Abstract
Acne vulgaris is a chronic condition affecting more than 85% of adolescents and young adults. It is one of the most common diseases affecting humanity and its impact on quality of life (QoL) is important. The impact of acne on QoL in Indian patients remains undocumented. The study was undertaken to detect the impact of acne vulgaris and related factors that may influence the QoL.
Materials and Methods:
This was a hospital-based, prospective, cross-sectional, prestructured, questionnaire-based study done on 140 consenting individuals, who attended the Dermatology outpatient department. Acne vulgaris was graded using simple grading system. QoL was measured using a combination of skin disease-specific (Dermatological Life Quality Index (DLQI)) and acne-specific (Cardiff Acne Disability Index (CADI)) questionnaires.
Results:
Majority of our study population were students (103, 73.6%). Face (139, 99.3%) was the commonest site of acne and comedones 133, 95% were the commonest type of lesion. Most of the individuals 66, 47.1% were observed to have grade 1 acne. The mean DLQI score was 6.91 and the mean CADI score was 5.2. Association between the scores was statistically significant. Age, occupation, marital status, family, and treatment history played a role in affecting the QoL. Diet, smoking, and alcohol did not influence the QoL.
Conclusion:
Though acne had impact on patient's QoL, it was less severe in our study. It is important for health professionals to incorporate QoL measurements when managing acne patients to provide better and appropriate care.
Keywords: Acne, influencing factors, quality of life, young adults
What was known?
Acne vulgaris affects the quality of life more in adolescents. Females are affected more than males. Facial acne is associated with severe negative impact on the quality of life. The impact of acne on quality of life in Indian patients remains undocumented.
Introduction
Acne vulgaris is a chronic condition affecting more than 85% of adolescents and two-third of adults aged 18 years and older. It is a chronic inflammatory disease of pilosebaceous units, characterized by seborrhea; open and closed comedones; papules; pustules; and in more severe cases nodules, pseudocysts, and scarring.[1] Acne is associated with greater psychological burden.[2] Patients experience psychological burdens like depression, anxiety, and low self-esteem because of acne. Acne vulgaris remains one of the most common diseases affecting humanity and measurement of its impact on patient's quality of life QoL is important using validated and age appropriate measures along with an objective assessment of acne status.
WHO defines QoL as the “individual's perception of their position in the context of culture and value systems in which they live and in relation to their goals, expectations, standards, and concerns”.[3] It provides a valuable insight into the debilitating effects of acne, that patients do not address themselves. Several general health-related quality of life (HRQoL) measures and acne specific HRQoL questionnaires have been developed.
General HRQOL measures compare the effect of different conditions on patient's life. These are Dermatological Life Quality Index (DLQI), Skindex, Skindex-29, Skindex-16, Dermatological Quality of Life Scale DQOLS, and Questionnaire of Chronic Skin Disorders CSD.[4] Finlay and Khan developed the widely used DLQI for use in research studies and routine clinical practice to assess changes in HRQoL, as it is a sensitive measure.[4] Although the general measures serve the purpose of assessing the impact of dermatologic conditions on patient's life, acne-specific HRQoL assessment can offer a focused insight into the negative effects of acne. It is the most sensitive way to determine the impact of acne and its effects on patients while excluding irrelevant symptoms.
Acne specific HRQoL measures include Acne Disability Index (ADI), Cardiff Acne Disability Index (CADI), Assessment of the Psychological and Social Effects of Acne APSEA, Acne Quality of Life AQOL, Acne-Quality of Life Index QOLI, Acne-QoL, and Acne Q4.[4] The CADI was developed to quickly assess the level of disability caused by acne. Clinical trials indicate that use of global and specific scales together has complementary benefits.[5]
The impact of acne on QoL and in Indian patients remains undocumented. Compared to France,[6] where adolescents worry even about getting acne, Indians appear to accept acne more readily. So there is a dearth of published information on the impact of acne on Indian patients which need to be assessed. The aim of the study was to study the impact of acne vulgaris and related factors that may influence the QoL.
Materials and Methods
This was a hospital-based, prospective, cross-sectional, prestructured, questionnaire-based study done in 140 consenting individuals, conforming to the inclusion and exclusion criteria who attended the Dermatology outpatient department of A. J. Institute of Medical Sciences AJIMS, Mangalore, Karnataka from March 2013 to July 2013, after obtaining Institute Ethics Committee clearance. Individuals between 18 and 30 years with acne vulgaris were included in the study. Patients suffering from medical disorders or on drugs likely to interfere with assessment of acne were excluded from the study.
A detailed history pertaining to the following parameters like demographic data, presenting illness, personal history/factors aggravating acne, presence of medical/surgical diseases, family, and treatment history were elicited. A thorough dermatological examination was performed to look for the following: Type of lesion, site, and grading.
Acne vulgaris was graded using a simple grading system as follows:[7]
Grade 1 - comedones, occasional papules
Grade 2 - papules, comedones, few pustules
Grade 3 - predominant pustules, nodules, abscesses
Grade 4 - mainly cysts, abscesses, widespread scarring.
QoL was measured using a combination of skin disease (DLQI) and acne specific-questionnaire (CADI). The DLQI grades QoL by giving a score to each domain. The domains assessed were: a) physical symptoms and feelings (questions 1 and 2), b) daily activities (questions 3 and 4), c) leisure (questions 5 and 6), d) work/school (question 7), e) personal relationships (questions 8 and 9), and f) treatment (question 10). The patients should answer the questions keeping in mind the obstacles faced during previous week. Each question is scored as “very much” (score 3), “a lot” (score 2), “a little” (score 1), “not at all” (score 0). Question 7 has two parts, the first part has choice “yes” score 3 and “no” and “not relevant” and the second part has the choices “a lot”, “a little”, and “not at all”. The final scores range from 0 to 30. High scores indicate poor QoL. Results from 0–1 mean no effect of the disease on the patient's QoL, 2-5 mean small effect, 6-10 mean moderate effect, 11-20 mean great effect, and 21-30 mean a very important effect.[8]
CADI is a well-validated, self-reported questionnaire consisting of five questions with a Likert scale and four response categories (0-3). The five questions relate to feeling of aggression, frustration, interference with social life, avoidance of public changing facilities, and appearance of the skin all over the last month and an indication of how bad the acne is now. The final score ranges from 0 to 15. CADI scores were graded as low (0-4), medium (5-9), and high 10-15. High scores indicate a higher level of disability. CADI identifies the area of concern in patients with acne. The patient's response to the questionnaire is significantly correlated with the clinician's assessment of acne severity.[9]
Statistical analysis
The data collected was tabulated in Microsoft Excel Worksheet and computer-based analysis was performed using the Statistical product and service solutions (SPSS) 16.0 software (SPSS, Chicago, Illinois, USA). The categorical variables were summarized as proportions and percentages. The continuous variables were summarized as mean and standard deviation. For comparison of proportions, Fisher's exact test was used. The association between parameters were found using Spearman rank-order correlation coefficient test.
Results
A total of 140 consenting individuals were included in the study with a female to male ratio of 1.55:1. The mean age of the study population was 21.46 ± 2.814 years (ranging from 18 to 30 years with a median of 21 years). The other demographic details of the study population are shown in Table 1. Majority of our study population were students (103, 73.6%).
Table 1.
Demographic data
Site of lesion
Face (139, 99.3%) was the commonest site of acne followed by back (5, 3.6%) and chest (3, 2.1%). Both facial acne independently, facial and truncal acne together were not associated with DLQI/CADI scores. There was no significant association between facial acne and gender.
Type of lesion
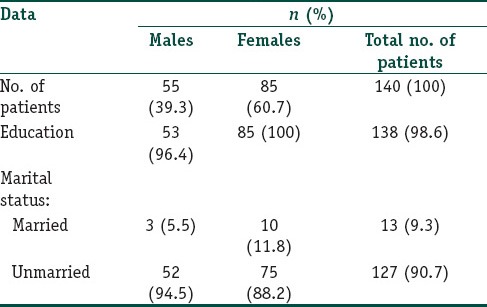
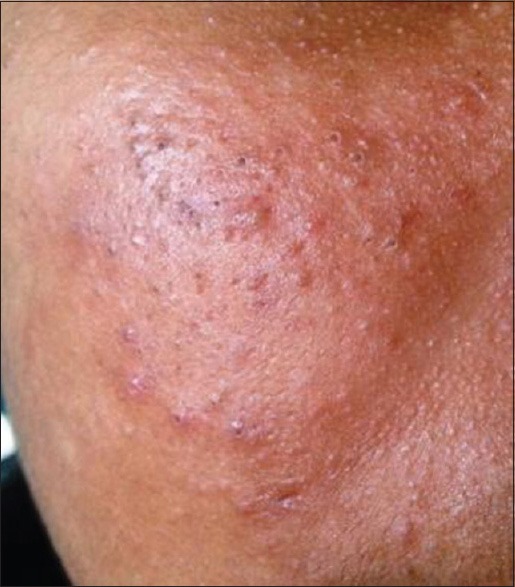
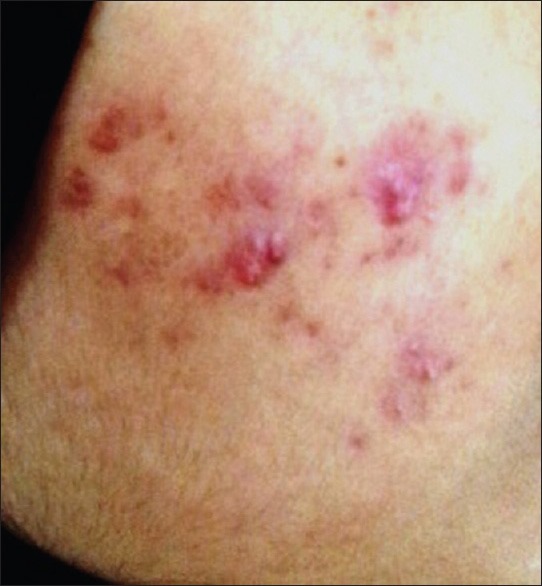
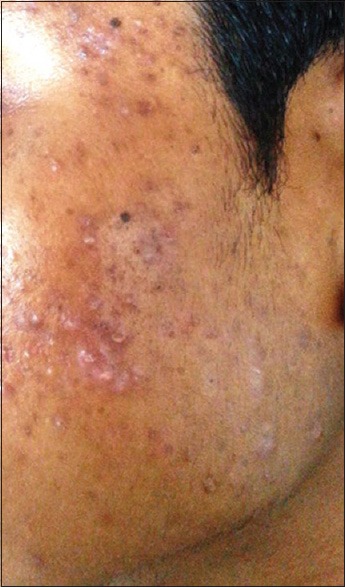
Comedones (133, 95%) [Figure 1] were the most common type of lesion. Papules [Figure 2] were seen in 82 (58.6%) patients followed by pustules (20, 14.3%) [Figure 3], scar (13, 9.3%) [Figure 4] and nodules (8, 5.7%) [Figure 5].
Figure 1.
Open and closed comedones seen over the malar region
Figure 2.
Erythematous papules seen over the face
Figure 3.
Erythematous papules with pustules and hyperpigmentation over the face
Figure 4.
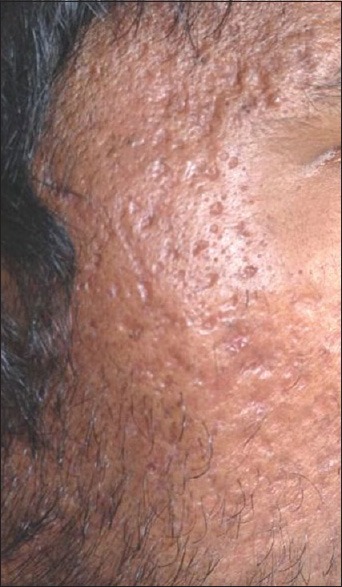
Scarring seen over the temple of face
Figure 5.
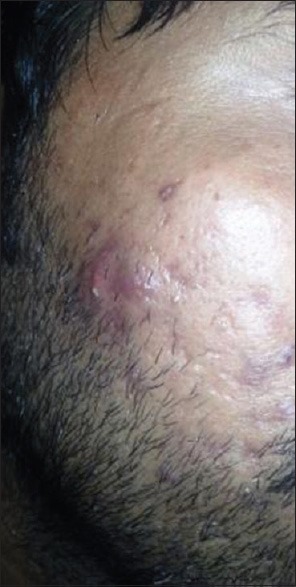
Nodule with few scars over the face
Grading of acne
Most of the individuals (66, 47.1%) were observed to have grade 1 acne [Chart 1]. There was statistically significant association between grade of acne and DLQI/CADI scores r = 0.3034, P = 0.0003 for DLQI and r = 0.3768, P = < 0.0001 for CADI. The two P values correspond to DLQI and CADI. The distribution of grading based on DLQI and CADI scores are tabulated in Tables 2 and 3, respectively.
Chart 1.
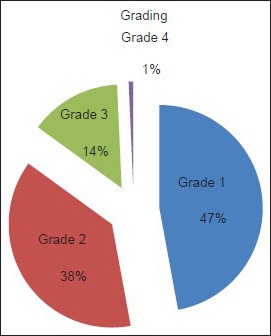
Grading of acne vulgaris
Table 2.
Distribution of acne grading based on DLQI score
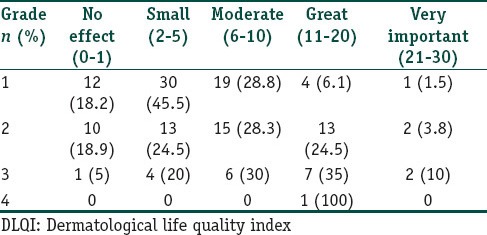
Table 3.
Distribution of acne grading based on CADI score
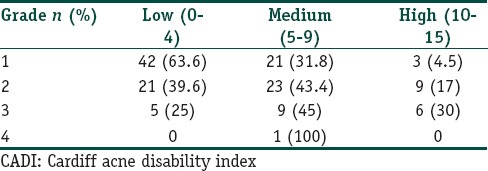
Scoring of acne based on DLQI and CADI
The mean DLQI score was 6.91 ± 5.74 (ranging from 0 to 26 with a median of 5.5) and the mean CADI score was 5.2 ± 3.14 (ranging from 0 to 15 with a median of 5). The most common DLQI score observed was in the range of 2-5 (small effect) in 47 (33.6%) patients and that of CADI was 0-4 (low) in 68 (48.6%) patients.
Association between DLQI and CADI scores were statistically significant (r = 0.7376 P < 0.0001, Spearman r correlation coefficient). The frequency distribution was done based on gender and significance was to be found based on gender for the scores DLQI/CADI. So Fisher's test was used. (Table 4).
Table 4.
Frequency distribution of DLQI and CADI scores based on gender
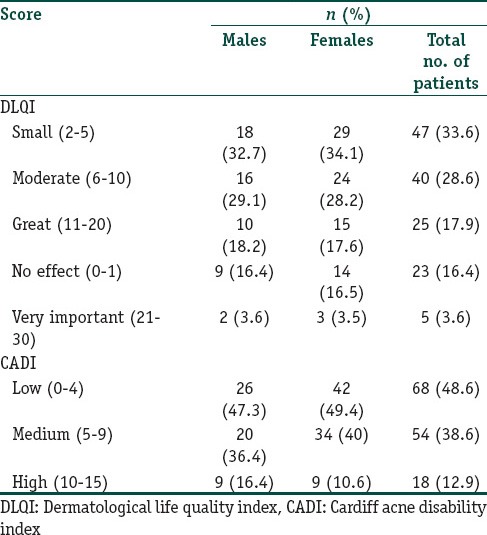
Factors Influencing Acne and QoL
Age
Age of the individuals was found to be statistically significant with the CADI scores (P = 0.0314, Spearman r correlation coefficient). The age groups 18-21 and 26-30 years were significantly associated with CADI score r = -0.2098, P = 0.0129 for ages 18-21yrs and r = 0.1847, P = 0.0289 for ages 26-30 years. The two p values correspond to the two age groups (18-21 years, 26-30 years).
Occupation
Statistically significant association was seen between occupation and DLQI/CADI scores Spearman r correlation coefficient test was done to find the association between occupation and DLQI/CADI.
Education
Educational status was assessed with the patient's ability to read and write in their language. Education and QoL was not significantly associated.
Marital status
Spearman r correlation coefficient test was done to find the association between marital status and CADI. CADI score with statistical significance was 0-4 (low) in both married (2, 15.4%) and unmarried (66, 52%) individuals (P = 0.0174, Fisher's exact test).
Diet
Majority (127, 90.7%) of the study population consumed mixed diet. Increased frequency of oily food consumption was seen in 60 (42.9%) patients, chocolates in 43 (30.7%) patients, ice cream in 36 (25.7%) patients, and milk consumption in nine (6.4%) patients. Association between diet and DLQI/CADI scores were not found to be statistically significant.
Smoking and alcohol
Out of the 140 individuals, four (2.85%) males had history of smoking and six (4.2%) males had history of alcohol consumption. Smoking and alcohol were not significantly associated with acne scores.
Family history
Thirty individuals (21.4%) had a positive family history, of whom 14 (46.6%) were females and 16 (53.3%) were males. Family history and DLQI/CADI scores were associated, which was statistically significant (P = 0.017, P = 0.0039 Spearman r correlation coefficient). The common scores with statistical significance were DLQI score of 21-30 (very important) in four (13.3%) patients and CADI score of 10-15 (high) in 10 (33.3%) patients (P = 0.007, P = 0.0007, Fisher's exact test).
Treatment history
Positive treatment history was present in 67 (47.9%) patients, majority of whom were females (41, 61.1%). Association between treatment history and DLQI/CADI scores were statistically significant (P = 0.002, P = 0.008, Spearman r correlation coefficient). Positive treatment history and DLQI score of 2-5 (small) was seen in 16 (23.9%) patients and CADI score of 0-4 (low) in 25 (37.3%) patients, which was statistically significant P = 0.031, P = 0.012, Fisher's exact test.
Discussion
Acne vulgaris is a chronic inflammatory disease of pilosebaceous unit.[1] It affects 85% of adolescents and young adults.[10] The pathogenesis is attributed to multiple factors such as increased sebum production, follicular hyperkeratinization, proliferation of Propionibacterium acnes within the follicle, alteration of the quality of sebum lipids, regulation of cutaneous steroidogenesis, androgen activity, interaction with neuropeptides, and exhibition of pro- and anti-inflammatory properties. The increased sebum excretion [Figure 6] is a major concurrent event associated with the development of acne.[1,11] Inflammation plays a key role and may be the primary process in the pathogenesis, led by the perifollicular T-helper cells through IL-1. It also upregulates sebum production through leukotriene B4.[2]
Figure 6.
Seborrhea with papules over the face
Additionally, fatty acids act as ligands of nuclear receptors such as the peroxisome proliferator activated receptors (PPARs). Sebaceous gland lipids exhibit direct pro- and anti-inflammatory properties. Furthermore, hormones like androgens control the proliferation/differentiation of sebocytes, infundibular keratinocytes, and sebum secretion. On the other hand, keratinocytes and sebocytes may be activated by P. acnes via Toll-like receptors (TLR2), CD14, CD1, interleukin (IL)-8 and IL-12.[1,11]
Acne lesions modify the individual's perception. It is essential to meet the rising demand on acne management. The Indian Acne Alliance (IAA) has been formulated to further the cause of acne in India and provides acne management guidelines which will help achieve rationalization, improvement in outcomes, and more uniform therapeutic approaches.[12] So the need to evaluate the impact on patient's life makes it necessary for the clinicians to use appropriate health-related QoL measures, which can improve physician patient relationship and also give an added perspective to the assessment of newer therapies.
There are studies assessing the impact of acne on QoL from various countries such as Cleveland,[13] USA,[10,14] Spain,[15] UK,[16] Iran,[17] Malaysia,[9] southern Brazil,[8] and Greece;[18] whereas, studies on Indian patients are reported less frequently. Comparison of prevalence between different studies is difficult because of differences in the questionnaire design, study setting, and population characteristics.
The age group of acne vulgaris patients included in different studies done in this regard are variable. Most of the studies[5,6,9,16,19] have included an age group between 13 and 18 years and some studies[10,15,20] from 11 years and some[13,21] from 17 years. The present study included an age group between 18 and 30 years.
The peak incidence of acne occurs at the age of 17 years.[10] In late adolescents, above the age of 16 years, they move towards young adult roles and appearance is given more importance than at an earlier age.[18] Physiological acne may persist even after the age of 25 years and in a study done in France and USA have shown that some level of acne activity may persist even into the 30-40 years age range.[1] Majority (60.7%) of our study population were females as in other studies,[13,14,18,20] which may be because females are more conscious of their appearance than the males.
Acne commonly involves the face. It affects the appearance of the individual which makes it more bothersome than acne at other sites. We observed facial acne (99.3%) to be the commonest in our study and there was no significant association with the QoL either alone or with facial and truncal acne together. This may be due to the mild nature of acne the patients had. No gender differences were observed.
Martin et al.[22] observed that the QoL in facial acne correlated with the patient reported severity (25%) and the QoL scores worsen with increasing severity. There was association between the acne severity of face and trunk than with face alone. Hanisah et al.[9] reported facial acne in 67.5% of individuals, being more common in males (71.1%) and QoL was affected by the severity, which was also reported by Hassan et al.[23]
We had a higher prevalence of comedones (133, 95%) [Figure 1] than that reported by Tasoula et al.[18] (65.82%) and majority of the study population (66, 47.1%) had grade 1 acne. There was significant correlation between the grade and QoL scores (P = 0.0003, P < 0.0001, Spearman r correlation coefficient) and the scores worsened with increasing severity. The mean DLQI score was 6.91 (of maximum 26) and the mean CADI score was 5.2 (of maximum 15) when compared with an Iranian study (7.57) which had higher score.[5] The QoL scores (DLQI/CADI) were significantly associated P < 0.0001, Spearman r correlation coefficient.
The mean scores in our study were low as majority had grade 1 acne. There was no gender difference between the scores which shows that both males and females were concerned about their acne when compared to a study done by Cotterill et al.,[24] Ismail et al.[20] and Halvorsen et al.[25] where females had higher scores.
Jankovic et al.[5] observed a low mean CADI (3.57) due to clinically mild acne and gender difference was significant with CADI score being more in females. They also showed correlation between Children's Dermatology Life Quality Index (CDLQI) and CADI scores. Study done by Walker and Lewis-Jones[16] showed low mean CADI score (1.9) and no gender differences, but with good correlation between CDLQI/CADI. Study by Srivastava et al. also showed correlation between acne severity and the scores and patients perception of the disease as an important factor.[26]
Eleni et al.[18] showed that moderate/severe acne had more impact on QoL (CDLQI) than mild acne and there were no gender differences. Studies done in Iraq,[20] Turkey,[20] and France[20] showed worsening QoL with increasing grade of acne. No association between acne severity and QoL was reported in some studies.[27,28]
Shahin et al.[17] evaluated CADI scores in patients with acne which showed a mean CADI score of 7.57. Majority (78%) of patients had moderately severe acne and the grade correlated with the CADI scoring as observed by Salek et al.[27] and Clark et al.[29] The score was greater in those with facial acne (7.9) and married individuals (8.8) and there were no gender differences.
In our study age of patients were significantly associated with the CADI scoring and the age groups 18-21 and 26-30 years had more significant correlation with CADI score, which indicates that severity of acne worsens as age advances, affecting the QoL. This may be because of the increased exposure to social, occupational functioning, and the treatment seeking behavior being at higher rates than before. Kameran et al.[20] observed association between age and QoL and most of the patients aged 21-25 years had higher scores. The negative impact being more as age advances was reported by various other studies.[9,13,14,17,18] In a study done by Pruthi and Babu in adult females, it was observed that acne has impact on both the physical and psychosocial aspects of life as was observed in the present study using DLQI and CADI scores.[30] Conversely Salek et al.[27] found no correlation between age and QoL.
Acne impact on the QoL based on the type of occupation was significant in our study similar to studies done by Rapp et al.[10] and Caballero et al.[15] who reported 3.05 and 2.8% unemployment, respectively, though there was no unemployment reported in our study. Probably as most of them were students. Conversely no association between occupation and scores were observed in a study done in Greece.[20]
The level of education did not affect the QoL in our study as was seen in a study done by Kameran et al.[20] which may indicate that whether educated or not, the importance for acne is the same. The marital status of an individual affects the QoL due to the cosmetic appearance. Our study showed significant association between marital status and CADI scores (P = 0.0049, Spearman r correlation coefficient), as our study population comprised mostly of unmarried individuals, but a low CADI score was significant in both married (15.4%) and unmarried (52%) which may be because, most had grade 1 acne. Similar association was observed in a study done by Aghaei et al.[17]
Acne is not an inherited condition, though has an inherited predisposition. Involvement of cytochrome P-450-1A1 and gene for steroid 21-hydroxylase are documented.[31] Positive family history is obtained in 40% of patients and correlates with more severe disease.[32] There is higher concordance rate among identical twins.[2] In our study, positive family history was observed in 21.4% of patients when compared to a study done by Eleni et al.[18] who reported 26.7%. Significant correlation was obtained with QoL scores in our study similar to that obtained by Eleni et al.[18] Scores 21-30 (very important; 13.3%) of DLQI and 10-15 (high; 33.3%) of CADI were statistically significant, showing the association of family history with acne severity.
Relationship between diet and acne is highly controversial. Dairy products influence acne through hormonal mediators and by increasing plasma insulin-like growth factor (IGF-1) level. Both testosterone precursors and 5α-reductase molecules are thought to contribute to the comedogenicity of milk by stimulating sebum production and hyperkeratinization of the pilosebaceous unit.[33] High glycemic load diets also have an impact on testosterone and IGF-1 metabolism.[34] One of the most important dietary factor that influence inflammation is the relative intake of omega 6 to omega 3 polyunsaturated fatty acids (PUFAs).[33]
We observed increased frequency of consumption of oily food (42.9%), chocolates (30.7%), ice creams (25.7%), and milk (6.4%) not to be statistically associated with the QoL scores. The reason may be that though there is consumption of foods which may be associated with acne, the quantity and frequency of high glycemic food intake are much less when compared with the western countries due to the difference in dietary habits. Fulton et al.[33] and Anderson[33] had observed negative association between food and acne. Adebamowo[33] showed milk was positively associated with acne. Smith et al.[33] showed the association of acne with high glycemic index. Ismail et al.[35] found that frequency of milk and ice cream intake was positively associated with acne occurrence.
Relationship between smoking and acne is unclear. The pathogenesis may be due to the effect of nicotine on nicotinic cholinergic receptors.[36] Presence of acetyl choline receptor (AChR) and nicotinic activity found in the infundibulum of the pilosebaceous unit can promote infundibular epithelial hyperplasia and follicular plugging, suggesting an important role for the cholinergic system in acne vulgaris.[11]
Another mechanism is arachidonic acid and polycyclic aromatic hydrocarbons present in the cigarette smoke induce phospholipase A2 dependent inflammatory pathway.[1] Beer, wine, and alcohol in large amounts induce worsening of acne, possibly due to the generally increased inflammatory reactivity of the skin after alcohol.[37]
We did not observe any correlation between smoking, alcohol and acne. Negative correlation between smoking and acne was also observed by Eleni et al.[18] and Mills et al.[38] for the first time studied the association between acne and smoking. He studied 156 patients and found that 19.7% of 96 males and 12.1% of 69 females were smokers, which was significantly less than national statistics (34.5 and 32.7%, respectively). So he suggested that some component of cigarette, possibly nicotine, has an anti-inflammatory action on acne. Another study by Schäfer et al.[39] showed an association between smoking and acne.
In our study, 47.9% of patients had taken treatment which was commoner in females. Positive treatment history and DLQI/CADI scores were strongly correlated (P = 0.002, P = 0.008, Spearman r correlation coefficient). QoL scores were low among patients who had taken treatment and this was statistically significant (P = 0.031, P = 0.012, Fisher's exact test); similar to that studied by Martin et al.[22] Walker and Lewis-Jones[16] observed among the 20 patients treated that 66% had overall improvement in the QoL. In contrast, study done by Tejada et al.[8] found treatment to be least affecting the QoL.
The differences seen in various studies underscore the racial influences, population characteristics, study settings, and questionnaire design. The varied pattern of impact of acne on the QoL helps us in understanding the ways in which the individual may be affected. QoL worsens with the severity of acne and also based on the individuals perception.
Conclusion
Our study has shown that there was significant correlation between the QoL and acne as age advances due to the impact of social and occupational functioning. Occupation and the marital status played a role in affecting the QoL. Positive family history was associated with acne severity and patients with positive treatment history had low scores. Facial acne did not have a negative impact, as most of patients had grade 1 acne. Grading of acne and DLQI/CADI scores correlated significantly showing increase in score with worsening lesions. There was good correlation between the two questionnaires. There were no gender differences in the QoL scores. Dietary factors, smoking, and alcohol did not influence the QoL.
The assessment of impact of acne on the QoL is essential, to detect those patients who are at increased risk of being negatively affected so as to treat them in a more integrated manner. Hence it is important for health professionals to incorporate QoL measurements when managing acne patients to provide better and appropriate care.
What is new?
The impact of acne on quality of life is becoming more common among young adults due to the social and occupational functioning and worsens as age advances. Facial acne did not affect the scores. There were no gender differences between the scores which states that both males and females were concerned about acne. The scores worsen as severity increases which increase as age advances.
Footnotes
Source of support: Nil
Conflict of Interest: Nil.
References
- 1.Layton AM. Disorders of sebaceous glands. In: Burns T, Breathnach S, Cox N, Griffiths C, editors. Rook's Textbook of Dermatology. 8th ed. Vol. 42. Oxford: Wiley-Blackwell publication; 2010. pp. 1–89. [Google Scholar]
- 2.Kubba R, Bajaj AK, Thappa DM, Sharma R, Vedamurthy M, Dhar S, et al. Indian Acne Alliance (IAA) Acne in India: Guidelines for management IAA consensus document Genetics in acne Acne and quality of life Pathogenesis of acne. Indian J Dermatol Venerol Leprol. 2009;75:S4–5. [PubMed] [Google Scholar]
- 3.The World Health Organization Quality Of Life assessment (WHOQOL): Position paper from the World Health Organization. Soc Sci Med. 1995;41:1403–9. doi: 10.1016/0277-9536(95)00112-k. [DOI] [PubMed] [Google Scholar]
- 4.Barnes LE, Levender MM, Fleischer AB, Feldman SR. Quality of life measures for acne patients. Dermatol Clin. 2012;30:293–300. doi: 10.1016/j.det.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Jankovic S, Vukicevic J, Djordjevic S, Jankovic J, Marinkovic J. Quality of life among school children with acne: Results of a cross sectional study. Indian J Dermatol Venerol Leprol. 2012;78:454–8. doi: 10.4103/0378-6323.98076. [DOI] [PubMed] [Google Scholar]
- 6.Pawin H, Chivot M, Beylot C, Faure M, Poli F, Revuz J, et al. Living with acne. A study of adolescent's personal experiences. Dermatology. 2007;215:308–14. doi: 10.1159/000107624. [DOI] [PubMed] [Google Scholar]
- 7.Adityan B, Kumari R, Thappa DM. Scoring systems in acne vulgaris. Indian J Dermatol Venerol Leprol. 2009;75:323–6. doi: 10.4103/0378-6323.51258. [DOI] [PubMed] [Google Scholar]
- 8.Tejada CDS S, Mendoza-Sassi RA, Almeida HL, Jr, Figueiredo PN, Tejada VF. Impact on the quality of life of dermatological patients in southern Brazil. An Bras Dermatol. 2011;86:1113–21. doi: 10.1590/s0365-05962011000600008. [DOI] [PubMed] [Google Scholar]
- 9.Hanisah A, Omar K, Shah SA. Prevalence of acne and its impact on the quality of life in school-aged adolescents in Malaysia. J Prim Health Care. 2009;1:20–5. [PubMed] [Google Scholar]
- 10.Rapp SR, Feldman SR, Graham G, Fleischer AB, Brenes G, Dailey M. The Acne Quality of Life Index (Acne-QOLI): Development and validation of a brief instrument. Am J Clin Dermatol. 2006;7:185–92. doi: 10.2165/00128071-200607030-00005. [DOI] [PubMed] [Google Scholar]
- 11.Kurokawa I, Danby FW, Ju Q, Wang X, Xiang LF, Xia L, et al. New developments in our understanding of acne pathogenesis and treatment. Exp Dermatol. 2009;18:821–32. doi: 10.1111/j.1600-0625.2009.00890.x. [DOI] [PubMed] [Google Scholar]
- 12.Kubba R, Bajaj AK, Thappa DM, Sharma R, Vedamurthy M, Dhar S, et al. Indian Acne Alliance (IAA). Acne in India: Guidelines for management-IAA consensus document. Introduction. Indian J Dermatol Venerol Leprol. 2009;75:S1–2. [PubMed] [Google Scholar]
- 13.Lasek RJ, Chre MM. Acne vulgaris and the quality of life of adult dermatology patients. Arch Dermatol. 1998;134:454–8. doi: 10.1001/archderm.134.4.454. [DOI] [PubMed] [Google Scholar]
- 14.Rapp DA, Brenes GA, Feldman SR, Fleischer AB, Jr, Graham GF, Dailey M, et al. Anger and acne: Implications for quality of life, patient satisfaction and clinical care. Br J Dermatol. 2004;151:183–9. doi: 10.1111/j.1365-2133.2004.06078.x. [DOI] [PubMed] [Google Scholar]
- 15.Jones-Caballero M, Chren MM, Soler B, Pedrosa E, Penas PF. Quality of life in mild to moderate acne: Relationship to clinical severity and factors influencing change with treatment. J Eur Acad Dermatol Venereol. 2007;21:219–26. doi: 10.1111/j.1468-3083.2006.01907.x. [DOI] [PubMed] [Google Scholar]
- 16.Walker N, Lewis-Jones MS. Quality of life and acne in Scottish adolescent school children: Use of the Children's Dermatology Life Quality Index (CDLQI) and the Cardiff Acne Disability Index (CADI) J Eur Acad Dermatol Venereol. 2006;20:45–50. doi: 10.1111/j.1468-3083.2005.01344.x. [DOI] [PubMed] [Google Scholar]
- 17.Aghaei S, Mazharinia N, Jafari P, Abbasfard Z. The Persian version of the Cardiff Acne Disability Index. Reliability and validity study. Saudi Med J. 2006;27:80–2. [PubMed] [Google Scholar]
- 18.Tasoula E, Gregoriou S, Chalikias J, Lazarou D, Danopoulou I, Katsambas A, et al. The impact of acne vulgaris on quality of life and psychic health in young adolescents in Greece. Results of a population survey. An Bras Dermatol. 2012;87:862–9. doi: 10.1590/S0365-05962012000600007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uslu G, Sendur N, Uslu M, Savek E, Karaman G, Eskin M. Acne: Prevalence, perceptions and effects on psychological health among adolescents in Aydin, Turkey. J Eur Acad Dermatol Venereol. 2008;22:462–9. doi: 10.1111/j.1468-3083.2007.02497.x. [DOI] [PubMed] [Google Scholar]
- 20.Ismail KH, Mohammed-Ali KB. Quality of life in patients with acne in Erbil city. Health Qual Life Outcomes. 2012;10:60. doi: 10.1186/1477-7525-10-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balkrishnan R, McMichael AJ, Hu JY, Camacho FT, Shew KR, Bouloc A, et al. Correlates of health-related quality of life in women with severe facial blemishes. Int J Dermatol. 2006;45:111–5. doi: 10.1111/j.1365-4632.2004.02371.x. [DOI] [PubMed] [Google Scholar]
- 22.Martin AR, Lookingbill DP, Botek A, Light J, Thiboutot D, Girman CJ. Health-related quality of life among patients with facial acne 2 assessment of a new acne-specific questionnaire. Clin Exp Dermatol. 2001;26:380–5. doi: 10.1046/j.1365-2230.2001.00839.x. [DOI] [PubMed] [Google Scholar]
- 23.Hassan J, Grogan S, Clark-Carter D, Richards H, Yates VM. The individual health burden of acne: Appearance- related distress in male and female adolescents and adults with back, chest and facial acne. J Health Psychol. 2009;14:1105–18. doi: 10.1177/1359105309342470. [DOI] [PubMed] [Google Scholar]
- 24.Cotterill JA, Cunliffe WJ. Suicide in dermatological patients. Br J Dermatol. 1997;137:246–50. doi: 10.1046/j.1365-2133.1997.18131897.x. [DOI] [PubMed] [Google Scholar]
- 25.Halvorsen JA, Stern RS, Dalgard F, Thoresen M, Bjertness E, Lien L. Suicidal ideation, mental health problems, and social impairment are increased in adolescents with acne: A population-based study. J Invest Dermatol. 2011;131:363–70. doi: 10.1038/jid.2010.264. [DOI] [PubMed] [Google Scholar]
- 26.Srivastava S, Bhatia MS, Das P, Bhattacharya SN. A cross sectional study of quality of life and psychiatric morbidity in patients with acne vulgaris. J Pak Psychiatr Soc. 2008;5:86. [Google Scholar]
- 27.Salek MS, Khan GK, Finlay AY. Questionnaire techniques in assessing acne handicap: Reliability and validity study. Qual Life Res. 1996;5:131–8. doi: 10.1007/BF00435978. [DOI] [PubMed] [Google Scholar]
- 28.Gupta MA, Gupta AK. Psychiatric and psychological co-morbidity in patients with dermatologic disorders: Epidemiology and management. Am J Clin Dermatol. 2003;4:833–42. doi: 10.2165/00128071-200304120-00003. [DOI] [PubMed] [Google Scholar]
- 29.Clark SM, Goulden V, Finlay AY, Cunliffe WJ. The psychological and social impact of acne: A comparison study using three acne disability questionnaire. Br J Dermatol. 1997;137:41. [Google Scholar]
- 30.Pruthi GK, Babu N. Physical and psychosocial impact of acne in adult females. Indian J Dermatol. 2012;57:26–9. doi: 10.4103/0019-5154.92672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herane MI, Ando I. Acne in infancy and acne genetics. Dermatology. 2003;206:24–8. doi: 10.1159/000067819. [DOI] [PubMed] [Google Scholar]
- 32.Cunliffe WJ, Gollnick HP. London: Martin Dunitz; 2001. Acne: Diagnosis and Management; pp. 1–46. [Google Scholar]
- 33.Bowe WP, Joshi SS, Shalita AR. Diet and acne. J Am Acad Dermatol. 2009;63:124–41. doi: 10.1016/j.jaad.2009.07.043. [DOI] [PubMed] [Google Scholar]
- 34.Danby FW. Diet and acne. Clin Dermatol. 2008;26:93–6. doi: 10.1016/j.clindermatol.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 35.Ismail NH, Manaf ZA, Azizan NZ. High glycemic load diet, milk and ice cream consumption are related to acne vulgaris in Malaysian young adults: a case control study. BMC Dermatol. 2012;12:13. doi: 10.1186/1471-5945-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klaz I, Kochba I, Shohat T, Zarka S, Brenner S. Severe acne vulgaris and tobacco smoking in young men. J Invest Dermatol. 2006;126:1749–52. doi: 10.1038/sj.jid.5700326. [DOI] [PubMed] [Google Scholar]
- 37.Michaelsson G. Diet and acne. Nutr Rev. 1981;39:104–6. doi: 10.1111/j.1753-4887.1981.tb06740.x. [DOI] [PubMed] [Google Scholar]
- 38.Mills CM, Peters TJ, Finlay AY. Does smoking influence acne? Clin Exp Dermatol. 1993;18:100–1. doi: 10.1111/j.1365-2230.1993.tb00986.x. [DOI] [PubMed] [Google Scholar]
- 39.Schäfer T, Nienhaus A, Vieluf D, Berger J, Ring J. Epidemiology of acne in the general population: The risk of smoking. Br J Dermatol. 2001;145:100–4. doi: 10.1046/j.1365-2133.2001.04290.x. [DOI] [PubMed] [Google Scholar]


