Abstract
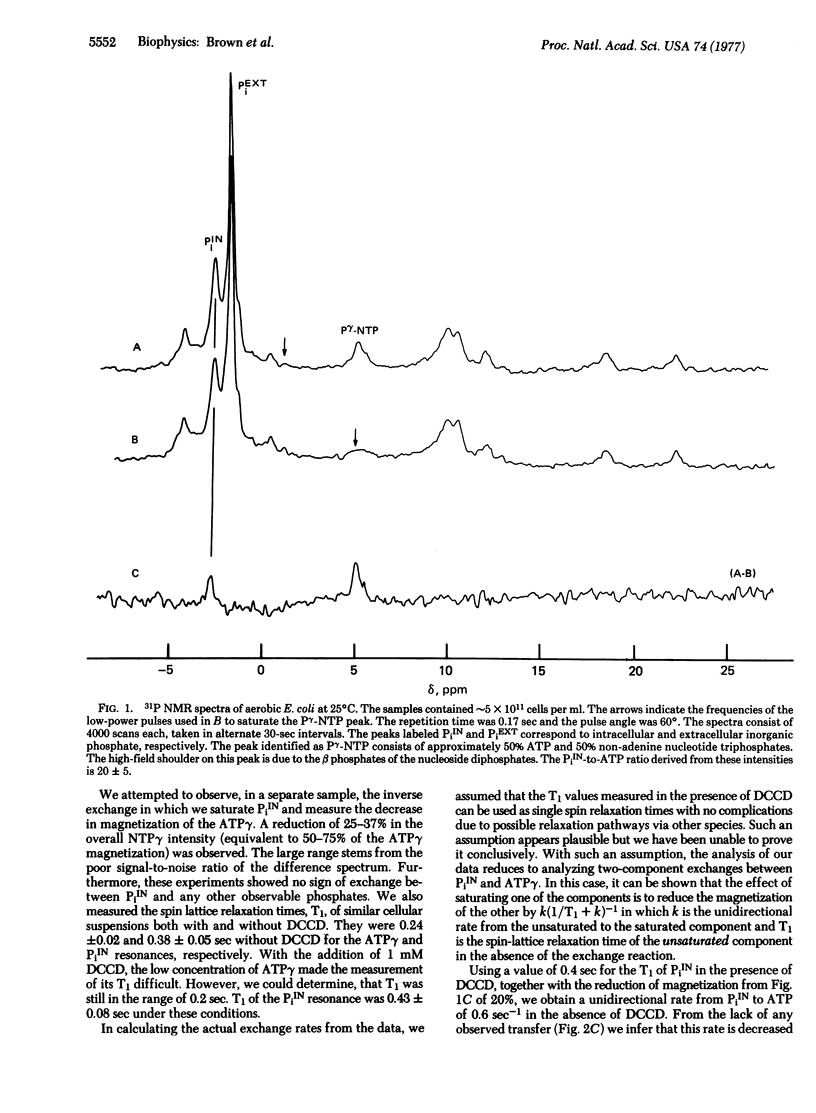
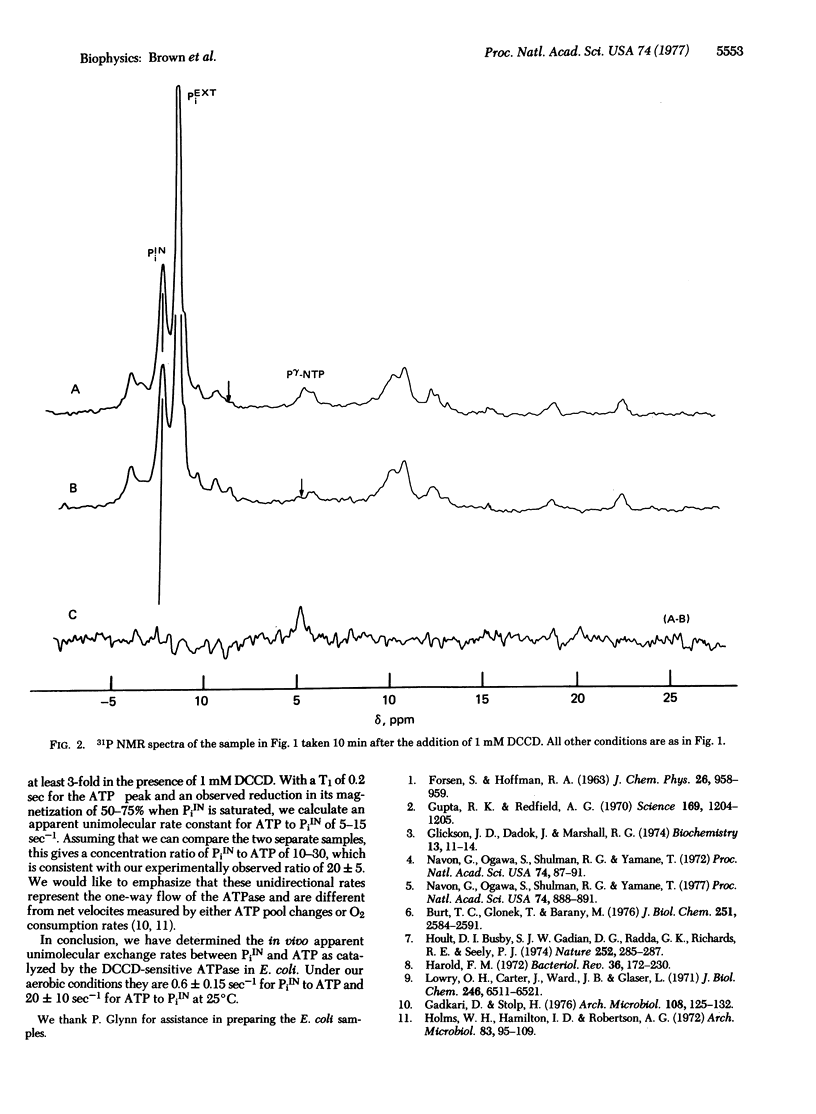
We have measured the in vivo unidirectional rates between the terminal phosphate of ATP and intracellular inorganic phosphate (PiIN) in aerobic suspensions of Escherichia coli cells using 31P nuclear magnetic resonance saturation transfer techniques. Typically, the measurements consisted of saturating the ATPgamma resonance and observing a 20 +/- 5% reduction in the intensity of the PiIN resonance. No saturation transfer was observed after incubation with 1 mM dicyclohexylcarbodiimide, an ATPase inhibitor. From the measured decrease in intensity of PiIN coming from saturation transfer, the apparent unimolecular rate constant of PiIN to ATP was calculated to be 0.6 +/- 0.15 sec-1.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burt C. T., Glonek T., Bárány M. Analysis of phosphate metabolites, the intracellular pH, and the state of adenosine triphosphate in intact muscle by phosphorus nuclear magnetic resonance. J Biol Chem. 1976 May 10;251(9):2584–2591. [PubMed] [Google Scholar]
- Gadkari D., Stolp H. Energy metabolism of Bdellovibrio bacteriovorus. II. P/O ratio and ATP pool turnover rate. Arch Microbiol. 1976 May 3;108(1):125–132. doi: 10.1007/BF00425102. [DOI] [PubMed] [Google Scholar]
- Glickson J. D., Dadok J., Marshall G. R. Proton magnetic double-resonance study of angiotensin II (Asn1Val5) in aqueous solution employing correlation spectroscopy. Assignment of peptide NH resonances and transfer of saturation from water. Biochemistry. 1974 Jan 1;13(1):11–14. doi: 10.1021/bi00698a002. [DOI] [PubMed] [Google Scholar]
- Gupta R. K., Redfield A. G. Double nuclear magnetic resonance observation of electron exchange between ferri- and ferrocytochrome c. Science. 1970 Sep 18;169(3951):1204–1206. doi: 10.1126/science.169.3951.1204. [DOI] [PubMed] [Google Scholar]
- Harold F. M. Conservation and transformation of energy by bacterial membranes. Bacteriol Rev. 1972 Jun;36(2):172–230. doi: 10.1128/br.36.2.172-230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holms W. H., Hamilton I. D., Robertson A. G. The rate of turnover of the adenosine triphosphate pool of Escherichia coli growing aerobically in simple defined media. Arch Mikrobiol. 1972;83(2):95–109. doi: 10.1007/BF00425016. [DOI] [PubMed] [Google Scholar]
- Hoult D. I., Busby S. J., Gadian D. G., Radda G. K., Richards R. E., Seeley P. J. Observation of tissue metabolites using 31P nuclear magnetic resonance. Nature. 1974 Nov 22;252(5481):285–287. doi: 10.1038/252285a0. [DOI] [PubMed] [Google Scholar]
- Lowry O. H., Carter J., Ward J. B., Glaser L. The effect of carbon and nitrogen sources on the level of metabolic intermediates in Escherichia coli. J Biol Chem. 1971 Nov;246(21):6511–6521. [PubMed] [Google Scholar]
- Navon G., Ogawa S., Shulman R. G., Yamane T. 31P nuclear magnetic resonance studies of Ehrlich ascites tumor cells. Proc Natl Acad Sci U S A. 1977 Jan;74(1):87–91. doi: 10.1073/pnas.74.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navon G., Ogawa S., Shulman R. G., Yamane T. High-resolution 31P nuclear magnetic resonance studies of metabolism in aerobic Escherichia coli cells. Proc Natl Acad Sci U S A. 1977 Mar;74(3):888–891. doi: 10.1073/pnas.74.3.888. [DOI] [PMC free article] [PubMed] [Google Scholar]



