Abstract
The DNA of the F plasmid is joined to bacterial DNA sequences in the F' ORF203 by directly repeated insertion sequence 2 (IS2) elements. The rate of excision of the F plasmid form this F' (presumably by recombination at the directly repeated IS2s) has been estimated in both recA+ and recA- strains. Normal F is produced in the recA+ strain, but is not detected in recA-. The autonomous plasmids produced in the recA- background were F's having deletions. F excision in this particular recA+ case is specific in the sense that the directly repeated IS2s appear to be more active in recombination than similarly disposed IS3 direct repetitions in this F'.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg C. M., Curtiss R., 3rd Transposition derivatives of an Hfr strain of Escherichia coli K-12. Genetics. 1967 Jul;56(3):503–525. doi: 10.1093/genetics/56.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D. E., Gallant J. A. Tests of reciprocality in crossingover in partially diploid F strains of Escherichia coli. Genetics. 1971 Aug;68(4):457–472. doi: 10.1093/genetics/68.4.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtiss R., 3rd Genetic manipulation of microorganisms: potential benefits and biohazards. Annu Rev Microbiol. 1976;30:507–533. doi: 10.1146/annurev.mi.30.100176.002451. [DOI] [PubMed] [Google Scholar]
- Deonier R. C., Davidson N. The sequence organization of the integrated F plasmid in two Hfr strains of Escherichia coli. J Mol Biol. 1976 Nov 5;107(3):207–222. doi: 10.1016/s0022-2836(76)80002-2. [DOI] [PubMed] [Google Scholar]
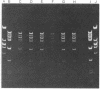
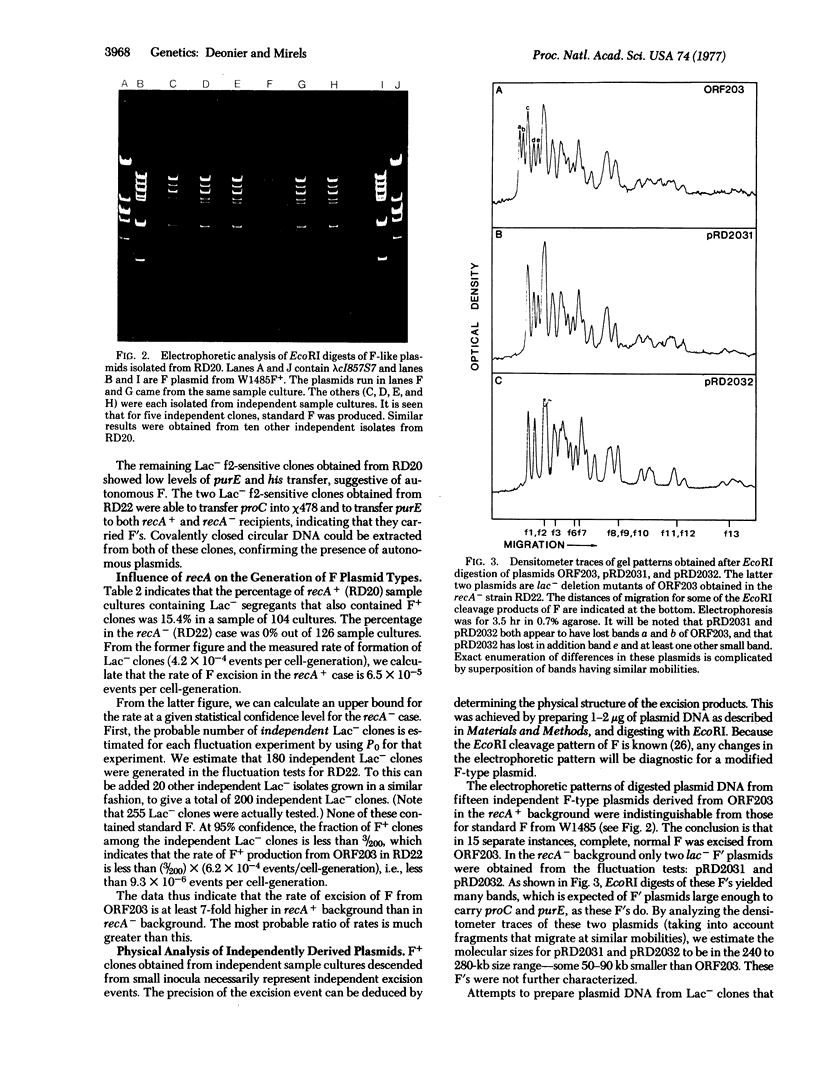
- Deonier R. C., Oh G. R., Hu M. Further mapping of IS2 and IS3 in the lac-purE region of the Escherichia coli K-12 genome: structure of the F-prime ORF203. J Bacteriol. 1977 Feb;129(2):1129–1140. doi: 10.1128/jb.129.2.1129-1140.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helling R. B., Goodman H. M., Boyer H. W. Analysis of endonuclease R-EcoRI fragments of DNA from lambdoid bacteriophages and other viruses by agarose-gel electrophoresis. J Virol. 1974 Nov;14(5):1235–1244. doi: 10.1128/jvi.14.5.1235-1244.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu M. T., Davidson N. Structure of inserted bacteriophage Mu-1 DNA and physical mapping of bacterial genes by Mu-1 DNA insertion. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2823–2827. doi: 10.1073/pnas.69.10.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S., Ohtsubo E., Davidson N. Electron microscopic heteroduplex studies of sequence relations among plasmids of Escherichia coli: structure of F13 and related F-primes. J Bacteriol. 1975 May;122(2):749–763. doi: 10.1128/jb.122.2.749-763.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S., Ptashne K., Cohen S. N., Davidson N. alphabeta sequence of F is IS31. J Bacteriol. 1975 Aug;123(2):687–692. doi: 10.1128/jb.123.2.687-692.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaskunas S. R., Lindahl L., Nomura M. Isolation of polar insertion mutants and the direction of transcription of ribosomal protein genes in E. coli. Nature. 1975 Jul 17;256(5514):183–187. doi: 10.1038/256183a0. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Lieb M. Forward and Reverse Mutation in a Histidine-Requiring Strain of Escherichia Coli. Genetics. 1951 Sep;36(5):460–477. doi: 10.1093/genetics/36.5.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low K. B. Escherichia coli K-12 F-prime factors, old and new. Bacteriol Rev. 1972 Dec;36(4):587–607. doi: 10.1128/br.36.4.587-607.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luria S. E., Delbrück M. Mutations of Bacteria from Virus Sensitivity to Virus Resistance. Genetics. 1943 Nov;28(6):491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polisky B., Greene P., Garfin D. E., McCarthy B. J., Goodman H. M., Boyer H. W. Specificity of substrate recognition by the EcoRI restriction endonuclease. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3310–3314. doi: 10.1073/pnas.72.9.3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saedler H., Heiss B. Multiple copies of the insertion-DNA sequences IS1 and IS2 in the chromosome of E. coli K-12. Mol Gen Genet. 1973 May 9;122(3):267–277. doi: 10.1007/BF00278602. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Hsu M. T., Otsubo E., Davidson N. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli. I. Structure of F-prime factors. J Mol Biol. 1972 Nov 14;71(2):471–497. doi: 10.1016/0022-2836(72)90363-4. [DOI] [PubMed] [Google Scholar]
- Thompson R., Hughes S. G., Broda P. Plasmid identification using specific endonucleases. Mol Gen Genet. 1974;133(2):141–149. doi: 10.1007/BF00264835. [DOI] [PubMed] [Google Scholar]