Abstract
Background
As articular cartilage is unable to repair itself, there is a tremendous clinical need for a tissue engineered replacement tissue. Current tissue engineering efforts using the self-assembly process have demonstrated promising results, but the biomechanical properties remain at roughly 50% of native tissue.
Methodology/Principal Findings
The objective of this study was to determine the feasibility of using exogenous crosslinking agents to enhance the biomechanical properties of a scaffoldless cartilage tissue engineering approach. Four crosslinking agents (glutaraldehyde, ribose, genipin, and methylglyoxal) were applied each at a single concentration and single application time. It was determined that ribose application resulted in a significant 69% increase in Young's modulus, a significant 47% increase in ultimate tensile strength, as well as a trend toward a significant increase in aggregate modulus. Additionally, methylglyoxal application resulted in a significant 58% increase in Young's modulus. No treatments altered the biochemical content of the tissue.
Conclusions/Significance
To our knowledge, this is the first study to examine the use of exogenous crosslinking agents on any tissue formed using a scaffoldless tissue engineering approach. In particular, this study demonstrates that a one-time treatment with crosslinking agents can be employed effectively to enhance the biomechanical properties of tissue engineered articular cartilage. The results are exciting, as they demonstrate the feasibility of using exogenous crosslinking agents to enhance the biomechanical properties without the need for increased glycosaminoglycan (GAG) and collagen content.
Keywords: Cartilage, tissue engineering, biomechanical testing, crosslinking
1. Introduction
Following injury, articular cartilage is unable to repair itself, thus resulting in its replacement with mechanically inferior fibrocartilage,1 which will eventually be degraded over time. As such, there is a tremendous clinical need for a viable replacement tissue, and tissue engineering appears to be a promising avenue for replacement tissue formation.
Our laboratory has had success with a scaffoldless approach to articular cartilage tissue engineering, called the self-assembly process.2 For instance, we have produced engineered constructs with glycosaminoglycans (GAGs) and collagen content on par with native tissue following stimulation with modalities including hydrostatic pressure, growth factor application, and chondroitinase ABC treatment.3–7 However, the tissue's biomechanical properties, particularly the tensile properties, remained less than half those of native tissue. Therefore, additional treatment modalities will be required to obtain biomechanical properties on par with native tissue.
Several studies have examined the effects of collagen crosslinking agents on biomechanical properties and have demonstrated promising results. For instance, glutaraldehyde has been widely used as a protein crosslinking agent for tissue fixation as well as stabilization, and has been successfully used to enhance the biomechanical properties of knee meniscus explants at low concentrations.8
An alternative crosslinking paradigm is the use of glycation, in which collagen amine groups are crosslinked with reducing sugars, leading to advanced glycation end products (AGEs).9 Although the presence of AGEs is generally a sign of aging that is detrimental to diabetics,10,11 glycation has been shown to be an interesting approach for enhancing tissue functional properties with minimal toxicity.12 Based on a review of the literature,8,12–16 ribose, genipin, and methylglyoxal were selected as glycation crosslinkers, and were compared to glutaraldehyde. Each of the four crosslinking agents was applied at a single concentration selected from the literature. Additionally, a single application time of 3.5 h with a 0.5-h wash was selected as 4 h was shown to be the maximum time of construct incubation prior to the loss of GAG.17
Though several studies have assessed the effects of crosslinking agents on explant tissue, to the best of our knowledge, no study has assessed the effects of crosslinking agents on tissue formed in a scaffoldless tissue engineering approach. Therefore, the objective of this study was to determine the feasibility of using exogenous crosslinking agents to enhance biomechanical properties in a scaffoldless cartilage tissue engineering approach. As such, the effects of the crosslinking agents on construct compressive and tensile biomechanical properties, GAG and collagen content, and cellularity, were assessed following tissue formation during a 4-week culture period. It was hypothesized that the one-time application of a crosslinking agent would enhance the biomechanical properties of the engineered cartilage tissue without affecting the biochemical properties of the tissue.
2. Materials and Methods
2.1. Chondrocyte isolation and seeding
Articular cartilage was obtained from the distal femur of week-old male calves18–20 (Research 87, Boston, MA) after slaughter, and chondrocytes were isolated after tissue digestion with collagenase type 2 (Worthington, Lakewood, NJ). To normalize animal variability, each leg was obtained from a different animal, and cells from all legs were combined together to create a mixture of chondrocytes; a mixture of cells from six legs was used in the study. Cell number was measured on a hemocytometer, and a trypan blue exclusion test indicated that viability remained >85%. Chondrocytes were frozen in culture medium supplemented with 20% FBS (Biowhittaker, Walkersville, MD) and 10% DMSO at −80°C for one week prior to use. After thawing, viability was greater than 90%. A stainless steel mold consisting of 5 mm diameter × 10 mm long cylindrical prongs was placed into a row of a 48-well plate. For construction of each agarose well, sterile and molten 2% agarose was added to wells containing the die. The agarose solidified at room temperature for 60 min and the mold was then removed from the agarose. Culture medium was exchanged twice to completely saturate the agarose well by the time of cell seeding. The medium was DMEM with 4.5 g/l-glucose and l-glutamine (Biowhittaker), 100 nM dexamethasone (Sigma, St. Louis, MO), 1% penicillin/streptomycin/fungizone (P/S/F) (Biowhittaker), 1% ITS+ (BD Scientific, Franklin Lakes, NJ), 50 μg/ml ascorbate-2-phosphate, 40 μg/ml l-proline, and 100 μg/ml sodium pyruvate (Fisher Scientific, Pittsburgh, PA). For each construct, 5.5 × 106 cells were added in 100 μl of culture medium. Constructs assembled within 24 h in the agarose wells and were cultured in the same well until t = 10 days, after which they were cultured unconfined for the remainder of the study, as described previously;21 t = 0 was defined as 24 h after seeding.
2.2. Crosslinking treatment
At t = 4 weeks, self-assembled constructs (n = 6−7/group) were removed from culture and exposed to one of four crosslinking treatments, for 3.5 h. The crosslinking treatments, all obtained from Sigma, included:
0.2% glutaraldehyde,
0.33% genipin,
30 mM ribose, and
100 mM methylglyoxal.
All treatments included 0.02% EDTA, and 1% P/S/F, in PBS. A control group was exposed to this same solution for 3.5 h without a crosslinking agent added. These treatments were applied at 37°C with agitation. Following the 3.5 h crosslinking treatment, the constructs were washed for 30 min in PBS at 37°C with agitation, and then all construct assessments were performed.
2.3. Histology
Following freezing, samples were sectioned at 14 μm. To determine construct GAG distribution, a safranin-O/fast green stain was used,22,23 and to examine collagen content, a picrosirius-red stain was employed.
2.4. Quantitative biochemistry
Samples were frozen overnight and lyophilized for 48 h. This was followed by digestion with 125 μg/ml papain (Sigma) in 50 mM phosphate buffer (pH = 6.5) containing 2 mM N-acetyl cysteine (Sigma) and 2 mM EDTA (Sigma) at 65°C overnight. A Picogreen® Cell Proliferation Assay Kit (Molecular Probes) was used to measure total DNA content. GAG content was determined using the Blyscan Glycosaminoglycan Assay kit (Biocolor), based on 1,9-dimethylmethylene blue binding.24,25 Finally, after hydrolysis with 2-N NaOH for 20 min at 110°C, total collagen content was quantified using a chloramine-T hydroxyproline assay.26
2.5. Indentation testing
Samples were assessed with an indentation apparatus, as previously described.27 A 0.7 g (0.007 N) mass was applied with a 1-mm flat-ended porous indenter tip, and specimens crept until equilibrium, as described elsewhere.2 Strains generally ranged from 2 to 5%. Preliminary estimations of the aggregate modulus of the samples were obtained using the analytical solution for the axisymmetric Boussinesq problem with Papkovich potential functions.28,29 The sample biomechanical properties were then calculated using the linear biphasic theory.30
2.6. Tensile testing
A uniaxial materials testing system (Instron Model 5565, Canton, MA) was used to measure tensile properties with a 50-N load cell, as described previously.31 Briefly, samples were cut into a dog-bone shape with a 1-mm-long gauge length. Samples were glued to paper tabs with cyanoacrylate glue outside of the gauge length. The 1-mm-long sections were pulled at a 1% constant strain rate, and samples broke within the gauge length. Stress–strain curves were generated from the load– displacement curve and the cross-sectional area of each sample, and Young's modulus and ultimate tensile strength were calculated from each stress–strain curve.
2.7. Statistical analysis
All biomechanical and biochemical assessments were made using n = 6−7. To compare among treatment groups, a single factor ANOVA was used, and a Tukey's HSD post-hoc test was used when warranted. Significance was defined as p < 0.05.
3. Results
3.1. Gross appearance and histology
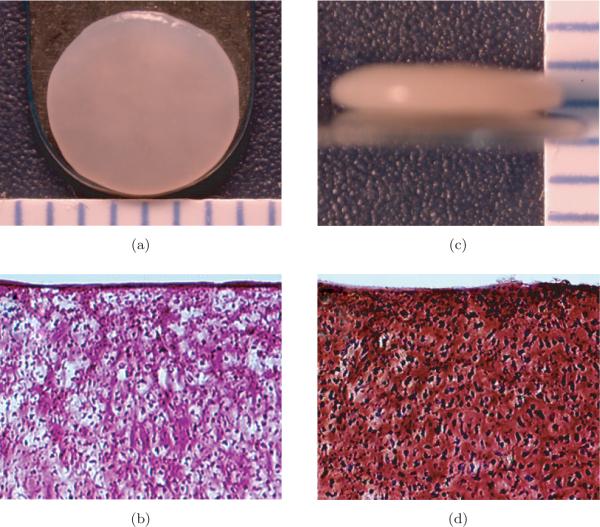
Construct diameter was approximately 6 mm in all studies. The construct wet weights for the control, glutaraldehyde, genipin, ribose, and methylglyoxal treated groups were 21.4 ± 1.5, 23.8 ± 1.2, 22.6 ± 1.3, 20.6 ± 1.5, and 21.8 ± 2.1 mg, respectively; no groups were significantly different from control. The construct thicknesses for the control, glutaraldehyde, genipin, ribose, and methylglyoxal treated groups were 0.79 ± 0.06, 0.98 ± 0.10, 0.91 ± 0.06, 0.79 ± 0.06, and 0.89 ± 0.07 mm, respectively. The thicknesses for the glutaraldehyde and genipin groups were significantly higher than control. Figure 1 depicts the construct gross morphological and histological properties of the tissue. All constructs stained positive for collagen and GAG throughout their thickness.
Fig. 1.
Gross morphological and histological properties representative of all self-assembled constructs (10x original magnification). (a) Construct gross morphology surface view (each bar is 1 mm). (b) Construct gross morphology profile view (each bar is 1 mm). (c) Picrosirius red stained sections. (d) Safranin-O/fast green stained sections.
3.2. Quantitative biochemistry
There were no differences among the treatment groups in cellularity, GAG content, or collagen content. The cells/construct for the control, glutaraldehyde, genipin, ribose, and methylglyoxal treated groups were 6.1 ± 0.8 × 106, 6.0 ± 0.5 × 106, 5.4±0.31×106, 5.7 ± 0.4×106, and 6.1 ± 0.6 × 106 cells, respectively. The GAG/WWs for the control, glutaraldehyde, genipin, ribose, and methylglyoxal treated groups were 8.2 ± 0.4, 8.1 ± 0.6, 8.8 ± 0.2, 8.3 ± 0.4, and 8.7 ± 0.5%, respectively. The collagen/WW for the control, glutaraldehyde, genipin, ribose, and methylglyoxal treated groups were 9.1±1.7, 8.7±1.0, 8.0±0.8, 8.4±1.4 and 7.6±1.1%, respectively.
3.3. Biomechanical evaluation
The effects of the various crosslinking treatments on construct tensile properties are displayed in Fig. 2. Treatment with ribose resulted in a significant increase in both Young's modulus and ultimate tensile strength (p < 0.05), and treatment with methylglyoxal resulted in a significant increase in construct Young's modulus (p < 0.05). The Young's moduli for the control, glutaraldehyde, genipin, ribose, and methylglyoxal treated groups were 682 ± 190, 955 ± 241, 878 ± 244, 1152 ± 263, and 1082 ± 407 kPa, respectively. The ultimate tensile strengths for the control, glutaraldehyde-, genipin-, ribose-, and methylglyoxal-treated groups were 184 ± 44, 241 ± 38, 190 ± 54, 271 ± 52, and 213 ± 67 kPa, respectively.
Fig. 2.
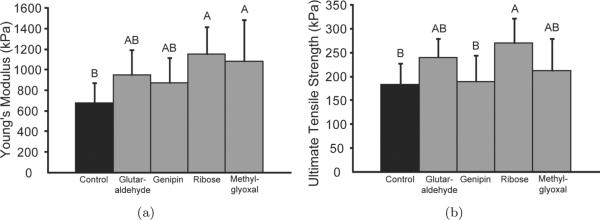
Tensile properties of self-assembled constructs. (a) Young's modulus. (b) Ultimate tensile strength. Exogenous application of ribose resulted in a significant increase in Young's modulus and ultimate tensile strength, and application of methylglyoxal resulted in a significant increase in Young's modulus. Columns and error bars represent means and standard deviations. Groups denoted by different letters are significantly different (p < 0.05).
The effects of the various crosslinking treatments on construct compressive properties are displayed in Fig. 3. There was a trend toward enhanced aggregate modulus with ribose treatment (p = 0.10). The aggregate moduli for the control, glutaraldehyde-, genipin-, ribose-, and methylglyoxal-treated groups were 237 ± 80, 307 ± 77, 230 ± 54, 334 ± 96, and 286 ± 51 kPa, respectively. The Poisson's ratios ranged from 0.28 to 0.35, with no differences among the groups. The permeability values for the control, glutaraldehyde-, genipin-, ribose-, and methylglyoxal-treated groups were 7.5 ± 2.6 × 10−13, 9.0 ± 7.8 × 10−13, 1.6 ± 0.6 × 10−12, 1.0 ± 0.6 × 10−12, and 1.8 ± 0.3 × 10−12 m4/Ns, with only methylglyoxal significantly different from control (p < 0.05).
Fig. 3.
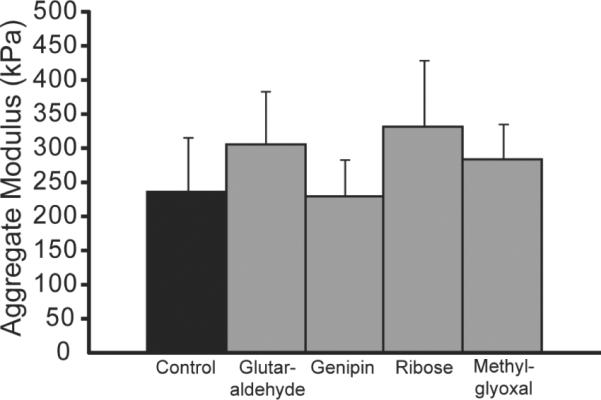
Compressive stiffness of self-assembled constructs. Exogenous application of ribose resulted in a trend toward a significant increase in aggregate modulus (p = 0.10). Columns and error bars represent means and standard deviations.
4. Discussion
This study examined the effects of four exogenous crosslinking treatments on self-assembled tissue-engineered cartilage constructs. It was hypothesized that exogenous crosslinking agents could be used to enhance the biomechanical properties of cartilage constructs after a one-time treatment, without altering the biochemical or histological composition of the tissue. To the best of our knowledge, no other study has demonstrated enhanced biomechanical properties of tissue formed in a scaffoldless tissue engineering approach, in this case self-assembled cartilage constructs, from the application of exogenous crosslinking agents following culture.
Ribose and methylglyoxal were found to be the winners of the study in terms of improving construct functionality, where functionality is defined by the construct biomechanical properties. Treatment with ribose appeared to be the most beneficial, as it led to a significant increase in construct tensile properties (69% increase in Young's modulus and 47% increase in ultimate tensile strength), as well as a trend toward a significant increase in construct aggregate modulus (41%). This result correlates with the findings of prior studies, in which it was demonstrated that ribose application to meniscal tissue32 or scaffolds14 significantly increased the tissue's biomechanical properties. Likewise, treatment with methylglyoxal resulted in a significant increase (58%) in construct Young's modulus, although no such increase in construct aggregate modulus was identified. Similarly, Wagner et al.16 observed an increase in annulus fibrosus tensile stiffness following treatment with methylglyoxal. The results of the present study can be attributed to the exogenous application of the crosslinking agents, as no changes in collagen or GAG content were demonstrated with either treatment. Although the objective of this study was to examine the effects of exogenous crosslinking agents and construct functional properties, future studies need to be conducted to examine and measure the resultant collagen crosslinks in the tissue following treatment.
Unfortunately, treatment with both genipin and glutaraldehyde did not result in significant changes in construct biomechanical properties, which differs from prior studies in the literature involving the knee meniscus and intervertebral disc. For instance, Hunter et al.8 demonstrated up to a 2.8-fold higher aggregate modulus with 0.02% glutaraldehyde application to meniscal explants. Additionally, Chuang et al.13 found up to a 151% increase in “low compressive stiffness modulus” and a 78% increase in Young's modulus when applying 0.33% genipin to annulus fibrosus explants; however, it must be noted that genipin was applied for 48 h in this study, as opposed to the 3.5 h in our study. Thus, it is possible that there is a significant dependence on treatment concentration and application, and it is likely that some of the crosslinking agents must be applied at higher concentrations or for longer periods of time than were used in this study in order to enhance the biomechanical properties of the tissue. This is especially likely given the demonstrated dose-dependent relationships of multiple crosslinking agents on tissue biomechanical properties.8,15 However, there also appears to be a ceiling in applied concentration, as ribose concentrations above 100 mM inhibit collagen crosslinking in bovine nasal cartilage.33 It is also possible that continued construct culture is required after crosslinking treatment before an increase in biomechanical properties is found, as was observed previously.34
In addition, the genipin and glutaraldehyde groups were significantly thicker than the control groups, and these were the only groups that did not have a significant increase in biomechanical properties following treatment, so it is possible that their increased thickness hindered the effects of the crosslinking agents. However, this is unlikely as these groups were only approximately 10–20% thicker than control, and all crosslinking treatments were applied for 3.5 h with agitation. Additionally, as indicated in the histological images, all constructs appeared uniform microscopically throughout their thickness. Furthermore, construct thickness was assessed after crosslinking, so it is possible that treatment with genipin and glutaraldehyde led to increased construct thickness immediately following treatment.
A potential drawback of this approach is the in vivo translatability of the approach used in the study. For instance, Speer et al.35 observed in vitro cytotoxicity and a foreign body giant cell reaction in vivo due to leaching of glutaraldehyde from the bioimplant at concentrations of only 3 ppm. Additionally, the failure of heart valve allografts has been associated with calcification resulting from glutaralde-hyde treatment.36 However, the use of glycation treatments mitigates some of these detrimental effects. For instance, Girton et al.12 found that ribose treatments up to 30 mM did not lead to cytotoxicity, and did not result in tissue calcification following a two-week subcutaneous implantation in rats. Furthermore, Lima et al.34 found that genipin treatment of chondrocyte-seeded agarose hydrogels was actually protective against cytokine degradation, and the use of genipin as a culture supplement had no effect on cell viability.
Although the results of this study are promising, future work must be performed to better elucidate the effects of these crosslinking agents. As this study served as a preliminary step to determine the feasibility of this approach in a scaffoldless system, future studies should be performed to assess the viability of the constructs following treatment with continued construct culture after crosslinking. Additionally, it would be exciting to examine if application of this treatment at regular time intervals would continually enhance the mechanical properties without compromising cell survival and tissue synthesis. In addition, the actual collagen crosslinks should be examined and quantified in future studies. Additionally, although only ribose and methylglyoxal were found to improve tissue biomechanical properties, these crosslinking agents should be examined at multiple higher concentrations and longer application times to further assess the feasibility of their use in future cartilage tissue engineering studies. Finally, the effects of these treatments need to be assessed in an in vivo model to assess the potential toxicity of each treatment.
In light of this, our study served an important role in assessing the feasibility of using exogenous crosslinking agents to improve the biomechanical properties of engineered cartilage constructs without the use of a scaffold, and provided guidance on which treatments to pursue for use in future studies. The results of this study are exciting, as they demonstrate the ability to significantly increase the tensile properties of self-assembled articular cartilage constructs after a short incubation period, without the need to enhance the biochemical properties of the tissue.
Contributor Information
BENJAMIN D. ELDER, Department of Neurosurgery The Johns Hopkins Hospital Baltimore, MD, USA
ARVIND MOHAN, Saint John School Houston, TX, USA.
KYRIACOS A. ATHANASIOU, Department of Biomedical Engineering University of California Davis One Shields Avenue, Davis, CA, USA 95616.
References
- 1.Buckwalter JA. Articular cartilage: Injuries and potential for healing. J Orthop Sports Phys Ther. 1998;28:192–202. doi: 10.2519/jospt.1998.28.4.192. [DOI] [PubMed] [Google Scholar]
- 2.Hu JC, Athanasiou KA. A self-assembling process in articular cartilage tissue engineering. Tissue Eng. 2006;12:969–979. doi: 10.1089/ten.2006.12.969. [DOI] [PubMed] [Google Scholar]
- 3.Elder BD, Athanasiou KA. Effects of temporal hydrostatic pressure on tissue-engineered bovine articular cartilage constructs. Tissue Eng Part A. 2009;15:1151–1158. doi: 10.1089/ten.tea.2008.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elder BD, Athanasiou KA. Synergistic and additive effects of hydrostatic pressure and growth factors on tissue formation. PLoS ONE. 2008;3:e2341. doi: 10.1371/journal.pone.0002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elder BD, Athanasiou KA. Systematic assessment of growth factor treatment on biochemical and biomechanical properties of engineered articular cartilage constructs. Osteoarthritis Cartilage. 2009;17:114–123. doi: 10.1016/j.joca.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu JC, Athanasiou KA. The effects of intermittent hydrostatic pressure on self-assembled articular cartilage constructs. Tissue Eng. 2006;12:1337–1344. doi: 10.1089/ten.2006.12.1337. [DOI] [PubMed] [Google Scholar]
- 7.Natoli RM, Responte DJ, Lu BY, Athanasiou KA. Effects of multiple chondroitinase ABC applications on tissue engineered articular cartilage. J Orthop Res. 2009;27:949–956. doi: 10.1002/jor.20821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hunter SA, Noyes FR, Haridas B, Levy MS, Butler DL. Meniscal material properties are minimally aff ected by matrix stabilization using glutaraldehyde and glycation with ribose. J Orthop Res. 2005;23:555–561. doi: 10.1016/j.orthres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Brownlee M. Nonenzymatic glycosylation of macromolecules. Prospects of pharmacologic modulation. Diabetes. 1992;41(Suppl 2):57–60. doi: 10.2337/diab.41.2.s57. [DOI] [PubMed] [Google Scholar]
- 10.King GL, Brownlee M. The cellular and molecular mechanisms of diabetic complications. Endocrinol Metab Clin North Am. 1996;25:255–270. doi: 10.1016/s0889-8529(05)70324-8. [DOI] [PubMed] [Google Scholar]
- 11.Monnier VM, Kohn RR, Cerami A. Accelerated age-related browning of human collagen in diabetes mellitus. Proc Natl Acad Sci USA. 1984;81:583–587. doi: 10.1073/pnas.81.2.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Girton TS, Oegema TR, Tranquillo RT. Exploiting glycation to stiffen and strengthen tissue equivalents for tissue engineering. J Biomed Mater Res. 1999;46:87–92. doi: 10.1002/(sici)1097-4636(199907)46:1<87::aid-jbm10>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 13.Chuang SY, Odono RM, Hedman TP. Effects of exogenous crosslinking on in vitro tensile and compressive moduli of lumbar intervertebral discs. Clin Biomech (Bristol, Avon) 2007;22:14–20. doi: 10.1016/j.clinbiomech.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Roy R, Boskey AL, Bonassar LJ. Non-enzymatic glycation of chondrocyte-seeded collagen gels for cartilage tissue engineering. J Orthop Res. 2008;26:1434–1439. doi: 10.1002/jor.20662. [DOI] [PubMed] [Google Scholar]
- 15.Verzijl N, DeGroot J, Ben ZC, et al. Crosslinking by advanced glycation end products increases the stiffness of the collagen network in human articular cartilage: A possible mechanism through which age is a risk factor for osteoarthritis. Arthritis Rheum. 2002;46:114–123. doi: 10.1002/1529-0131(200201)46:1<114::AID-ART10025>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 16.Wagner DR, Reiser KM, Lotz JC. Glycation increases human annulus fibrosus stiffness in both experimental measurements and theoretical predictions. J Biomech. 2006;39:1021–1029. doi: 10.1016/j.jbiomech.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Elder BD, Eleswarapu SV, Athanasiou KA. Extraction techniques for the decellularization of tissue engineered articular cartilage constructs. Biomaterials. 2009;30:3749–3756. doi: 10.1016/j.biomaterials.2009.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khalafi A, Schmid TM, Neu C, Reddi AH. Increased accumulation of superficial zone protein (SZP) in articular cartilage in response to bone morphogenetic protein-7 and growth factors. J Orthop Res. 2007;25:293–303. doi: 10.1002/jor.20329. [DOI] [PubMed] [Google Scholar]
- 19.Mauck RL, Nicoll SB, Seyhan SL, Ateshian GA, Hung CT. Synergistic action of growth factors and dynamic loading for articular cartilage tissue engineering. Tissue Eng. 2003;9:597–611. doi: 10.1089/107632703768247304. [DOI] [PubMed] [Google Scholar]
- 20.Saini S, Wick TM. Effect of low oxygen tension on tissue-engineered cartilage construct development in the concentric cylinder bioreactor. Tissue Eng. 2004;10:825–832. doi: 10.1089/1076327041348545. [DOI] [PubMed] [Google Scholar]
- 21.Elder BD, Athanasiou KA. Systematic assessment of growth factor treatment on biochemical and biomechanical properties of engineered articular cartilage constructs. Osteoarthritis Cartilage. 2009;17:114–123. doi: 10.1016/j.joca.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shimizu M, Minakuchi K, Kaji S, Koga J. Chondrocyte migration to fibronectin, type I collagen, and type II collagen. Cell Struct Funct. 1997;22:309–315. doi: 10.1247/csf.22.309. [DOI] [PubMed] [Google Scholar]
- 23.Rosenberg L. Chemical basis for the histological use of safranin O in the study of articular cartilage. J Bone Joint Surg Am. 1971;53A:69–82. [PubMed] [Google Scholar]
- 24.Brown AN, Kim BS, Alsberg E, Mooney DJ. Combining chondrocytes and smooth muscle cells to engineer hybrid soft tissue constructs. Tissue Eng. 2000;6:297–305. doi: 10.1089/107632700418029. [DOI] [PubMed] [Google Scholar]
- 25.Pietila K, Kantomaa T, Pirttiniemi P, Poikela A. Comparison of amounts and properties of collagen and proteoglycans in condylar, costal and nasal cartilages. Cells Tissues Organs. 1999;164:30–36. doi: 10.1159/000016640. [DOI] [PubMed] [Google Scholar]
- 26.Woessner JF., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 27.Athanasiou KA, Agarwal A, Dzida FJ. Comparative study of the intrinsic mechanical properties of the human acetabular and femoral head cartilage. J Orthop Res. 1994;12:340–349. doi: 10.1002/jor.1100120306. [DOI] [PubMed] [Google Scholar]
- 28.Sneddon I. The relaxation between load and penetration in the axisymmetric Boussinesq problem for a punch of arbitrary profile. Int J Eng Sci. 1965;3:47–57. [Google Scholar]
- 29.Hayes WC, Keer LM, Herrmann G, Mockros LF. A mathematical analysis for indentation tests of articular cartilage. J Biomech. 1972;5:541–551. doi: 10.1016/0021-9290(72)90010-3. [DOI] [PubMed] [Google Scholar]
- 30.Athanasiou KA, Agarwal A, Muffoletto A, Dzida FJ, Constantinides G, Clem M. Biomechanical properties of hip cartilage in experimental animal models. Clin Orthop Relat Res. 1995;316:254–266. [PubMed] [Google Scholar]
- 31.Aufderheide AC, Athanasiou KA. Assessment of a bovine co-culture, scaffold-free method for growing meniscus-shaped constructs. Tissue Eng. 2007;13:2195–2205. doi: 10.1089/ten.2006.0291. [DOI] [PubMed] [Google Scholar]
- 32.Hunter CJ, Levenston ME. Maturation and integration of tissue-engineered cartiages within an in vitro defect repair model. Tissue Eng. 2004;10:736–746. doi: 10.1089/1076327041348310. [DOI] [PubMed] [Google Scholar]
- 33.Pokharna HK, Pottenger LA. Nonenzymatic glycation of cartilage proteoglycans: An in vivo and in vitro study. Glycoconj J. 1997;14:917–923. doi: 10.1023/a:1018514727213. [DOI] [PubMed] [Google Scholar]
- 34.Lima EG, Tan AR, Tai T, et al. Genipin enhances the mechanical properties of tissue-engineered cartilage and protects against inflammatory degradation when used as a medium supplement. J Biomed Mater Res A. 2009;91:692–700. doi: 10.1002/jbm.a.32305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Speer DP, Chvapil M, Eskelson CD, Ulreich J. Biological effects of residual glutaralde-hyde in glutaraldehyde-tanned collagen biomaterials. J Biomed Mater Res. 1980;14:753–764. doi: 10.1002/jbm.820140607. [DOI] [PubMed] [Google Scholar]
- 36.Jayakrishnan A, Jameela SR. Glutaraldehyde as a fixative in bioprostheses and drug delivery matrices. Biomaterials. 1996;17:471–484. doi: 10.1016/0142-9612(96)82721-9. [DOI] [PubMed] [Google Scholar]