ground-glass opacities (11 of total 20 abnormal scans) followed by reticular pattern and traction bronchiectasis (6 of total 20 abnormal scans) [Figure 1]. Ground-glass appearance was the most common HRCT finding, observed in total 11 scans with it being an exclusive finding in 9 scans [Figures 2 and 4]. Of the 9 patients in whom an exclusive GGO was observed in HRCT scans near 50% lack clinical symptoms of pulmonary involvement. Against any other HRCT finding GGO is the only HRCT pattern which is as commonly found in clinically asymptomatic patients as it is found in symptomatic patients. This is in remarkable contrast to chest X-ray which was normal in all asymptomatic patients. Practically such observations would mean that presence of effort dyspnoea and/or dry cough in patients with SS reflects overt pulmonary fibrosis and that HRCT evaluation of the chest should document such findings in almost all such cases. Such results are in concurrence with what has been documented in the literature. In the long run, GGO on HRCT usually progress to overt fibrosis. Furthermore, the finding of ground glass appearance of pulmonary parenchyma on HRCT in a few patients who had no pulmonary symptoms and its presence in significant number of those patients who had HRCT evidence of frank fibrosis 18% (2/11) might hint at it representing a harbinger of pulmonary fibrosis. This inference has also been suggested by various authors.[5] In view of above finding the authors believe that GGO in HRCT of SS patients can be used as a radiological marker of impending ILD.
Figure 1.
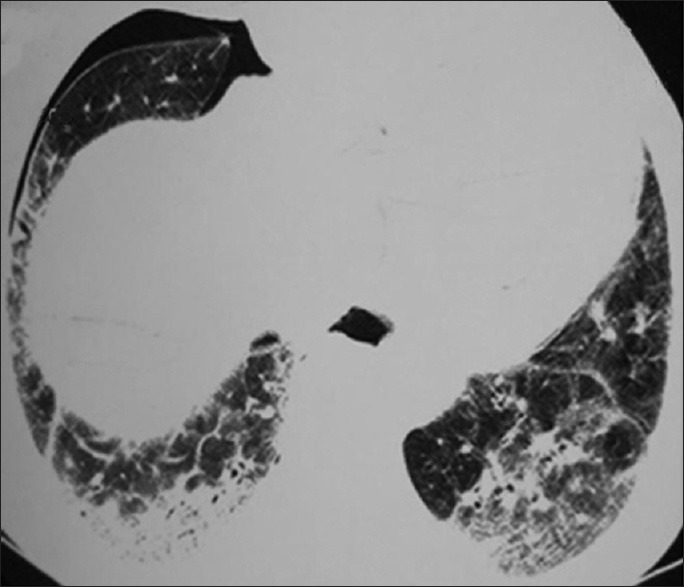
Traction bronchiectasis and honeycombing in association with fine reticular pattern and pnemothorax
Figure 2.
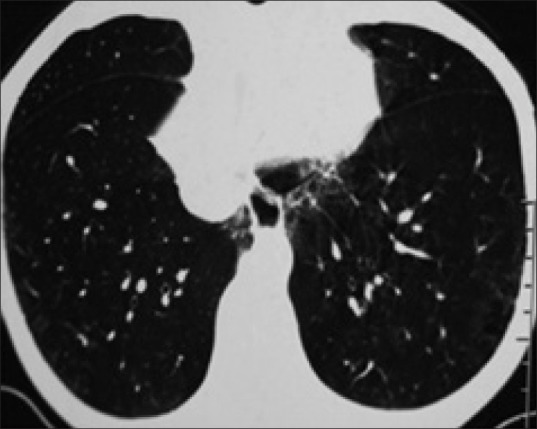
Ground glass opacities in association with fine reticular pattern and bronchiectasis changes
Figure 4.
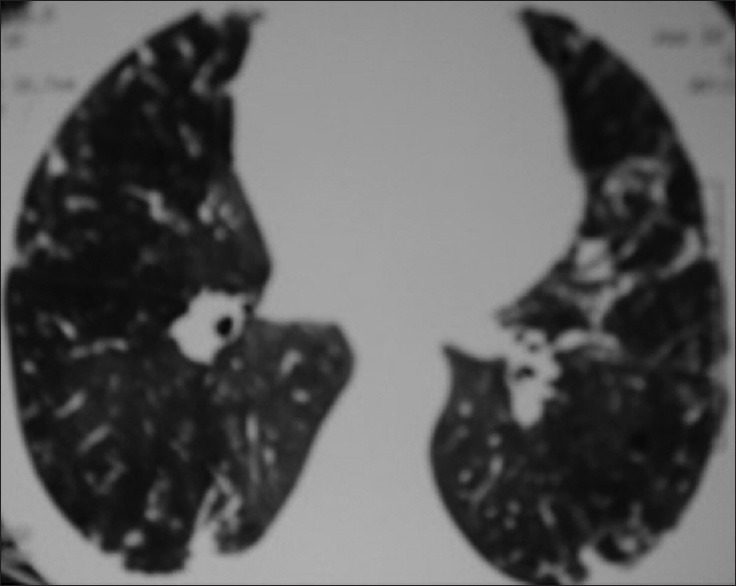
Ill-defined sub-pleural nodules and areas of bilateral ground glass opacities
In the group with less than 5 years of disease duration the discordance between chest skiagram and HRCT was observed in 9 out of total 15 (60%) of the group while in the group of more than 5 yrs disease duration such discordance was observed in 3 out of total 10 (30%) patients. Though the difference in discordance seems to decrease with disease duration but the figures in our study were not statistically significant (<0.06) for such kind of conclusion in this study of relatively smaller sample size. And the authors believe that a study with larger sample size is necessary for establishing the trend of the discordance with disease duration.
In the group with mean skin tethering index of ≤10 the discordance between chest skiagram and HRCT was observed in 8/15 (53%) patients while in the group with skin tethering index of >10 such discordance was observed 5/10 (50%) of the patients. Keeping in view with these figures the authors conclude that the discordance of chest X-ray and HRCT does not correlate with disease severity in the study. Because we lack the data of sequential follow up of the patients for the parameters of progression/remission so this study has a drawback of being unable to comment on the correlation of the discordance of chest X-ray and HRCT with the progression of the disease.
Tendency for screening SS patients with HRCT for pulmonary involvement or by any other clinical method is undermined by unavailability of a means for controlling the progression if not reversing the condition. Pre-symptomatic pharmacotherapy with drugs like cyclophosphamide aimed at such ground glass appearances has already been taken off for research. However face appearance of the results of such kind of work though seemingly discouraging but yet to be analytically conclusive.[6,7]
The pattern of distribution of Spirometric parameter (FEV1/FVC) in the study tends to match with distribution of HRCT abnormality in SS patients. Furthermore the involvement of musculoskeletal system, subjectivity of the maneuver for performing spirometry and the lack of absolute normal single value for the spirometric parameters dents further the use of FEV1/FVC parameter in SS patients. We believe that in the current HRCT era, spirometric analysis might lose its value in the evaluation of SS patients as it does not add any more information other than that what is evident from the pulmonary symptomatology of the patient.
We documented a mean skin tethering index of 11.05 and 10.4 for those with and without abnormal HRCT respectively [Table 2] with a P > 0.05, a finding endorsing the already established view of it being a non correlate of extent of pulmonary involvement in SS patients.[8] Parenchymal bands have been observed in as many as 26% of SS patients in some studies.[9] In one patient, we observed parenchymal band in one lung in association with interlobular fibrosis in both the lungs [Figure 3].
Figure 3.
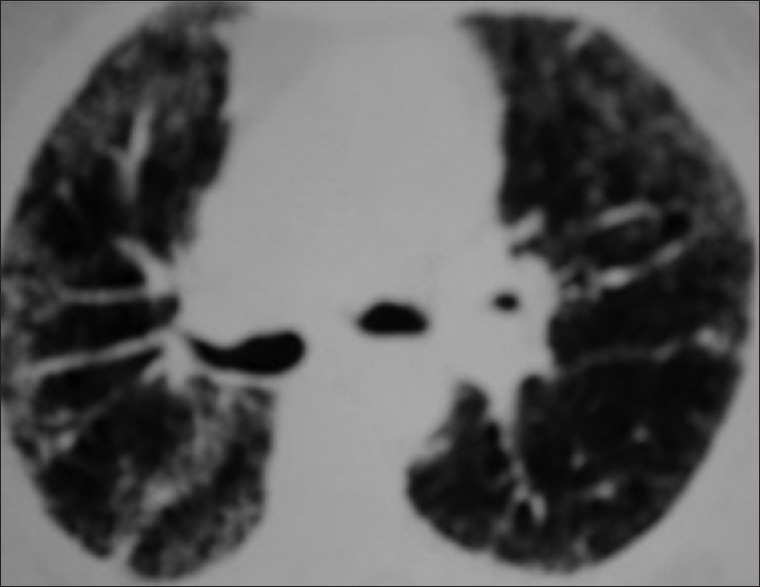
Interlobular septal thickening, parenchymal band in left lung and minimal right pleural effusion
Our study documented a solitary cyst in one lung of a patient [Figure 5]. Whether this cyst was present congenitally or developed during the course of SS remains to be elucidated. Our study also documented pneumothorax in one case [Figure 1]. Spontaneous pneumothorax has been reported, often, in association with subpleural blebs in the context of ILD. Interstitial fibrosis is not an essential underlying condition for the development of pneumomediastinum, as some cases with pneumomediastinum reported in the literature had no findings of ILD. Rupture of the alveoli and honeycomb cysts and subsequent air leakage into the surrounding interstitium could be regarded as a cause of pneumomediastinum in patients with pulmonary fibrosis.[10]
Figure 5.
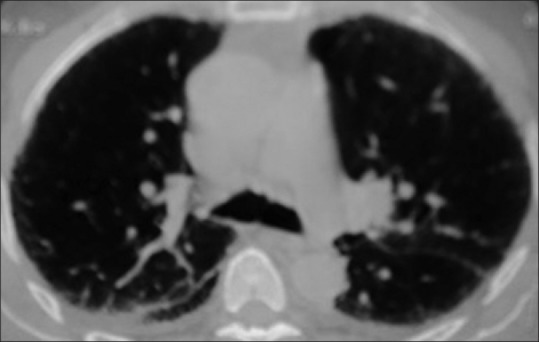
Thickened interlobular septae with sub-pleural cystic areas. Interstitial pulmonary fibrosis. Honey-combing. Pleural effusion ill-defined sub-pleural nodules, thickening of interstitium in both lungs with cyst in left lung
We also documented pleural effusion in one of our patients who had diffuse cutaneous involvement and had presented with dry cough along with dyspnea [Figure 3]. Pleural effusions are uncommon in scleroderma in the absence of clinical congestive heart failure or ILD, but have been reported in 15% of patients with an overlap syndrome and scleroderma. Thompson and Pope observed a few patients in his scleroderma population with significant pleural effusions that were not explained by other etiologies.[11] The pathogenesis of pleural effusion in SS is unknown, but may relate to vasculitis of the pleura, concomitant heart failure and infection. We observed parenchymal band in one lung in association with interlobular fibrosis in both lungs in one patient.
To conclude, pulmonary complications are very common and a leading cause of mortality in SS. Treatment of various lung complications which can be picked up at an early stage by HRCT is still disappointing. A better understanding of the pathophysiology of this disease is greatly needed so that more targeted approaches can be instituted at the earliest in order to decrease the morbidity and mortality associated with this disease.
What is new?
The study endorse the view that HRCT chest is far more sensitive than chest X-ray for identifying early involvement of lung parenchymal by SS especially in patients who do not have respiratory symptoms and probably also in those who have a disease duration of less than 5 years. Furthermore the HRCT pattern of ground-glass appearance may be considered as a radiological marker for early involvement of lung parenchyma by SS.
Footnotes
Source of support: Nil
Conflict of Interest: Nil.
References
- 1.Wells AU, Steen V, Valentini G. Pulmonary complications: One of the most challenging complications of systemic sclerosis. Rheumatology (Oxford) 2009;48(Suppl 3):iii40–4. doi: 10.1093/rheumatology/kep109. [DOI] [PubMed] [Google Scholar]
- 2.Ferri C, Valentini G, Cozzi F, Sebastiani M, Michelassi C, La Montagna G, et al. Systemic sclerosis: Demographic, clinical, and serologic features and survival in 1,012 Italian patients. Medicine (Baltimore) 2002;8:139–53. doi: 10.1097/00005792-200203000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Steen V. Predictors of end stage lung disease in systemic sclerosis. Ann Rheum Dis. 2003;62:97–9. doi: 10.1136/ard.62.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fischer A, Swigris JJ, Groshong SD, Cool CD, Sahin H, Lynch DA, et al. Clinically significant interstitial lung disease in limited scleroderma: Histopathology, clinical features, and survival. Chest. 2008;134:601–5. doi: 10.1378/chest.08-0053. [DOI] [PubMed] [Google Scholar]
- 5.Desai SR, Veeraraghavan S, Nikolakopolou A, Nicholson AG, Colby TV, Denton CP, et al. CT features of lung disease in patients with systemic sclerosis: comparison with idiopathic pulmonary fibrosis and nonspecific interstitial pneumonia. Radiology. 2004;232:560–7. doi: 10.1148/radiol.2322031223. [DOI] [PubMed] [Google Scholar]
- 6.Giacomelli R, Valentini G, Salsano F, Cipriani P, Sambo P, Conforti ML, et al. Cyclophosphamide pulse regimen in the treatment of alveolitis in systemic sclerosis. J Rheumatol. 2002;29:731–6. [PubMed] [Google Scholar]
- 7.Griffiths B, Miles S, Moss H, Robertson R, Veale D, Emery P, et al. Systemic sclerosis and interstitial lung disease: a pilot study using pulse intravenous methylprednisolone and cyclophosphamide to assess the effect on high resolution computed tomography scan and lung function. J Rheumatol. 2002;29:2371–8. [PubMed] [Google Scholar]
- 8.Morelli S, Barbieri C, Sqreccia A, Ferrante L, Pittoni V, Conti F, et al. Relationship between cutaneous and pulmonary involvement in systemic sclerosis. J Rheumatol. 1997;24:81–5. [PubMed] [Google Scholar]
- 9.Schurawitzki H, Stiglbauer R, Graninger W, Herold C, Polzleitner D, Burghuber OC, et al. Interstitial lung disease in progressive systemic sclerosis: High–resolution CT versus radiography. Radiology. 1990;176:755–9. doi: 10.1148/radiology.176.3.2389033. [DOI] [PubMed] [Google Scholar]
- 10.Teixeira Moreira Almeida Mdo S, Dias LT, Fernandes SJ, Almeida JV. Spontaneous pneumomediastinum and subcutaneous emphysema in systemic sclerosis. Rheumatol Int. 2007;27:675–7. doi: 10.1007/s00296-006-0260-y. [DOI] [PubMed] [Google Scholar]
- 11.Thompson AE, Pope JE. A study of the frequency of pericardial and pleural effusions in scleroderma. Br J Rheumatol. 1998;37:1320–3. doi: 10.1093/rheumatology/37.12.1320. [DOI] [PubMed] [Google Scholar]


