Abstract
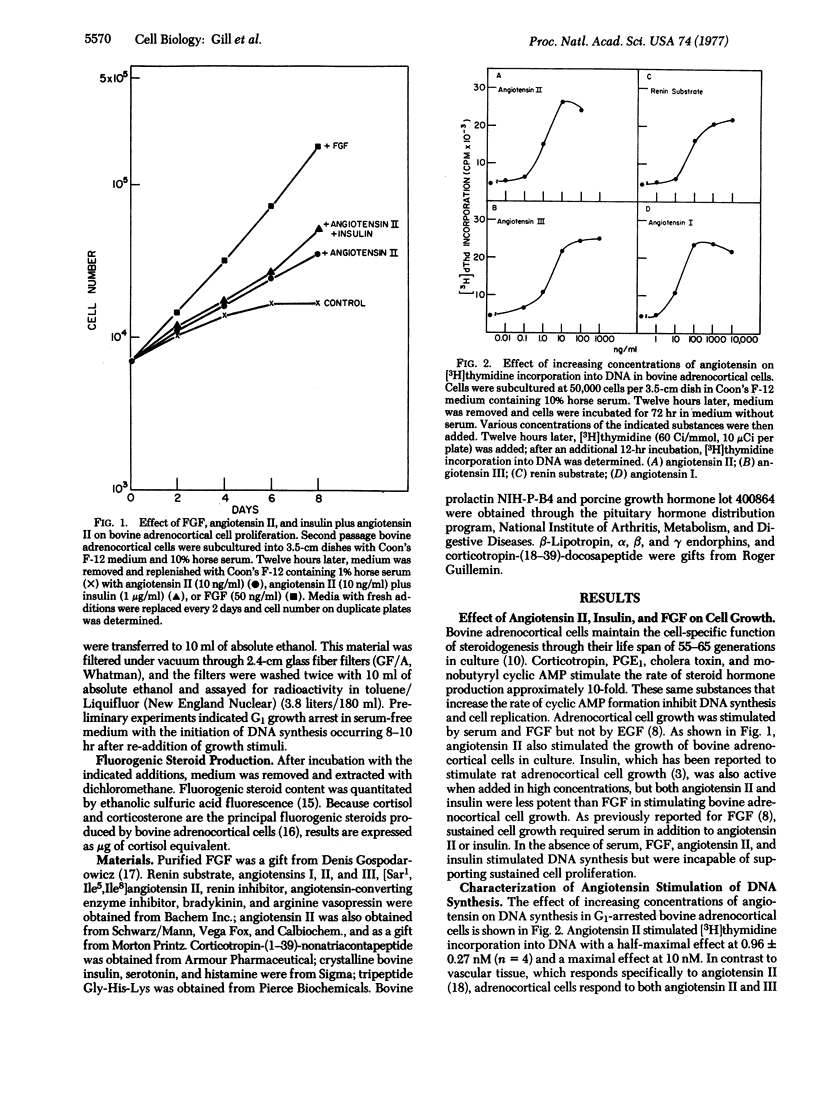
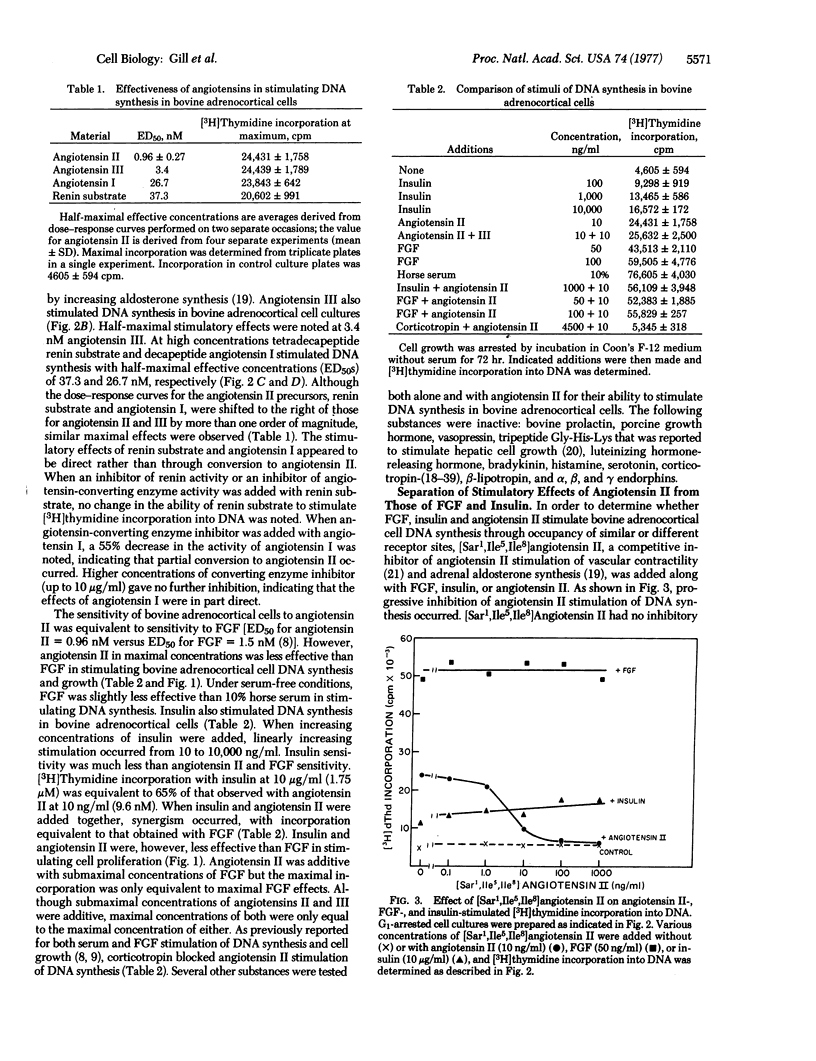
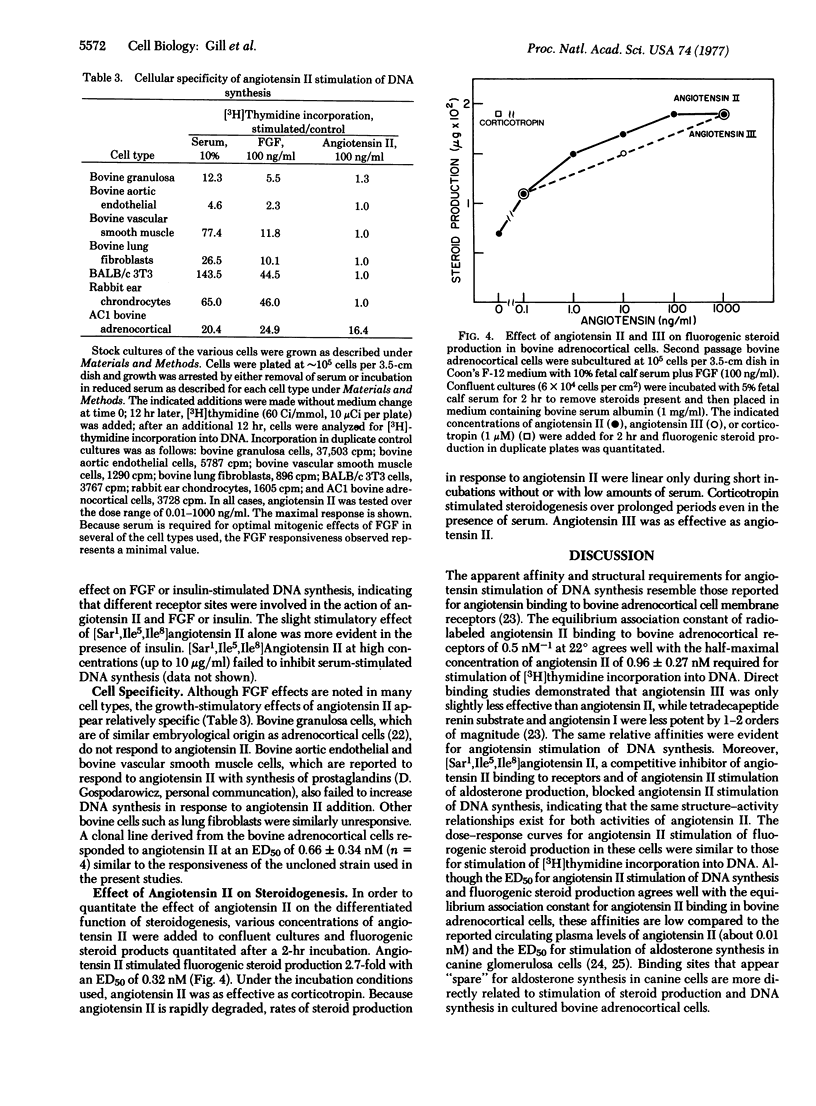
Factors controlling proliferation of adrenocortical cells have been studied in monolayer cultures of bovine adrenocortical cells. Angiotensin II stimulated cell proliferation and [3H]thymidine incorporation into DNA with a half-maximal effective concentration of 0.96 +/- 0.27 nM. Similar sensitivity to angiotensin III with reduced sensitivity to angiotensin I and tetradecapeptide renin substrate was observed. Although sensitivity to angiotensin II was equivalent to that for fibroblast growth factor (1.5 nM half-maximal effective concentration), maximal effects of angiotensin were less than for fibroblast growth factor and serum. High concentrations of insulin (1-10 micrometer) also stimulated [3H]thymidine incorporation into DNA and cell proliferation. [Sar1,Ile5,Ile8]Angiotensin II, a competitive antagonist of angiotensin II, blocked angiotensin II stimulation of DNA synthesis but did not affect fibroblast growth factor and insulin stimulation of DNA synthesis. Corticotropin (ACTH) blocked the stimulatory effects of both angiotensin II and fibroblast growth factor. The dose-response curves for angiotensin II stimulation of steroidogenesis were similar to those for stimulation of [3H]thymidine incorporation into DNA. Among the seven cell types examined, only adrenocortical cells responded to angiotension II with stimulation of DNA synthesis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander R. W., Gimbrone M. A., Jr Stimulation of prostaglandin E synthesis in cultured human umbilical vein smooth muscle cells. Proc Natl Acad Sci U S A. 1976 May;73(5):1617–1620. doi: 10.1073/pnas.73.5.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonassisi V., Venter J. C. Hormone and neurotransmitter receptors in an established vascular endothelial cell line. Proc Natl Acad Sci U S A. 1976 May;73(5):1612–1616. doi: 10.1073/pnas.73.5.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channing C. P. Influences of the in vivo and in vitro hormonal environment upon luteinization of granulosa cells in tissue culture. Recent Prog Horm Res. 1970;26:589–622. doi: 10.1016/b978-0-12-571126-5.50019-4. [DOI] [PubMed] [Google Scholar]
- Chiu A. T., Peach M. J. Inhibition of induced aldosterone biosynthesis with a specific antagonist of angiotensin II. Proc Natl Acad Sci U S A. 1974 Feb;71(2):341–344. doi: 10.1073/pnas.71.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devynck M. A., Meyer P. Angiotensin receptors in vascular tissue. Am J Med. 1976 Nov;61(5):758–767. doi: 10.1016/0002-9343(76)90157-1. [DOI] [PubMed] [Google Scholar]
- Fredlund P., Saltman S., Catt K. J. Aldosterone production by isolated adrenal glomerulosa cells: stimulation by physiological concentrations of angiotensin II. Endocrinology. 1975 Dec;97(6):1577–1586. doi: 10.1210/endo-97-6-1577. [DOI] [PubMed] [Google Scholar]
- Ganten D., Hutchinson J. S., Schelling P., Ganten U., Fischer H. The iso-renin angiotensin systems in extrarenal tissue. Clin Exp Pharmacol Physiol. 1976 Mar-Apr;3(2):103–126. doi: 10.1111/j.1440-1681.1976.tb00596.x. [DOI] [PubMed] [Google Scholar]
- Glossmann H., Baukal A. J., Catt K. J. Properties of angiotensin II receptors in the bovine and rat adrenal cortex. J Biol Chem. 1974 Feb 10;249(3):825–834. [PubMed] [Google Scholar]
- Gospodarowicz D., Gospodarowicz F. The morphological transformation and inhibition of growth of bovine luteal cells in tissue culture induced by luteinizing hormone and dibutyryl cyclic AMP. Endocrinology. 1975 Feb;96(2):458–467. doi: 10.1210/endo-96-2-458. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Ill C. R., Birdwell C. R. Effects of fibroblast and epidermal growth factors on ovarian cell proliferation in vitro. I. Characterization of the response of granulosa cells to FGF and EGF. Endocrinology. 1977 Apr;100(4):1108–1120. doi: 10.1210/endo-100-4-1108. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Ill C. R., Hornsby P. J., Gill G. N. Control of bovine adrenal cortical cell proliferation by fibroblast growth factor. Lack of effect of epidermal growth factor. Endocrinology. 1977 Apr;100(4):1080–1089. doi: 10.1210/endo-100-4-1080. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Moran J. S. Growth factors in mammalian cell culture. Annu Rev Biochem. 1976;45:531–558. doi: 10.1146/annurev.bi.45.070176.002531. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Moran J., Braun D., Birdwell C. Clonal growth of bovine vascular endothelial cells: fibroblast growth factor as a survival agent. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4120–4124. doi: 10.1073/pnas.73.11.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D. Purification of a fibroblast growth factor from bovine pituitary. J Biol Chem. 1975 Apr 10;250(7):2515–2520. [PubMed] [Google Scholar]
- Griswold M. D., Solari A., Tung P. S., Fritz I. B. Stimulation by follicle-stimulating hormone of DNA synthesis and of mitosis in cultured Sertoli cells prepared from testes of immature rats. Mol Cell Endocrinol. 1977 Apr;7(2):151–165. doi: 10.1016/0303-7207(77)90064-8. [DOI] [PubMed] [Google Scholar]
- Ham R. G., Sattler G. L. Clonal growth of differentiated rabbit cartilage cells. J Cell Physiol. 1968 Oct;72(2):109–114. doi: 10.1002/jcp.1040720205. [DOI] [PubMed] [Google Scholar]
- Hayashi I., Sato G. H. Replacement of serum by hormones permits growth of cells in a defined medium. Nature. 1976 Jan 15;259(5539):132–134. doi: 10.1038/259132a0. [DOI] [PubMed] [Google Scholar]
- Hornsby P. J., Gill G. N. Hormonal control of adrenocortical cell proliferation. Desensitization to ACTH and interaction between ACTH and fibroblast growth factor in bovine adrenocortical cell cultures. J Clin Invest. 1977 Aug;60(2):342–352. doi: 10.1172/JCI108782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosla M. C., Leese R. A., Maloy W. L., Ferreira A. T., Smeby R. R., Bumpus F. M. Synthesis of some analogs of angiotensin II as specific antagonists of the parent hormone. J Med Chem. 1972 Aug;15(8):792–795. doi: 10.1021/jm00278a003. [DOI] [PubMed] [Google Scholar]
- Masui H., Garren L. D. Inhibition of replication in functional mouse adrenal tumor cells by adrenocorticotropic hormone mediated by adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3206–3210. doi: 10.1073/pnas.68.12.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PUCK T. T., MARCUS P. I., CIECIURA S. J. Clonal growth of mammalian cells in vitro; growth characteristics of colonies from single HeLa cells with and without a feeder layer. J Exp Med. 1956 Feb 1;103(2):273–283. doi: 10.1084/jem.103.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickart L., Thaler M. M. Tripeptide in human serum which prolongs survival of normal liver cells and stimulates growth in neoplastic liver. Nat New Biol. 1973 May 16;243(124):85–87. [PubMed] [Google Scholar]
- Ramachandran J., Suyama A. T. Inhibition of replication of normal adrenocortical cells in culture by adrenocorticotropin. Proc Natl Acad Sci U S A. 1975 Jan;72(1):113–117. doi: 10.1073/pnas.72.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silber R. H. Fluorimetric analysis of corticoids. Methods Biochem Anal. 1966;14:63–78. doi: 10.1002/9780470110324.ch3. [DOI] [PubMed] [Google Scholar]


