Abstract
Background:
Many studies show a high prevalence of vitamin D deficiency across various populations the world over. There is relative lack of prevalence data in Punjab, India. This cross-sectional study was carried out to assess the prevalence of vitamin D deficiency in the north-west Punjab population.
Aim:
To study the prevalence of vitamin D deficiency in the north-west Punjab population across various population characteristics such as gender, education, locality, occupation, and dietary habits.
Materials and Methods:
Healthy volunteers (N = 150) of either sex were enrolled and their fasting plasma samples tested for 25-hydroxy vitamin D [25(OH) D] levels. Data were compiled as percentages and means across different population characteristics. Statistical analysis was done using Chi-square test and Fisher's exact test.
Results:
A high overall prevalence (90%) of vitamin D deficiency was observed in the study subjects. There was a significant difference in the prevalence of vitamin D insufficiency between rural and urban subjects (P < 0.05) and among the subjects pursuing different occupations (P < 0.001). A significant gender-specific difference was also recorded at the cut-off level of 25 (OH) D, with women showing higher prevalence of deficiency compared to men (P < 0.05).
Conclusions:
There is a high prevalence of vitamin D deficiency in the population of Punjab. Lower prevalence is displayed by those subjects who have greater opportunities for sunlight exposure, such as like rural individuals, farmers, and housewives.
Keywords: Calcium, deficiency, parathormone, prevalence, vitamin D
INTRODUCTION
Vitamin D has long been associated with calcium homeostasis and its deficiency is a known precursor to different bone disorders. Recent research, however, indicates many other biological roles of this vitamin, such as cardiovascular, endocrinal, and immune system homeostasis.[1,2,3,4] Thus, it opens up avenues for further research on potential disorders due to vitamin D deficiency.
Vitamin D is not a single entity but a group of antirachitic substances that are found in certain foods as well as synthesized in skin. Various forms of vitamin D are: vitamin D1, vitamin D2 (ergocalciferol), vitamin D3 (cholecalciferol), vitamin D4 (dihydroergocalciferol), and vitamin D5 (sitocalciferol). Two important forms in human context are vitamin D3 and vitamin D2. Vitamin D3 is derived from the precursor 7-dehydrocholesterol which is synthesized in skin. Vitamin D2, on the other hand, is obtained from ergosterol found in foods like bread, milk, yeast, mushrooms, and cod liver oil. Exposure of skin to the ultraviolet rays of sun is required for the conversion of these precursors into vitamins. These are then hydroxylated in two steps, first in liver and then in kidney, to give the final active forms: 1,25-dihydroxy vitamin D3 [1,25(OH)2 D3] and 1,25-dihydroxy vitamin D2 [1,25(OH)2 D2].[5,6,7] These active metabolites exert their effects through vitamin D receptor (VDR) which is a nuclear hormonal receptor that is expressed in most of the tissues.[5]
The recommended dietary allowance of vitamin D falls in the range of 400–800 IU/day, the need increasing as the age advances. But this has been challenged by many authors who recommend a higher allowance of 800–1000 IU/day for all ages in the absence of adequate sun exposure.[8,9] Also, to treat vitamin D deficiency, a higher dose of at least 50,000 IU/week is recommended to achieve optimal levels.[9] The Indian Council of Medical Research (ICMR) has stuck to the lower allowance of 400 IU/day, emphasizing a greater exposure to sunlight as a remedy to improve the levels.[10] However, it is difficult to ensure a higher sunlight exposure for every individual across the country. In spite of greater annual sunshine time in the region, prevalence of vitamin D deficiency is high as shown by some regional studies.[11,12] Factors such as skin hyperpigmentation in the Indian population compared to the Caucasian counterparts, high pollution levels, and relatively poor nutrient intake could be responsible for nullifying the effects of greater sunshine.
25-Hydroxy forms of both vitamin D2 and D3 are easily measured in plasma. We will refer these collectively as 25-hydroxy vitamin D [25(OH) D]. The levels of 25(OH) D are considered to be a precise indicator of the vitamin D status.[13] There has been a controversy about the optimum 25(OH) D levels required for normal homeostasis. According to the consensus reached at the 13th Vitamin D Workshop, the minimum 25 (OH) D level required was 50 nmol/L (20 ng/L), which was challenged at the 14th Vitamin D Workshop by several researchers.[14,15] Many authors, however, agree on levels >75 nmol/L (30 ng/L) to be optimal for overall vitamin D functioning.[16,17]
Vitamin D deficiency has been a neglected disorder and not much work has been done on its demographic patterns, especially in the Indian context. This study aims to evaluate the demographic pattern of vitamin D deficiency in the local population of north-west Punjab, India. Punjab has a flourishing middle class society with a mix of agrarian, business, and service communities. Economic growth has brought about changes in the lifestyles as regards the dietary habits, work types and schedules. Whether these population characteristics influence the vitamin D levels needs to be explored.
MATERIALS AND METHODS
Healthy volunteers (N = 150) of either sex, belonging to different backgrounds, were randomly enrolled after ethical permission was obtained. Informed consent was taken from all subjects. Average age of the subjects was 36 years (range, 17–68 years). The subjects who consented to participate were analyzed for plasma 25(OH) D levels. Non-consenting individuals; those taking vitamin D supplements or any other drugs influencing vitamin D levels; patients with renal insufficiency, chronic hepatic disease, or any other serious illness; and persons on prescribed dietary schedules due to any reason were excluded from the study.
Detailed records of dietary habits, education, occupation, and locality were maintained and taken into account during analysis. Fasting samples were collected for determining the plasma 25(OH) D levels (nmol/L). Plasma levels were categorized into three cut-offs: <25 nmol/L, <75 nmol/L, and > 75 nmol/L according to the broad agreement reached for deficient, insufficient, and optimal levels, respectively.[7,13] Levels more than 250 nmol/L were considered to be in the toxic range. Data were compiled in the form of percentages and averages. Chi-square test and Fisher's exact test were used for statistical analysis of proportions. P <0.05 was considered as significant and P < 0.001 as highly significant.
RESULTS
Of the total subjects enrolled (N = 150), 75 (50%) were males, 102 (68%) were from urban background, and 48 (32%) from rural background. Sixty-six (44%) subjects were vegetarian, while 84 (56%) were non-vegetarian.
One hundred and thirty-five (90%) subjects were found to have 25(OH) D levels <75 nmol/L which are considered as insufficient to carry out all the physiological functions of vitamin D appropriately. A large proportion (40%) were found to have levels <25 nmol/L, and thus, are prone to bone disorders. Only 15 (10%) subjects had the optimal levels of >75 nmol/L.
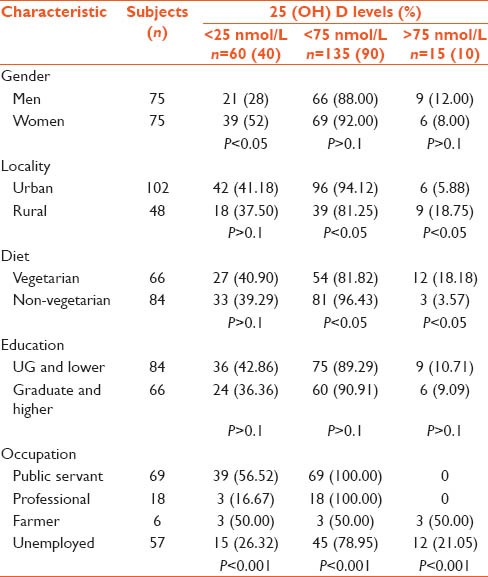
Gender-specific difference was significant at the lowest cut-off level (<25 nmol/L), and 21 (28%) men and 39 (52%) women displayed levels < 25 nmol/L (P < 0.05). However, there was no significant difference between the two groups with regard to the higher cut-off levels (<75 nmol/L). Sixty-six (88%) men and 69 (92%) women had levels less than 75 nmol/L (P > 0.1) [Table 1].
Table 1.
Plasma levels of 25 (OH) D across various demographic characteristics
Subjects in the age range of 51–60 years showed the least prevalence (25%) of vitamin insufficiency (levels <75 nmol/L), followed by the age range of 41–50 years (76.92%). Highest prevalence was seen in subjects of age less than 40 years as well as in subjects more than 60 years (95.83–100%) (P < 0.05) [Figure 1].
Figure 1.

Prevalence of vitamin D insufficiency across various age groups
Interestingly, a significantly high percentage (80.00%) of the subjects having optimal vitamin D levels was vegetarian (P < 0.05). Twelve of the total vegetarian subjects (18.18%) were having optimal levels, while only 3 (3.57%) of the non-vegetarians displayed optimal levels [Figure 2].
Figure 2.

Pattern of 25(OH) D levels across various population characteristics
A significantly different pattern was also observed in urban and rural populations. Ninety-six (94.12%) urban subjects displayed insufficient levels as compared to 39 (81.25%) rural subjects. Only 5.88% of the urban subjects had optimal vitamin D levels as compared to 18.75% of the rural subjects (P < 0.05). However, the difference was not significant for proportions showing the lowest cut-off of < 25 nmol/L (41.18% and 37.5%, respectively, with P > 0.1) [Figure 2].
An evaluation of educational background revealed no significant difference across different groups; 6 (9.09%) of the subjects who were graduates and above had optimal levels while 9 (10.71%) of the subjects who were undergraduates or illiterate had optimal levels (P > 0.1). Similarly, 36 (42.86%) of the former group and 24 (36.36%) of the latter had levels <25 nmol/L (P > 0.1) [Table 1].
On the other hand, occupation seems to have an influence, as all the subjects showing optimal levels were either non-working or farmers or housewives. Three out of 6 (50%) subjects who reported having agriculture as their occupation had optimal levels and 12 (21.05%) of the subjects who reported to be housewives or non-working had optimal levels. None of the subjects pursuing services or sedentary professions had optimal levels (P < 0.001) [Figure 3].
Figure 3.
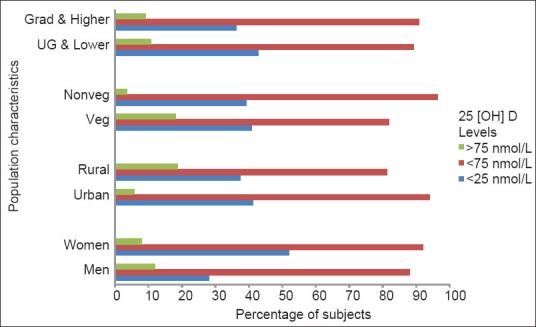
Pattern of 25(OH) D levels across various occupations
DISCUSSION
A large bulk of literature reports the high prevalence of vitamin D deficiency across the globe. Although many studies have been conducted in different communities and ethnic groups, there is a scarcity of searchable data on Indian populations. Some studies conducted in the region invariably report a high prevalence of vitamin D inadequacy contrary to popular belief.[18,19] Studies on northern populations have been scanty, more so on the Punjabi population.[20,21]
This study done on general random population of north-west Punjab found a high prevalence of vitamin D insufficiency across persons with different backgrounds. Of the total 150 study subjects, 135 (90%) were found to have vitamin D levels <75 nmol/L that are considered insufficient. Also, 60 (40%) individuals had very low (<25 nmol/L) levels and are thus prone to bone disorders. This is despite the fact that this region gets a good sunshine throughout the year and people are well off and can afford good nourishment. Most of the studies conducted on other Indian populations show a similar picture of dismal vitamin D status. Many authors suggest a revision of nutritional guidelines and even a national level fortified food scheme to improve the scenario.[22]
Urban subjects had significantly higher prevalence of insufficient 25(OH) D levels compared to their rural counterparts (94.12% vs. 81.25% with P < 0.05). It could perhaps be attributed to a greater exposure to sunlight in the rural as compared to urban areas. Also, there is a greater tendency among urbanites to use protective sunscreens which could prevent vitamin D synthesis by UV light. Other studies covering Indian population show similar trends. A study on North Indian rural population reports significant differences in the vitamin D levels of rural and urban individuals.[11] Another study done on vitamin D status in a southern region of India with a much larger study sample has shown a similar urban rural divide and urban subjects had significantly higher prevalence of 25(OH) D deficiency (P < 0.001).[23]
Men were slightly better off compared to women and lesser proportion of men had very low levels of <25 nmol/L (P < 0.05). But this difference dissipated at higher cut-off levels, such that none of the two groups fared better. A similar observation was made in a study done on British subjects wherein women showed greater prevalence of vitamin D deficiency at this particular threshold of level <25 nmol/L during winters. During summer or fall, however, higher cut-offs also displayed significant difference.[17] As our study duration coincided with the winter season in Punjab, the results of the two studies are in accordance with each other.
Prevalence of vitamin D deficiency was significantly different across various age groups. Middle age groups (41–60 years) displayed relatively lower prevalence compared to their younger (40 years and less) as well as older (61 years and more) counterparts (P < 0.05). Elderly people are known to be prone to vitamin D deficiency. Decreased exposure to sunlight, reduced cutaneous synthesis of vitamin D, and dietary inadequacy could be the factors contributing to lower vitamin D levels in the elderly.[24] Younger age group (40 years and less) of our study sample consisted mainly of employed persons with long indoor working hours and less duration of exposure to sunlight. This group is also likely to eat irregularly and junk food. These factors could translate into lower vitamin D levels.
Significant difference in the prevalence was seen between vegetarians and non-vegetarians. Oil-rich fish is one of the dietary sources of vitamin D.[24] Also, vegetarian diet rich in fiber and phytates is known to deplete the vitamin D stores.[25] Vitamin D fortified foods are not readily available in this region. In that sense, non-vegetarians should have some advantage over vegetarians as far as the vitamin D levels are concerned. Our study, however, revealed significantly higher proportions of vegetarians having optimal levels, compared to non-vegetarians (P < 0.05). A careful look at the data revealed that frequency of having non-vegetarian meals varied heavily amongst our subjects and only few subjects had non-vegetarian food on a daily basis. Most of them had non-vegetarian food occasionally, and that too, mostly chicken or mutton. Oil-rich fish, considered to be a source of dietary vitamin D, was consumed very rarely. Thus, our division of subjects into vegetarian and non-vegetarian can be considered superfluous.
Educational background of the subjects does not seem to have a bearing on the serum vitamin D levels and the differences across the groups were insignificant (P > 0.1). However, occupation did affect the levels. None of those serving as public servants or pursuing various professions and businesses had optimal levels. Subjects who reported to be farmers or housewives or unemployed showed a significantly different pattern; 50% of the farmers and 21.05% of others had optimal levels. This probably suggests more indoor-staying duration and lesser sunlight exposure of the service holders or professionals compared to their agrarian or non-working counterparts. Similar observations have been made by Hariharan et al.,[23] wherein rural agrarian subjects had lower prevalence of vitamin D inadequacy.
CONCLUSIONS
There is a high prevalence of hypovitaminosis D in the local population of north-west Punjab across different demographic characteristics. Subjects with chances of a higher exposure to sunlight, such as rural subjects, farmers, and housewives, have relatively lower prevalence of vitamin D insufficiency. There is a need for public awareness regarding the need for dietary rectifications and lifestyle changes providing opportunities for greater exposure to sunlight. Many countries have the policy of food fortification with vitamin D. Such a strategy could also help improving the scenario in India. Further research is required in this region to find the factors that are responsible for such an overall higher prevalence and also any association with various diseases and disorders. We would also like to add that with such high proportions of apparently healthy persons showing the defined levels of vitamin D deficiency globally, there may be a need to reassess the definitions of optimal and insufficient levels.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80(Suppl 6):1678–88S. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- 2.Talaei A, Mohamadi M, Adgi Z. The effect of vitamin D on insulin resistance in patients with type 2 diabetes. Diabetol Metab Syndr. 2013;5:8. doi: 10.1186/1758-5996-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coussens AK, Wilkinson RJ, Hanifa Y, Nikolayevskyy V, Elkington PT, Islam K, et al. Vitamin D accelerates resolution of inflammatory responses during tuberculosis treatment. Proc Natl Acad Sci USA. 2012;109:15449–54. doi: 10.1073/pnas.1200072109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hilger J, Friedel A, Herr R, Rausch T, Roos F, Wahl DA, et al. A systematic review of vitamin D status in populations worldwide. Br J Nutr. 2014;111:23–45. doi: 10.1017/S0007114513001840. [DOI] [PubMed] [Google Scholar]
- 5.Tripathi KD, editor. Essentials of Medical Pharmacology. 7th ed. New Delhi: Jaypee Brothers; 2013. Drugs affecting calcium balance; pp. 340–1. [Google Scholar]
- 6.Calvo MS, Whiting SJ, Barton CN. Vitamin D intake: A global perspective of current status. J Nutr. 2005;135:310–6. doi: 10.1093/jn/135.2.310. [DOI] [PubMed] [Google Scholar]
- 7.Norman AW. From vitamin D to hormone D: Fundamentals of the vitamin D endocrine system essential for good health. Am J Clin Nutr. 2008;88:491–9S. doi: 10.1093/ajcn/88.2.491S. [DOI] [PubMed] [Google Scholar]
- 8.Vieth R, Bischoff-Ferrari H, Boucher BJ, Dawson-Hughes B, Garland CF, Heaney RP, et al. The urgent need to recommend an intake of vitamin D that is effective. Am J Clin Nutr. 2007;85:649–50. doi: 10.1093/ajcn/85.3.649. [DOI] [PubMed] [Google Scholar]
- 9.Holick MF, Chen TC. Vitamin D deficiency: A worldwide problem with health consequences. Am J Clin Nutr. 2008;87:1080–6S. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]
- 10.Indian Council of Medical Research [www.icmr.nic.in] New Delhi: Nutrient Rquirements and Recommended Dietary Allowance for Indians. A report of the expert group of the Indian Council of Medical research. 2009. [Last accessed on 2014 Mar 24]. Available from: http://www.icmr.nic.in/final/RDA-2010.pdf .
- 11.Goswami R, Kochupillai N, Gupta N, Goswami D, Singh N, Dudha A. Presence of 25(OH) D deficiency in a rural North Indian village despite abundant sunshine. J Assoc Physicians India. 2008;56:755–7. [PubMed] [Google Scholar]
- 12.Zargar AH, Ahmad S, Masoodi SR, Wani AI, Bashir MI, Laway BA, et al. Vitamin D status in apparently healthy adults in Kashmir Valley of Indian subcontinent. Postgrad Med J. 2007;83:713–6. doi: 10.1136/pgmj.2007.059113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80(Suppl 6):1689–96S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 14.Norman AW, Bouillon R, Whiting SJ, Vieth R, Lips P. 13th workshop consensus for vitamin D nutritional guidelines. J Steroid Biochem Mol Biol. 2007;103:204–5. doi: 10.1016/j.jsbmb.2006.12.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henry HL, Bouillon R, Norman AW, Gallagher JC, Lips P, Heaney RP, et al. vitamin D workshop consensus on vitamin D nutritional guidelines. J Steroid Biochem Mol Biol. (14th) 2010;121:4–6. doi: 10.1016/j.jsbmb.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 16.Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporos Int. 2005;16:713–6. doi: 10.1007/s00198-005-1867-7. [DOI] [PubMed] [Google Scholar]
- 17.Hyppönen E, Power C. Hypovitaminosis D in British adults at age 45 y: Nationwide cohort study of dietary and lifestyle predictors. Am J Clin Nutr. 2007;85:860–8. doi: 10.1093/ajcn/85.3.860. [DOI] [PubMed] [Google Scholar]
- 18.Harinarayan CV, Sachan A, Reddy PA, Satish KM, Prasad UV, Srivani P. Vitamin D status and bone mineral density in women of reproductive and postmenopausal age groups: A cross-sectional study from south India. J Assoc Physicians India. 2011;59:698–704. [PubMed] [Google Scholar]
- 19.Marwaha RK, Tandon N, Garg MK, Kanwar R, Narang A, Sastry A, et al. Vitamin D status in healthy Indians aged 50 years and above. J Assoc Physicians India. 2011;59:706–9. [PubMed] [Google Scholar]
- 20.Marwaha RK, Sripathy G. Vitamin D and bone mineral density of healthy school children in northern India. Indian J Med Res. 2008;127:239–44. [PubMed] [Google Scholar]
- 21.Kotwal SK, Laway BA, Shah ZA. Pattern of 25 Hydroxy Vitamin D status in North Indian people with newly detected type 2 diabetes-A prospective case control study. J Med Sci. 2013;16(Suppl 1):4–10. doi: 10.4103/2230-8210.139242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Babu US, Calvo MS. Modern India and the vitamin D dilemma: Evidence for the need of a national food fortification program. Mol Nutr Food Res. 2010;54:1134–47. doi: 10.1002/mnfr.200900480. [DOI] [PubMed] [Google Scholar]
- 23.Harinarayan CV, Ramalakshmi T, Prasad UV, Sudhakar D. Vitamin D status in Andhra Pradesh: A population based study. Indian J Med Res. 2008;127:211–8. [PubMed] [Google Scholar]
- 24.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81:353–73. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- 25.Khadilkar AV. Vitamin D deficiency in Indian adolescents. Indian Pediatr. 2010;47:755–6. doi: 10.1007/s13312-010-0110-6. [DOI] [PubMed] [Google Scholar]


