Abstract
Context:
Gastric ulcer is one of the most prevalent gastrointestinal disorders. A number of studies have been carried out to determine the efficacy of herbal medicines in the treatment of gastric ulcer.
Objectives:
The present study was undertaken to evaluate the anti-ulcerogenic activity of methanol extract of Gomphrena celosioides (GC) in male Wistar rats.
Materials and Methods:
The rats were divided into eight groups, methanol extract of GC was administered orally, for seven consecutive to five groups. On the 7th day, indomethacin was administered to induce gastric ulceration. Gastric secretions were collected and analyzed.
Results:
Animals pretreated with GC extract showed a significant reduction in ulcer score, ulcer index, gastric volume, and gastric total acidity in indomethacin-induced ulcer models in a dose dependent manner when compared to the ulcerated control group.
Conclusion:
The study revealed gastroprotective activity of the extract in dose-dependent manner. Methanol extract of the leaves of GC was significantly effective in protecting the gastric mucosa against indomethacin-induced ulcers at all the dose level studied.
Keywords: Cimetidine, anti-ulcerogenic, indomethacin, nonsteroidal anti-inflammatory drugs, peptic ulcer
INTRODUCTION
Medicinal plants are herbal preparations produced by subjecting plant materials to extraction, fractionation, purification, and other physical or biological processes, which may be produced for immediate consumption or as a basis of herbal drugs.[1,2] The therapeutic properties of medicinal plants are conditioned by the presence in their organs of active substances, such as alkaloids, flavonoids, glycosides, vitamins, tannins, and coumarin compounds, which physiologically affect the bodies of humans and animals.[3] Aromatic plants are a source of fragrances, flavors, cosmeceuticals, health beverages and chemical terpenes.[4]
With the onset of scientific research in medicinal herbs, it is becoming clearer that the medicinal herbs have the potential in today's synthetic era as numbers of medicines are becoming resistant.[5]
The signs and symptoms of peptic ulcer can be constant or sporadic, and the disease cause varies among individuals.[6] The most common symptoms of peptic ulcer are known collectively as dyspepsia. Dyspepsia may be persistent or recurrent and can lead to a variety of upper abdominal symptoms such as pain, difficulty in swallowing or regurgitation, vomiting blood or vomit with the appearance of coffee grounds, black or tar-like stools, unintended and unexplained weight loss weight loss and anemia.
Indomenthacin is a synthetic Non-steroidal anti-inflammatory drug (NSAID) with analgesic and antipyretic activity.[6] It is a potent inhibitor of postaglandins synthesis that are important mediators of the inflammatory response.[7] The anti-inflammatory action of indomethacin is due to inhibition of vasodilator prostaglandin E2 and prostaglandin I2 synthesized from arachidonic acid through cyclo-oxygenase pathway by inhibiting cyclo-oxygenase I (COX-I) and cyclo-oxygenase-II (COX-II). Deficiency of COX-I is of pivotal importance in anti-inflammatory response of NSAIDs.[8]
Gomphrena celosioides (GC) also commonly called Soft khaki weed or White-eye is a short lived perennial plant.[9] It belongs to the Amaranthaceae family, and over 120 species of the family are found in America, Australia and Indo-Malaysia, while 46 species occur in Brazil. Few species occur in the East and West of Africa.[10]
Gomphrena species in different parts of the world are used for various folkloric medicinal purposes.[10] In Brazil, some species are employed in the treatment of bronchial infections, diarrhea, and malaria fever, while others had found the application as analgesic, tonic/carminative and diuretics.[10,11] Earlier research work by Botha and Gerritsma-Van der Vijver[12] on GC extracts revealed the presence of saponins, steroids, amino acids, nonreducing sugars, phenols, and flavonoids.[10]
MATERIALS AND METHODS
The reagents and chemicals used for this experiment include di-potassium orthophosphate, potassium di-hydrogen orthophosphate (FBC Industries.Inc.), sodium hydroxide (The Stutz.co-Chicago), magnesium chloride (Weifang Haibin Chemical.co), Folin-Ciocalteau reagent (Sigma Aldrich), formalin (Sigma Aldrich) and bovine serum albumin (Sigma Aldrich). Indomethacin (Iroko Pharm.) and cimetidine (Glaxosmithkline) used were obtained from Pharama Aid, a licensed pharmaceutical store in Nigeria.
The plant, GC was obtained locally from the vegetative land of Bowen University, Iwo, authenticated at the Department of Botany, Bowen University, Iwo. The leaves were plucked, air-dried and then pulverized. 1 kg of powdered plant was then weighed and soaked in methanol for 24 h with intermittent shaking, the solvent was filtered and the filtrate was collected and concentrated into semi-solid using rotary evaporator at 40°C. The extract was then dried and stored at −4°C until ready to use.
Animal protocol
Thirty two adult male Wistar rats weighing about 150–200 g were obtained from the vet anatomy department, University of Ibadan, and housed in the animal house of the department of Biochemistry, Bowen University, Iwo. They were randomly placed into eight groups containing 4 rats each. They were kept in wire meshed cages, fed with commercial rat pellets, and had access to water ad libitum, and exposed to the natural daily photo-period of about 12 h light and 12 h darkness during the period of acclimatization and throughout the study.
The animals were grouped as - Group 1: 1% gum acacia solution; Group 2: 40 mg/kg body weight of indomethacin; Group 3: 50 mg/kg of body weight of cimetidine; Group 4: 500 mg/kg of body weight of GC extract; Group 5: 40 mg/kg body weight of indomethacin + 200 mg/kg of body weight of GC extract; Group 6: 40 mg/kg body weight of indomethacin + 500 mg/kg of body weight of GC extract; Group 7: 40 mg/kg body weight of indomethacin + 800 mg/kg of body weight of GC extract; Group 8: 40 mg/kg body weight of indomethacin + 50 mg/kg of body weight of cimetidine.
Therapeutic doses of drugs and plant extract were administered as prescribed per body weight. Stock solutions of indomethacin and cimetidine were made by dissolving 40 mg and 50 mg respectively in 1% gum acacia. The rats were pretreated for 7 days with the plant extract and cimetidine. On the 7th day all animals excluding the control group (Group 1) were orally given 40 mg/kg body weight of Indomethacin. The animals were fasted for 24 h before the start of drug administration.
Gastric ulcers were induced in the different groups of rats by indomethacin. Indomethacin was administered per os to different groups of rats in a dosage of 40 mg/kg of body weight each. The treated rats were sacrificed by cervical dislocation 4 h later, and the abdomen opened through midline incision. All stomachs were incised along the greater curvatures.
Determination of gastric volumes, pH and acid outputs
The rat's stomachs were excised out under ether, and the gastric contents were collected. The stomachs were washed with lukewarm sterile water, and the gastric volume was measure using 10 ml measuring cylinder. Both the washings and the gastric content were centrifuged together at 4000 rpm for 10 min, and the resulting supernatants were collected separately. The pH of all the supernatants was measured and their acidities were determined by titration to pH 7 with 0.1 N sodium hydroxide (NaOH) solution. The acid output was calculated by the following equation.
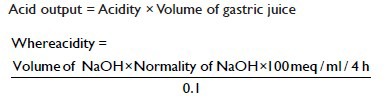
Determination of ulcer score and ulcer index
On each animal, a ventral midline incision was made, and the stomach exteriorized and opened through the greater curvature, rinsed, laid out on a flat surface and examined for the presence of mucosal lesions. The gastric damage in the glandular regions was located in the gastric mucosa as elongated black-red lines parallel to the long axis of stomachs using a 10× magnifying lens to locate and score the lesions, and this represents the ulcer score. Severity of the gastric mucosal damage was graded as grade 0 - No lesions, grade 0.5 - Hemorrhagic erosion (<5 nm), Grade 1 - hemorrhagic erosion (more than 5 nm) Grade 3 - Many small linear ulcers (shorter than 2 mm).
The ulcer index of each group was calculated by multiplying the number of rats in each grade by the number of grade divided by the number of rats in each group.
Estimation of pepsin activity
Four test tubes were arranged. To tubes 1 and 2, 2.5 ml of 1% bovine serum albumin in 0.01M HCL was added. To tubes 3 and 4, 2 ml of 0.35M trichloroacetic acid. The gastric juice was mixed with an equal volume of 0.01M HCL, warmed to 37°C. 0.2 ml of this mixture was added to each of the test tubes of 1 and 4 and incubated for 15 min. Tubes 1 + 3 were added together, and this represent the test solution, similarly, tubes 2 + 4 were added together, and the resulting solution is the blank. The mixture was filtered and to 1 ml of the filtrate, 5 ml of NaOH was added. 0.2 ml phenol reagent was added and mixed by gentle rotation. After 30 min, the absorbance was measured at 680 nm. The difference between the test and blank gives the measures of peptic activity.
Lipid peroxidation assessment
Lipid peroxidation (LPO) was assayed by measuring the thiobarbituric acid reactive substances products present in the test sample using the procedure of Vashney and Kale (1970) and expressed as micromolar of malondialdehyde.
The assay is based on the ratio of the chromogenic reagent (2-thiobarbituric acid) to malondidehyde (an end product of LPO) under acidic condition to yield a stable pink chromophore with maximum absorption at 532 nm wavelength. The malonaldehyde (MDA) level was calculated according to the method of Adam-Vizi and Seregi (1987).
Aliquots of 0.4 ml of the supernatant fractions of the test sample were mixed with 1.6 ml of Tris-KCI buffer of 30% trichloroacetic acid, and 0.5 ml of 0.75% thiobarbituric acid. The reaction mixture was placed in a water bath for 45 min at 80°C, cooled in ice, and then centrifuged at 3000 g for 5 min. The clear supernatant was collected, and the absorbance measured against a reference blank (distilled water) at 532 nm wavelength. LPO in units/mg or g tissue was computed with molar extinction coefficient 1.56 × 105/M cm.

Determination of protein in the sample
Protein concentration of the gastric juice was determined by Folin Lowry method (1951).
Protein reacts with folin ciocalteau reagent to give a colored complex. The color so formed is due to the reaction of alkaline copper with the tryptophan present in the protein. The intensity of the color depends on the amount of these aromatic amino acids present and will vary for different proteins
Reagents
Alkaline sodium carbonate solution (Reagent A), copper sulfate-sodium potassium tartrate solution (reagent B), alkaline solution–prepared on the day of use by mixing 50 cm3 of (A) and 1 cm3 of (B), Folin-ciocalteau reagent dilute, Standard proteins (Bovine serum albumin) 200 ug/ml, test solution sodium hydroxide (1 N), sodium potassium tartrate (4%).
The post mitochondrial fractions of stomach supernatants were diluted 20 times with distilled water. To 0.5 ml of the enzyme extract, 3 ml of alkaline copper reagent was added, vortexed and allowed to stand for 20 min. 3 ml of folin's phenol reagent was added and incubated for 30 min at room temperature. The blue color formed was measured at 650 nm in a spectrophotometer. Distilled water was used as blank solution. The protein content of the samples was extrapolated from the standard curve and multiplied by 20 to get the actual amount in the fraction.
Statistical analysis
Results are expressed as mean ± standard deviation. The data were analyzed by one-way analysis of variance. Means values were compared using Duncan test. The SPSS statistical package by IBM was used, and the value of P ≤ 0.01 was considered as statistically significant.
RESULTS
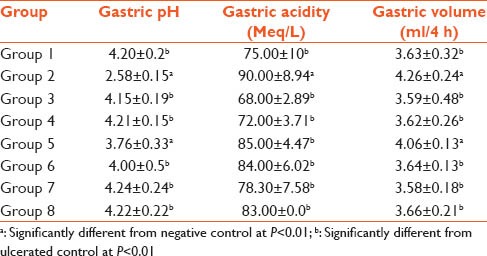
The animals that received indomethacin alone had the lowest gastric pH and there was a does dependent increase in gastric pH. Furthermore, administration of cimetidine-indomethacin increased the pH but not as significant as the administration of 800 mg/kg body weight administration of plant extract. Similarly the animals that received indomethacin alone had the highest total acidity. Also, administration of cimetidine-indomethacin reduces the acidity, but not as significant as the administration of 800 mg/kg body weight. Also, acidity was shown to decrease with increasing concentration of plant extract. The animals that received indomethacin alone had the highest gastric volume, and as the dosage of plant extract administered increases, gastric volume decreases. Also administration of cimetidine-indomethacin and reduces the gastric volume but not as significant as the administration of 800 mg/kg body weight of plant extract [Table 1].
Table 1.
Effect of methanolic extract of Gomphrena celosioides on various parameters
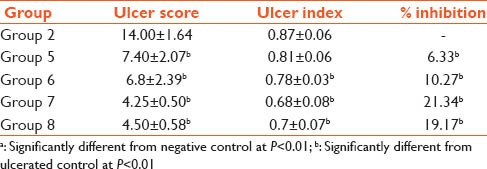
The ulcer index decreased as the dosage of the plant extract increased (0.87–0.68). Also, there was dose dependent decrease in the ulcer score of the rat's pretreated with plant extract. Percentage Inhibition increased as the dosage of plant extracts increased. Although the cimetidine-indomethacin group also had lower ulcer score and ulcer index when compared to the negative control group ulcer score and ulcer index; however, the percentage inhibition was low when compared to the group that received the highest dosage of plant extract [Table 2].
Table 2.
Effect on ulcer score, gastric ulcer index, % inhibition
The negative control group had the highest acid output, however, the acid output decreased with increasing concentration of the plant extract. The gastric pepsin activity was highest with the group treated with indomethacin alone and decreased as the dosage of plant extract administered increases. Also, pepsin activity of the cimetidine-indomethacin group decreased but not as significantly as the 800 mg/kg body weight administration of extract group. A significant increase in the level of MDA in the animals that took indomethacin alone was seen. However, MDA levels decrease progressively as the dosage of plant extract increases [Table 3].
Table 3.
Effects on various parameters in different groups
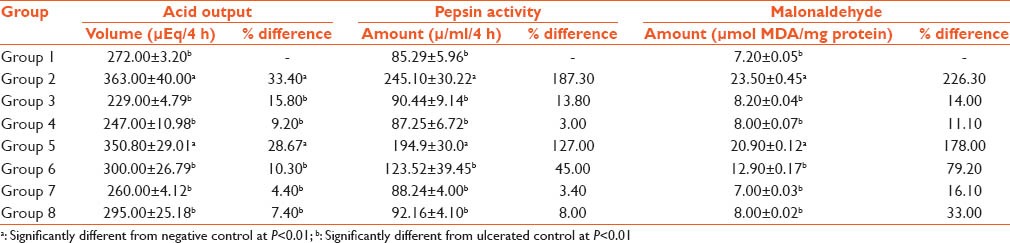
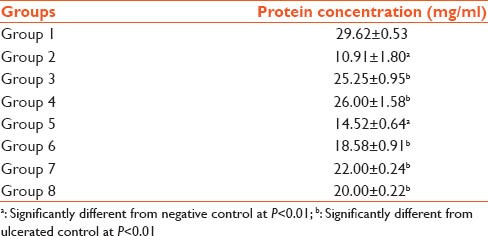
Table 4 shows a significant decrease in the protein level of the ulcerated control group. However, protein levels increased progressively as the dosage of plant extract increases.
Table 4.
Protein level in rats treated with indomethacin, gomphrena and cimetidine
DISCUSSION
Gomphrena celosioides is prevalently used among the rural people of West Africa to treat a wide variety of ailments, partly because of their rich history and also because synthetic drugs are quite expensive and may be beyond their reach.
Ulcer has become a global disease affecting people in all geographical regions. It is generally accepted that peptic ulcer results from an imbalance between aggressive factors and the maintenance of mucosal integrity through the endogenous defense mechanisms.[13] To regain the balance, different therapeutic agents including plant extracts may be used. Indomethacin is known to induce gastric ulcer by inhibition of prostaglandins which are cytoprotective to the gastric mucosa, particularly due to the inhibition of COX pathway of arachidonic acid metabolism resulting in excessive production of leukotrienes and other products of 5-lipoxygenase pathway.[14]
In the stomach, prostaglandins play a vital protective role, stimulating the secretion of bicarbonate and mucus, maintaining mucosal blood flow, and regulating mucosal cell turnover and repair.[7] Thus, the suppression of prostaglandins synthesis by NSAIDs results in increased susceptibility to mucosal injury and gastroduodenal ulceration. Several studies have indicated that gastroduodenal protection by prostaglandins is due to increase in the mucosal resistance as well as the decrease in aggressive factors like acid and pepsin secretion.[15] It is a known fact that indomethacin and other members of the NSAIDs can slow down the healing process of peptic ulcer, this is further established by the results obtained in this experiment. These actions of indomethacin are due to its anti-inflammatory activity.[16]
Summarily, the results of this study indicate that the methanolic extract of GC displays an antiulcerogenic effect related to its gastroprotective activity, it significantly reduced indomethacin induced gastric ulcers. Reports suggest that phytochemicals such as flavonoids and phenolic compounds, other antioxidant compounds could be active in producing antiulcerogenic effect.[17] Therefore, the observed ulcer preventive and ulcer curative activity of GC extract may be partially due to its relative antioxidant activity and its photochemical constituents.
The phyto-constituents like flavonoids, tannins, terpenoids, and Saponin have been reported in several anti-ulcer literatures as possible gastro protective agents.[17] Flavonoids, tannins and triterpenes are among the cytoprotective active materials for which anti ulcerogenic efficacy have been extensively confirmed. Tannins may prevent ulcer development due to their protein precipitating and vasoconstriction effects.[17]
The present study revealed that the extract has promising phytochemicals for the development of alternative treatment against gastric ulcer. Further studies are also needed to explore the antioxidant activity, gastroprotective effects and to determine the active ingredients in the plant GC.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Dfeudis FV. Paris, France: Elsevier Editions Scientifiques; 1991. Ginkgo Bloba Extract (EGb 761): Pharmacological Activities and Clinical Applications. [Google Scholar]
- 2.Joy PP, Thomas J, Mathew S, Skaria BP. Medicinal plants. Trop Hortic. 2001;2:449–632. [Google Scholar]
- 3.Cotton PB, Shorvon PJ. Analysis of endoscopy and radiography in the diagnosis, follow-up and treatment of peptic ulcer disease. Clin Gastroenterol. 1984;13:383–403. [PubMed] [Google Scholar]
- 4.Handa SS. An overview of extraction techniques for medicinal and aromatic plants. In: Handa SS, Khanuja SP, Longo G, Rakesh DD, editors. Extraction Technologies for Medicinal and Aromatic plants. Vol. 1. Trieste, Italy: ICS-UNIDO; 1997. pp. 21–52. [Google Scholar]
- 5.Kurekci C, Bishop-Hurley SL, Vercoe PE, Durmic Z, Al Jassim RA, McSweeney CS. Screening of Australian plants for antimicrobial activity against Campylobacter jejuni. Phytother Res. 2012;26:186–90. doi: 10.1002/ptr.3526. [DOI] [PubMed] [Google Scholar]
- 6.Traversa G, Walker AM, Ippolito FM, Caffari B, Capurso L, Dezi A, et al. Gastroduodenal toxicity of different nonsteroidal antiinflammatory drugs. Epidemiology. 1995;6:49–54. doi: 10.1097/00001648-199501000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Bolton JP, Palmer D, Cohen MM. Effect of the E2 prostaglandins on gastric mucus production in rats. Surg Forum. 1976;27:402–3. [PubMed] [Google Scholar]
- 8.Hinz B, Cheremina O, Brune K. Acetaminophen (paracetamol) is a selective cyclooxygenase-2 inhibitor in man. FASEB J. 2008;22:383–90. doi: 10.1096/fj.07-8506com. [DOI] [PubMed] [Google Scholar]
- 9.Allison MC, Howastson AG, Torrance CJ, Lee FD, Russel RI. The medical benefits of Gomphrena celosioides. N Engl J Med. 1992;327:749–54. doi: 10.1056/NEJM199209103271101. [DOI] [PubMed] [Google Scholar]
- 10.Vieira CC, Mercier H, Chu EP, Figueiredo-Ribeiro RC. Gomphrena species (globe amaranth): In vitro culture and production of secondary metabolites. Biotechnol Agric Forest. 1994;2:257–70. [Google Scholar]
- 11.Gessler MC, Nkunya MH, Mwasumbi LB, Heinrich M, Tanner M. Screening Tanzanian medicinal plants for antimalarial activity. Acta Trop. 1994;56:65–77. doi: 10.1016/0001-706x(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 12.Botha S, Gerritsma-van der Vijver LM. A pharmacochemical investigation of Gomphrena celosioides (Amaranthaceae) Planta Med. 1986;55:115–25. [Google Scholar]
- 13.Goel RK, Gupta S, Shankar R, Sanyal AK. Anti-ulcerogenic effect of banana powder (Musa sapientum var. paradisiaca) and its effect on mucosal resistance. J Ethnopharmacol. 1986;18:33–44. doi: 10.1016/0378-8741(86)90041-3. [DOI] [PubMed] [Google Scholar]
- 14.Baron JH, Sinai MT, Baillie M. The morbid anatomy of some of the most important parts of human body. J Med. 2000;10:85–97. [Google Scholar]
- 15.Hart FD, Boardman PL. Indomethacin: A new non-steroid anti-inflammatory agent. Br Med J. 1963;2:965–70. doi: 10.1136/bmj.2.5363.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brodie DA. The mechanism of gastric hyperacidity produced by pylorus ligation in the rat. Am J Dig Dis. 1966;11:231–41. doi: 10.1007/BF02233904. [DOI] [PubMed] [Google Scholar]
- 17.Pandian RS, Anuradha CV, Viswanathan P. Gastroprotective effect of fenugreek seeds (Trigonella foenum graecum) on experimental gastric ulcer in rats. J Ethnopharmacol. 2002;81:393–7. doi: 10.1016/s0378-8741(02)00117-4. [DOI] [PubMed] [Google Scholar]


