Abstract
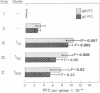
Mice were immunized intravenously with suboptimal numbers (3.5-5 X 10(5)) of sheep erythrocytes together with various anti-Ia antisera or with sheep erythrocytes alone, and the primary IgM and IgG plaque-forming cell responses were assayed 6 days later9 A/J (H-2a) mice given 5 X 10(5) sheep erythrocytes together with as little as 0.4 mul of a (129 X A.TH)F1 anti-A.TL (anti-Iak) antiserum developed 2-3 times as many IgM and IGG plaque-forming cells as mice injected with antigen alone or together with various antisera not containing anti-Ia antibodies. Similar results were obtained with BALB/c (H-2d) mice and a (C3H X LG/Ckc)F1 anti-C3H. OH (anti-Iad) antiserum plus sheep erythrocytes. In the case of the anti-Iad antiserum, the potentiating activity could be absorbed with C3H. OH (Id) but not C3H(Ik) spleen cells, demonstrating that the active antibodies were specific for the Id region. Antiserum to I-Jk subregion-coded determinants was tested in A/J (I-Jk) mice and found to also potentiate 2- to 3-fold the plaque-forming cell response to suboptimal erythrocyte immunization. This antiserum [(BIO.A(3R) X DBA/2)F1 anti-B10.(5R)] failed to potentiate responses in BALB/c (I-Jd) mice, as expected on a genetic basis. The potentiating antibodies could be removed by absorption with B10.BR (I-Jk) but not B10 (I-Jb) spleen cells, also confirming the I-J specificity of the activity. The interference of anti-I-J antibodies with T lymphocyte suppressor mechanisms is prposed as a possible explanation for this phenomenon.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augustin A., Cosenza H. Expression of new idiotypes following neonatal idiotypic suppression of a dominant clone. Eur J Immunol. 1976 Jul;6(7):497–501. doi: 10.1002/eji.1830060710. [DOI] [PubMed] [Google Scholar]
- Benacerraf B., Katz D. H. The histocompatibility-linked immune response genes. Adv Cancer Res. 1975;21:121–173. doi: 10.1016/s0065-230x(08)60972-0. [DOI] [PubMed] [Google Scholar]
- Debré P., Waltenbaugh C., Dorf M., Benacerraf B. Genetic control of specific immune suppression. III. Mapping of H-2 complex complementing genes controlling immune suppression by the random copolymer L-glutamic acid50-L-tyrosine50 (GT). J Exp Med. 1976 Jul 1;144(1):272–276. doi: 10.1084/jem.144.1.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichmann K. Idiotype suppression. I. Influence of the dose and of the effector functions of anti-idiotypic antibody on the production of an idiotype. Eur J Immunol. 1974 Apr;4(4):296–302. doi: 10.1002/eji.1830040413. [DOI] [PubMed] [Google Scholar]
- Eichmann K., Rajewsky K. Induction of T and B cell immunity by anti-idiotypic antibody. Eur J Immunol. 1975 Oct;5(10):661–666. doi: 10.1002/eji.1830051002. [DOI] [PubMed] [Google Scholar]
- Erb P., Feldmann M. The role of macrophages in the generation of T-helper cells. II. The genetic control of the macrophage-T-cell interaction for helper cell induction with soluble antigens. J Exp Med. 1975 Aug 1;142(2):460–472. doi: 10.1084/jem.142.2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann M., Beverley P. C., Woody J., McKenzie I. F. T-T interactions in the induction of suppressor and helper T cells: analysis of membrane phenotype of precursor and amplifier cells. J Exp Med. 1977 Apr 1;145(4):793–801. doi: 10.1084/jem.145.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frelinger J. A., Niederhuber J. E., Shreffler D. C. Inhibition of immune responses in vitro by specific antiserums to Ia antigens. Science. 1975 Apr 18;188(4185):268–270. doi: 10.1126/science.1118728. [DOI] [PubMed] [Google Scholar]
- Huber B., Gershon R. K., Cantor H. Identification of a B-cell surface structure involved in antigen-dependent triggering: absence of this structure on B cells from CBA/N mutant mice. J Exp Med. 1977 Jan 1;145(1):10–20. doi: 10.1084/jem.145.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz D. H., Benacerraf B. The function and interrelationships of T-cell receptors, Ir genes and other histocompatibility gene products. Transplant Rev. 1975;22:175–195. doi: 10.1111/j.1600-065x.1975.tb01559.x. [DOI] [PubMed] [Google Scholar]
- Kölsch E., Stumpf R., Weber G. Low zone tolerance and suppressor T cells. Transplant Rev. 1975;26:56–86. doi: 10.1111/j.1600-065x.1975.tb00175.x. [DOI] [PubMed] [Google Scholar]
- Murphy D. B., Herzenberg L. A., Okumura K., Herzenberg L. A., McDevitt H. O. A new I subregion (I-J) marked by a locus (Ia-4) controlling surface determinants on suppressor T lymphocytes. J Exp Med. 1976 Sep 1;144(3):699–712. doi: 10.1084/jem.144.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisonoff A., Bangasser S. A. Immunological suppression of idiotypic specificities. Transplant Rev. 1975;27:100–134. doi: 10.1111/j.1600-065x.1975.tb00186.x. [DOI] [PubMed] [Google Scholar]
- Nowinski R. C., Klein P. A. Anomalous reactions of mouse alloantisera with cultured tumor cells. II. Cytotoxicity is caused by antibodies to leukemia viruses. J Immunol. 1975 Nov;115(5):1261–1268. [PubMed] [Google Scholar]
- Okumura K., Herzenberg L. A., Murphy D. B., McDevitt H. O., Herzenberg L. A. Selective expression of H-2 (i-region) loci controlling determinants on helper and suppressor T lymphocytes. J Exp Med. 1976 Sep 1;144(3):685–698. doi: 10.1084/jem.144.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce C. W., Johnson B. M., Gershon H. E., Asofsky R. Immune responses in vitro. 3. Development of primary gamma-M, gamma-G, and gamma-A plaque-forming cell responses in mouse spleen cell cultures stimulated with heterologous erythrocytes. J Exp Med. 1971 Aug 1;134(2):395–416. doi: 10.1084/jem.134.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce C. W., Kapp J. A., Solliday S. M., Dorf M. E., Benacerraf B. Immune responses in vitro. XI. Suppression of primary IgM and IgG plaque-forming cell responses in vitro by alloantisera against leukocyte alloantigens. J Exp Med. 1974 Oct 1;140(4):921–938. doi: 10.1084/jem.140.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shreffler D. C., David C. S. The H-2 major histocompatibility complex and the I immune response region: genetic variation, function, and organization. Adv Immunol. 1975;20:125–195. doi: 10.1016/s0065-2776(08)60208-4. [DOI] [PubMed] [Google Scholar]
- Tada T., Taniguchi M., David C. S. Properties of the antigen-specific suppressive T-cell factor in the regulation of antibody response of the mouse. IV. Special subregion assignment of the gene(s) that codes for the suppressive T-cell factor in the H-2 histocompatibility complex. J Exp Med. 1976 Sep 1;144(3):713–725. doi: 10.1084/jem.144.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taussig M. J., Munro A. J., Campbell R., David C. S., Staines N. A. Antigen-specific T-cell factor in cell cooperation. Mapping within the I region of the H-2 complex and ability to cooperate across allogeneic barriers. J Exp Med. 1975 Sep 1;142(3):694–700. doi: 10.1084/jem.142.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thèze J., Waltenbaugh C., Dorf M. E., Benacerraf B. Immunosuppressive factor(s) specific for L-glutamic acid50-L-tyrosine50 (GT) II. Presence of I-J determinants on the GT-suppressive factor. J Exp Med. 1977 Jul 1;146(1):287–292. doi: 10.1084/jem.146.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]