Introduction
In recent years, outcomes for pediatric cardiac transplantation (PCTx) have steadily improved, with current 5-year overall survival rates estimated at 83%.1 This progress may be attributed to improvements in pre-transplant management, selection of donor hearts, surgical technique, prevention and treatment of rejection, and minimization of treatment-related adverse events. Despite these advances, children who receive cardiac transplants suffer significant morbidity and mortality, and there is considerable variability in outcomes, much of which cannot be explained by known clinical risk factors.
One source of inter-individual variability is genetic variation in the host. There is growing evidence that genomic variation leads to differences in immune response, response to therapies, and susceptibility to adverse outcomes such as malignancy and infection.2–4 This review will focus on the effect of variation in genes encoding enzymes, transporters and drug target molecules influencing drug response. After review of the foundational principles of pharmacogenomics, evidence for pharmacogenomic effects on cyclosporine, tacrolimus, azathioprine, mycophenolate mofetil (MMF), and the mammalian target of rapamycin (mTOR) inhibitors sirolimus and everolimus will be reviewed and clinical implications of these findings discussed. Given the limited nature of evidence from studies of PCTx patients, support will also be drawn from non-pediatric cardiac transplant research and pediatric renal transplantation. Genetic studies of non-pharmacogenes such as those in immunomodulatory pathways and variation in the donor genotype may contribute to risk prediction, but are beyond the scope of this review.
Principles of Pharmacogenomics
Genetic Variation
DNA variation can alter biologic function through several mechanisms (Figure 1). Variation within the coding sequence or in intron/exon borders can change the protein product through changes in start codons, stop codons, splice sites, or within the coding sequence, leading to nonsense mediated decay or altered protein function. Other variants associated with pharmacogenomic outcomes are not predicted to change the encoded protein. These synonymous and intergenic variants may have direct functional effects via altered regulation of expression, or they may serve as a marker for the presence of another functional variant. Thus, important pharmacogenomic variation can occur anywhere in the genome. Individual variants are denoted by their Reference SNP cluster ID (rsID, e.g. rs1045642). The pattern of variants in an allele defines the haplotype, indicated by the “star allele” designation (e.g. CYP3A5*3). The rates of occurrence for specific genotypes and haplotypes vary across populations; some variants are ancestry-specific, while others have been identified with varying frequency in all ancestries studied to date.
Figure 1.
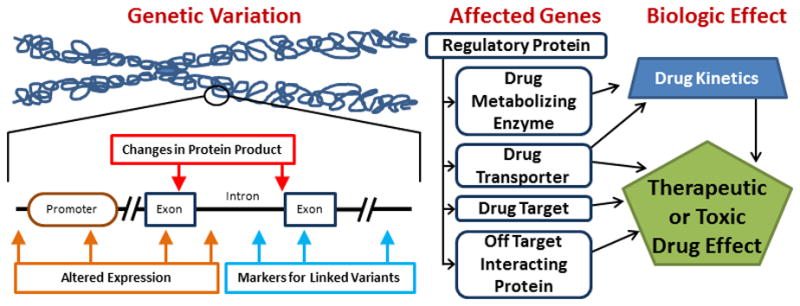
Mechanisms of Pharmacogenomic Effect. Several types of genetic variation may lead to alterations in gene products. Pharmacogenes include those coding for a variety of cellular functions, and may alter drug response via pharmacokinetic (absorption, distribution, metabolism or excretion) or pharmacodynamic (direct drug effect) pathways.
Pharmacogenomic Pathways
Pharmacogenomic variation may influence drug effect through pharmacokinetic mechanisms, affecting absorption, distribution, metabolism, and excretion, or via pharmacodynamic mechanisms, altering drug effects (Figure 1). Variations in drug metabolism genes are among the most well characterized sources of pharmacogenomic variability. For example, cytochrome P450 (CYP) 2D6 enzyme activity is required for O-demethylation of the prodrug codeine to the active metabolite, morphine. Due to genetic variation, a subset of the population has no CYP2D6 activity (“poor metabolizers”), resulting in greatly reduced morphine formation and insufficient pain relief.5,6 Conversely, individuals with multiple functional copies of CYP2D6 (“ultrarapid metabolizers”) rapidly convert codeine to morphine, causing symptoms similar to those of overdose. Variations in drug metabolism genes may also effect conversion of active drugs or metabolites to inactive compounds. Warfarin is inactivated by CYP2C9; individuals with loss-of-function CYP2C9 variants require lower doses of warfarin to reach target levels of anticoagulation.7
Pharmacogenomic variants are not restricted to drug metabolism genes, but can also be found in genes encoding drug transporters and targets. The OATP1B1 transporter (encoded by SLCO1B1) facilitates hepatocellular uptake of statin medications. Individuals with inactive OATP1B1 are at increased risk for muscle toxicity, specifically with high doses of simvastatin.8 Vitamin K epoxide reductase (VKORC1) is the target of warfarin. Variation in the promoter region of the VKORC1 gene affects gene transcription and thus modulates the warfarin dose required to achieve therapeutic effect.7 With increasing knowledge of the pathways regulating the expression and function of enzymes, transporters, and drug targets, variation in these important components of pharmacologic pathways are being discovered.
Pharmacogenomic Outcomes
Many pharmacogenomic studies have focused on pharmacokinetic outcomes such as drug concentration or dose to achieve therapeutic levels. These outcomes are clinically and biologically relevant, particularly for drugs with a narrow therapeutic index where maintenance of appropriate concentration is critical to achieve benefit without toxicity. However, pharmacodynamic studies of drug efficacy, taking serum drug levels into account, are required to determine genetic risk factors for ultimate clinical outcomes. Adverse drug events such as drug intolerance due to side effects or drug toxicity are also crucial events with individual differences in susceptibility, sometimes mediated by genetic variation (Figure 1). A complete personalized therapeutic plan must consider the full spectrum of drug effects, from therapeutic benefit to adverse event, in order to accurately determine the safest, most effective combination of agents.
Special Considerations for Pediatric Cardiac Transplantation
The vast majority of pharmacogenomic data are from adult studies. While genomes are stable throughout life, gene expression and function may vary with age. The developmental ontogeny of drug metabolism and response genes is a topic of active research, as pathways unique to children may contribute to individual differences in drug response. In addition, developmental changes in the pediatric age range can lead to specific drug effects and toxicities in children. For these reasons, it is important to validate pharmacogenomic associations in children rather than extrapolating data from adults.
The specific case of cardiac transplantation also demands consideration of factors unique to organ transplantation. After transplant, the patient has two genomes: their host genome, present in the majority of cells relevant to drug response including the liver, kidneys, immune cells, and vasculature; and the donor genome, present in the heart and passenger cells (e.g. leukocytes). Specific variants affecting drug action or toxicity via action in heart cells will be associated with donor, not host, genotype. The interaction of variants in the host and donor genomes is an important topic of current research, but with very limited information at this time in this patient population. Finally, given the need to balance risks for rejection vs. drug toxicity, a broad spectrum of clinical outcomes must be studied including rejection, cardiac function, graft loss and mortality, as well as infection, malignancy, and tissue / organ toxicities in order to fully evaluate the effects of variants altering cellular response to therapy.
Maintenance Immunosuppressive Agents in Pediatric Cardiac Transplantation
Although immune suppression protocols vary among centers, broad principles apply. Therapies include induction therapy (generally intravenous), maintenance therapies (usually multi-drug oral medications), and rescue therapy for acute rejection events. Induction therapy use is increasing; currently 71% of PCTx patients receive induction therapy with either interleukin-2 receptor antagonists or T cell depleting polyclonal anti-thymocyte antibodies.9 The goals of induction therapy include protection against early rejection and delayed introduction of nephrotoxic medications such as calcineurin inhibitors, as renal function is typically impaired in patients immediately after transplant. The influence of pharmacogenomic variation on the efficacy of induction therapies has not been reported for PCTx patients. However, use of these agents can influence the interpretation and application of pharmacogenomic associations for maintenance therapy as described below. Many studies reported herein were conducted in patients who did not receive induction therapy. Whether the influence of genotype on outcomes such as acute rejection events, long-term steroid dependency and drug toxicity is attenuated or amplified by induction regimens is the focus of ongoing research.
Medications used for maintenance of immunosuppression in PCTx patients include long-term use of a calcineurin inhibitor (CNI, tacrolimus or cyclosporine) in almost all cases. Tacrolimus is currently used in approximately 78% of patients at the time of initial hospital discharge (Table 1, Figure 2).9 Most centers also use an antimetabolite agent (MMF or azathioprine) as adjunctive therapy, with MMF being the most commonly prescribed adjunctive agent at the present time and the use of azathioprine on the decline. In some cases, a proliferation signal inhibitor (sirolimus or everolimus) is used as the adjunctive agent in lieu of MMF or azathioprine.1 These proliferation signal inhibitors are only very rarely used as primary immunosuppressant as there is insufficient evidence that they offer adequate protection against early rejection in the PCTx population. There is increasing use of steroid avoidance or early steroid withdrawal after pediatric transplantation due to significant adverse effects of long-term use of corticosteroids, including growth failure. Steroid avoidance may be facilitated by the use of induction therapy. For each of these agents, the clinical use profile, mechanism of action, and potential adverse events will be discussed, followed by a summary of pharmacogenomic associations to date based on review of published findings from cohorts of PCTx patients, pediatric renal transplant patients, and/or adult cardiac transplant patients (Table 2, Supplemental Table 1).
Table 1.
Maintenance Immunotherapy for Pediatric Cardiac Transplantation
| Drug Name | Clinical Use | Mechanism of Action | Side Effects* | Pharmaco-kinetic Pathways | Genes Associated with Drug Response |
|---|---|---|---|---|---|
| Tacrolimus | Most commonly used calcineurin inhibitor | Binds FK binding protein, causing inhibition of calcineurin, T-lymphocyte signal transduction, and IL-2 transcription | Nephrotoxicity, hypertension, dyslipidemia, neurotoxicity, glucose intolerance, diabetes mellitus, hyperkalemia, hypomagnesemia, gastrointestinal side effects | Substrate for ABCB1 transporter Metabolized by CYP3A enzymes |
ABCB1 CYP3A4 CYP3A5 |
| Cyclosporine | Calcineurin inhibitor with decreasing use | Binds cyclophilin, inhibits calcineurin, decreasing T- lymphocyte signal transduction and IL-2 transcription | Nephrotoxicity, hypertension, dyslipidemia, hypokalemia, hypomagnesemia, neurotoxicity, gingival hyperplasia, hirsutism, gastrointestinal side effects | Substrate for ABCB1 transporter Metabolized by CYP3A enzymes |
ABCB1 CYP3A5 NR1I2 |
| MMF/MPA | Increasing use, very common | Binds inosine-5′-monophosphate dehydrogenase (IMPDH) causing decreased B- and T-cell proliferation and antibody production | Gastrointestinal symptoms, bone marrow suppression | Metabolized by CES-1, CES-2, UGT enzymes Substrate for organic anion transport polypeptides |
ABCC2 IMPDH1 IMPDH2 UGT2B7 TNF-alpha |
| Azathioprine | Infrequent, decreasing use | Inhibits purine synthesis, decreasing proliferation of leukocytes and lymphocytes | Gastrointestinal side effects, bone marrow suppression | Metabolized by TPMT | TPMT |
| Sirolimus & Everolimus | Rarely used | Bind to the FK binding protein, blocking T-and B- cell activation via modulation of mammalian target of rapamycin (mTOR) | Bone marrow suppression, hyperlipidemia, gastrointestinal symptoms, progressive interstitial pneumonitis, nephrotoxicity; hemolytic uremic syndrome with cyclosporine | Substrate for ABCB1 transporter Metabolized by CYP3A4 |
None reported in pediatric patients |
All agents associated with risk of infection and malignancy
MMF – mycophenolate mofetil; MPA – mycophenolic acid
Figure 2.
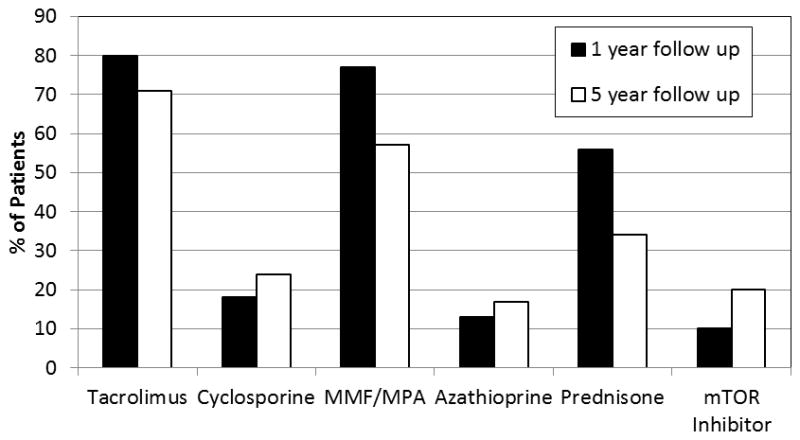
Maintenance Immunosuppressant Use in Pediatric Heart Transplantation. Maintenance immunosuppression at 1 year (black bars) and 5 year (white bars) follow up in pediatric heart transplant recipients with visits from 2007 onwards. Percent of all patients receiving each drug is plotted on the Y axis. Sum across medications is greater than 100% due to concomitant use of two or more medications. MMF – mycophenolate mofetil; MPA – mycophenolic acid; mTOR – mammalian target of rapamycin. Based on published data.9
Table 2.
Pharmacogenes and Variants in Pediatric Cardiac Transplantation
| Gene Name(s) | Protein Names | Cellular Functions | Pharmaco-variants | Alternate Variant Name(s) | Associations | MAF in EA* | MAF in AA† |
|---|---|---|---|---|---|---|---|
|
ABCB1 MDR1 P-gp |
ATP-binding cassette, sub-family B, member 1 | Membrane efflux pump and drug transporter | rs1045642 | C3435T Ile1145Ile |
CC homozygotes on tacrolimus with increased steroid dependency, lower tacrolimus concentration10–12 | 0.571 | 0.205 |
| rs2032582 | G2677C/T Ser893Ala/T hr |
GG homozygotes with lower tacrolimus concentration11 | 0.469 | 0.105 | |||
|
ABCC2 DJS MRP2 cMRP |
ATP-binding cassette, sub-family C, member 2 | Membrane efflux pump and drug transporter | rs717620 | −24C>T | CC homozygotes on MMF/MPA with lower discontinuation, less GI intolerance13 | 0.181 | 0.035 |
| CYP3A4 | Cytochrome P450, family 3, subfamily A, polypeptide 4 | Steroid hormone and drug metabolism | rs35599367 | CYP3A4*22 | *22 carriers with lower tacrolimus doses to reach target concentration14 | NR | NR |
| CYP3A5 | Cytochrome P450, family 3, subfamily A, polypeptide 5 | Steroid hormone and drug metabolism | rs776746 | CYP3A5*3 | *3 homozygotes with higher tacrolimus concentration, lower tacrolimus dose requirement, higher tacrolimus dose- adjusted concentration11,15 | 0.036 | 0.64 |
| IMPDH1 | Inosine 5′-monophosphate dehydrogenase 1 | Guanine nucleotide synthesis | rs2278294 | 250-106G>A | GG homozygotes on MMF/MPA with reduced dose-holding or discontinuation, less GI intolerance13 | 0.323 | 0.377 |
| rs2228075 | 1245G>A/C Ala492Ala |
GG homozygotes on MMF/MPA with less GI intolerance13 | 0.248 | 0.202 | |||
| IMPDH2 | Inosine 5′-monophosphate dehydrogenase 2 | Guanine nucleotide synthesis | rs11706052 | 819+10T>C | C carriers on MMF/MPA with possible increased bone marrow toxicity13 | 0.115 | 0.018 |
Based on HapMap CEU Population
Based on HapMap ASW Population
NR – Not reported in HapMap Data; MAF – Minor Allele Frequency; EA – European-Americans; AA – African-American; MMF – mycophenolate mofetil; MPA – mycophenolic acid
Tacrolimus
Tacrolimus, the most commonly used CNI in PCTx, is a macrolide antibiotic compound derived from the fungus Streptomyces tsukubaensis.1 Tacrolimus binds FK binding protein; this complex binds to and inhibits the phosphatase activity of the calcineurin-calmodulin complex, leading to decreased translocation of NF-AT transcription factors to the nucleus. This inhibits the transcription of cytokines including IL-2, IL-3, IL-4, TNF-alpha, granulocyte-macrophage colony-stimulating factor (GMCSF), and interferon-gamma, causing blunted T-cell activation and proliferation. Adverse events associated with tacrolimus include nephrotoxicity such as acute reversible azotemia, chronic irreversible renal disease, tubular disease or hemolytic uremic syndrome; hypertension; dyslipidemia; neurotoxicity such as tremor; metabolic derangements such as glucose intolerance, diabetes mellitus, hyperkalemia or hypomagnesemia; and gastrointestinal side effects such as anorexia, nausea, vomiting, diarrhea, and abdominal discomfort. Like all immunosuppressive agents, tacrolimus is also associated with increased risk for infection and malignancy.
Tacrolimus is inactivated in the liver by cytochrome P450 enzymes in the 3A family (CYP3A). Functional CYP3A alleles have been associated with more rapid tacrolimus inactivation and higher dose requirements in pediatric renal transplant patients16–21 and adult cardiac transplant patients.22,23 The most well characterized CYP3A variant is CYP3A5*3, which includes a splicing variant that prevents translation of active enzyme. One or more copies of the functional enzyme (encoded by CYP3A5*1) are present in the majority of African-American individuals, but homozygosity for the non-functional genotype, denoted by CYP3A5*3/*3, is more common among individuals of European and Asian descent and Hispanic Americans. Several studies have investigated the influence of CYP3A5*3 on tacrolimus disposition in PCTx, consistently finding significant associations of CYP3A5*3 with lower required tacrolimus doses and higher tacrolimus dose-adjusted trough levels.11,14,15 Gijsen et al. investigated the effect of CYP3A4*22 (defined by a variation in intron 6) and CYP3A5*3, finding that CYP3A poor metabolizers required 17% less tacrolimus than intermediate metabolizers and 48% less than extensive metabolizers.14
Tacrolimus is also a substrate for the drug transporter p-glycoprotein, encoded by the gene ABCB1. Homozygosity for the C allele for the synonymous rs1045642 variant has been associated with lower dose-adjusted serum tacrolimus levels and increased steroid dependency at one year post-transplant in cohorts of over 60 patients.10–12 The mechanism whereby this synonymous variant influences outcomes is uncertain. Patients homozygous for the missense G allele at rs2032582 had lower dose-adjusted tacrolimus levels measured at 6 and 12 months after transplantation.11 A smaller cohort did not find a statistically significant difference in tacrolimus dose adjusted concentrations or per-kilogram dose to achieve therapeutic goal by rs1045642, rs2032582, or rs1128503 genotype.15 In one study of 38 pediatric renal transplant patients, ABCB1 variants were associated with early post-transplant dose-adjusted tacrolimus levels, but other studies of pediatric renal and adult cardiac transplant patients found no effect.19,22,24 The inconsistent impact of ABCB1 variation on tacrolimus may be due to small sample size or unique genetic structure in specific populations. Alternately, the observation of increased steroid dependency with ABCB1 variation without differences in serum tacrolimus concentration led to the hypothesis that functional p-glycoprotein pumps tacrolimus out of the target cells, leading to decreased effect despite therapeutic blood levels.10
Cyclosporine
Cyclosporine, the older of the two CNIs, is an 11 amino acid cyclic peptide derived from the fungus Tolypocladium inflatum. Use is decreasing due to the side effect profile and data indicating higher incidence of rejection when compared to tacrolimus.1,25,26 Cyclosporine inhibits calcineurin through binding to cyclophilin, with downstream mechanisms as described for tacrolimus. Oral bioavailability is low because of poor absorption, metabolism by enzymes in the bowel mucosa, and first-pass hepatic metabolism, though improved with microemulsion formulations compared to the oil-based form. Toxicities are similar to tacrolimus, including infection, malignancy, nephrotoxicity, hypertension, dyslipidemia, hypokalemia, hypomagnesemia, tremor, and gastrointestinal side effects. The incidence of hypertension and hyperlipidemia may be higher than with tacrolimus, with lower risk of new onset diabetes mellitus, tremor, and gastrointestinal effects in some series. Cyclosporine is unlike tacrolimus in causing gingival hyperplasia and hirsutism.
Pharmacogenomic studies of cyclosporine in PCTx patients have not been reported, perhaps owing to the predominant use of tacrolimus in recent years. Cyclosporine is metabolized to inactive compounds by the CYP3A family of enzymes, and one study of 25 adult cardiac transplant patients reported an increase in dose- and weight-corrected cyclosporine concentrations in CYP3A5 poor metabolizers.22 However, in larger cohorts, including 87 teenagers and 104 pediatric patients who underwent renal transplant, CYP3A5*3 was not associated with variation in cyclosporine pharmacokinetics.19,27 Cyclosporine is also a substrate for p-glycoprotein; the influence of ABCB1 variants on cyclosporine pharmacokinetics have been studied in pediatric renal19,27,28 and adult cardiac transplant22,29,30 patients. In all three pediatric studies, ABCB1 genotype influenced cyclosporine concentrations, though in the adult cardiac transplant studies the effect was inconsistent and dependent on the time point studied.
An additional candidate gene, NR1I2, encodes the steroids and xenobiotics receptor (SXR) which regulates CYP3A4 expression. Three studies in pediatric renal transplant patients have demonstrated that carriers of rs3842689, a 6 base-pair deletion in the NR1I2 promoter, require lower cyclosporine doses.28,31,32
Mycophenolate mofetil and mycophenolate sodium
MMF is a prodrug that is rapidly metabolized to the active form, mycophenolic acid (MPA). Enteric-coated mycophenolate sodium delivers MPA in the small intestine. MPA reversibly inhibits inosine monophosphate dehydrogenase (IMPDH), which catalyzes purine synthesis. Because activated lymphocytes are dependent upon the de novo synthesis of purine nucleotides, IMPDH inhibition causes decreased B- and T-cell proliferation and decreased antibody production. MPA preferentially binds to IMPDH isoform type II, expressed in active lymphocytes. In addition to malignancy and infection (specifically CMV and herpes zoster infections), MMF toxicities include nausea, diarrhea, abdominal cramping, and bone marrow suppression. MMF is not nephrotoxic.
Published pharmacogenomic studies of MMF have focused on drug-related toxicities. Adverse events are common, especially in the infant population. In a cohort of 59 PCTx patients, those homozygous for A at rs11706052 in IMPDH2 experienced less bone marrow toxicity leading to MMF dose-holding, although the effect was no longer statistically significant after adjustment for age, race and gender.13 In the same patient population, homozygous G genotype at IMPDH1 rs2278294 or rs2228075 was associated with decreased rates of gastrointestinal toxicity.13 Use of the IMPDH1 haplotype did not lead to greater statistical confidence.33 These observations have not yet been validated in larger cohorts.
The organic anion transporter multi-drug resistance protein 2, encoded by ABCC2, is involved in the enterohepatic circulation of MPA. In the same 59 PCTx patients, ABCC2 rs717620 GG genotype was protective against MMF discontinuation secondary to gastrointestinal side effects, attributed to decreased enterohepatic circulation and lower intestinal MPA concentrations.13 In 290 patients enrolled in the Pediatric Heart Transplant Study, rs717620 GG genotype conferred increased risk of rejection with hemodynamic compromise and late rejection with hemodynamic compromise, consistent with lower drug exposure in this subset of patients.34 An evaluation of four ABCC2 variants (rs717620, rs2273697, rs8187694, and rs3740066) in 89 PCTx patients found no associations to MMF pharmacokinetics.35 A study of 32 adult cardiac transplant and 36 lung transplant recipients had mixed results with respect to ABCC2, finding no association for rs717620, although rs3740066 and rs17222723 in ABCC2 were associated with anemia and leukopenia.36
MPA is metabolized through phase II glucuronidation by UDP glucuronosyl transferases (UGT’s). In pediatric renal transplant patients and adult cardiac transplant patients, polymorphisms in UGT2B7 and UGT1A8 have influenced the metabolism, clearance, and side effect profile.35–37 The rs1800629 variant in TNF-alpha has also been studied in pediatric renal transplant patients treated with MMF and was associated with increased rates of myelotoxicity.38
Azathioprine
Azathioprine is an alternate antimetabolite agent used less frequently than MMF.1 A prodrug that is metabolized by glutathione in red blood cells to 6-mercaptopurine, this agent inhibits adenine and guanine ribonucleotide production, resulting in decreased numbers of circulating B and T lymphocytes, reduced immunoglobulin synthesis, and diminished interleukin-2 secretion. Thiopurine methyltransferase (TPMT) is important for 6-mercaptopurine metabolism. Side effects of azathioprine include gastrointestinal effects (anorexia, nausea, vomiting), infection, malignancy, and bone marrow suppression.
No pharmacogenomic studies of azathioprine in PCTx have been reported. In multiple other patient populations, TPMT variants conferring decreased enzyme function have been shown to increase formation of 6-thioguanine in patients treated with azathioprine, an outcome associated with higher risk for side effects such as bone marrow suppression.39–41 However, a recent study of 93 adult cardiac transplant patients paradoxically found that patients with decreased TMPT activity experienced earlier, more severe rejection without an increase in incidence of leukopenia;42 these data indicate that azathioprine may be less efficacious in patients with decreased TPMT activity, perhaps due to decreased production of active metabolites.42 Guidelines for the use of TPMT genotype data in clinical care have been published, and the FDA label recommends TPMT genotype or enzyme function testing in patients treated with azathioprine.43
Sirolimus & Everolimus
Sirolimus and everolimus are macrocyclic triene antibiotics that block the response of T- and B-cell activation by cytokines through the binding of FK binding protein and modulation of mTOR. These drugs have infrequent and decreasing use in PCTx.1 Toxicities include anemia, thrombocytopenia, leukopenia, hyperlipidemia, nephrotoxicity, progressive interstitial pneumonitis, and gastrointestinal effects (constipation, diarrhea, dyspepsia, nausea, vomiting). Delayed wound healing is sometimes observed, raising concerns about early use after transplantation surgery. When sirolimus is used in conjunction with cyclosporine, hemolytic uremic syndrome has been reported. These are substrates for CYP3A and p-glycoprotein. CYP3A5 and ABCB1 variants have been studied in adult cardiac transplant patients treated with everolimus (N=30 and 59 patients), but no significant associations of genotypes to pharmacokinetics were found.23,44
Clinical Implications
In the current literature, the evidence for the influence of pharmacogenetic variation on response to maintenance immunotherapy used in PCTx is sparse and in some cases contradictory. Consistent findings from multiple studies in distinct populations are required for clinical implementation of genotype-guided therapy. Consistent evidence for pharmacokinetic outcomes has been established for azathioprine and TPMT, and tacrolimus and CYP3A5. Additional data, pending validation, suggest that variation in drug transporters and drug targets affect drug disposition and effect, including ABCB1’s influence on tacrolimus and IMPDH effects on MMF intolerance. All of these data are limited by small sample size, evaluation of a limited number of variants, and lack of statistical correction for multiple comparisons. There are several unanswered questions regarding the clinical relevance of these associations. The impact of pharmacovariants on outcomes beyond drug trough concentrations has not been firmly established. Prior knowledge of drug metabolism genotype may facilitate individualized dosing to achieve early therapeutic concentrations and avoid toxic supratherapeutic levels; however, this effect has not been proven. The impact of pharmacovariants on clinical outcomes must be assessed. Given that both morbidity and mortality are increasingly due to medication toxicities rather than rejection, accurate prediction models for efficacy and toxicity have the potential to individualize therapy and further improve PCTx outcomes.
Potential Clinical Implementation: Tacrolimus and Azathioprine
The Pharmacogenomic Resource for Enhanced Decisions in Care & Treatment (PREDICT) program at Vanderbilt University Medical Center was launched to evaluate evidence for clinical pharmacogenomic testing, develop genotype-guided clinical decision support (CDS), and implement pharmacogenomic testing.45,46 Given the well-established associations of drug metabolism variants to tacrolimus and azathioprine disposition and the potential to reduce drug toxicity, the PREDICT program has implemented testing and CDS for CYP3A5 and TPMT variants. Beyond the burden of scientific evidence, obstacles to implementation include technical challenges (e.g. laboratory testing protocols and reporting, genotype data storage, development and maintenance of clinical decision support), practical challenges (e.g. test turnaround time, physician workflow, communication of results to patients and providers, reimbursement), and educational challenges (for both providers and patients). PREDICT provides proof-of-principle that preemptive genotyping and real time CDS can be operationalized in clinical practice, and offers the opportunity to evaluate the impact of pharmacogenomic testing.
For azathioprine, the FDA label recommends testing for TPMT genotype or phenotype and use of alternate agents or reduced dosage in patients with low or absent TPMT activity. Clinical guidelines for use of TPMT genotype or phenotype information for dosage adjustment of these drugs in adults and children have been published and are also available at the Pharmacogenomics Knowledgebase (PharmGKB).43,47,48 Given the low prevalence of nonfunctional TPMT alleles, patients rarely have two such alleles (1 in 178 to 1 in 3736 patients); however, intermediate TPMT activity due to heterozygosity for functional and nonfunctional alleles is much more common (3–14% of patients), and also merits dosage adjustment. Pre-prescription testing for TPMT status prior to initiation of azathioprine therapy is advocated for many clinical contexts.49–53 Through PREDICT, clinical testing for TPMT genotype is available at this institution, with CDS activated for patients with genotypes indicating low or intermediate TPMT activity (Figure 3A). A website has also been developed to provide genotype-specific information and a review of evidence for patients and providers outside of Vanderbilt.54
Figure 3.

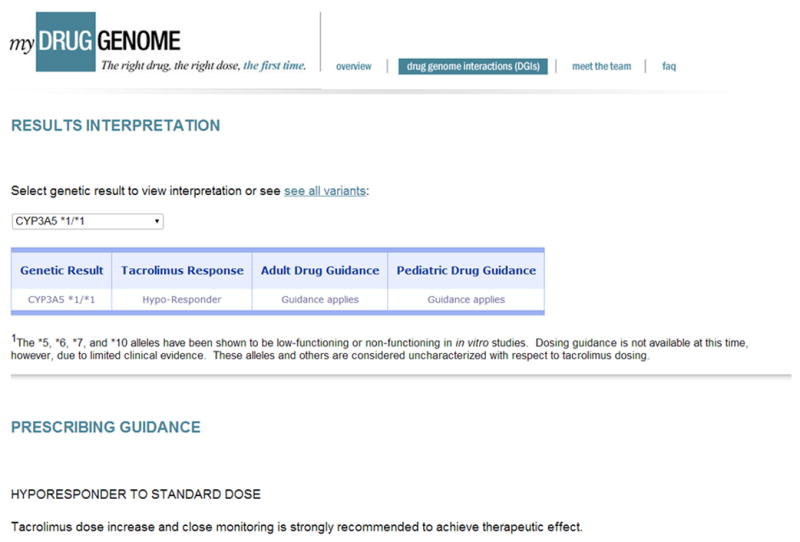
Clinical Implementation of Pharmacogenomics for Pediatric Cardiac Transplantation. A) Example of clinical decision support implemented by the Vanderbilt University Medical Center Pharmacogenomic Resource for Enhanced Decisions in Care & Treatment. This warning is shown in the inpatient order system for individuals with one functional and one nonfunctional TPMT allele if an order is entered for a thiopurine drug. B) Result interpretation and prescribing guidance for CYP3A5*1 homozygosity, available at MyDrugGenome.org.54
For tacrolimus, the presence of CYP3A5*1 genotype has been established to increase the tacrolimus dose required to achieve target drug concentrations in multiple populations, including children.11,14–18,20–23,55 In pediatric renal transplant patients, CYP3A5*1 was associated with lower tacrolimus serum concentration despite therapeutic drug monitoring, perhaps due to clinician hesitancy to prescribe the high doses of tacrolimus required in these patients.17,18 Through PREDICT, clinical CYP3A5 genotyping is available for both pediatric and adult patients receiving tacrolimus. CDS alerts providers of the potential for drug resistance in CYP3A5*1 carriers and homozygotes; further information is available at the MyDrugGenome.org website (Figure 3B).54
Biologic Factors Contributing to Health Disparity
Transplant outcomes have improved considerably, but significant differences in morbidity and mortality are evident when comparing outcomes across race and ethnicity, especially for late mortality rates.56–60 Many factors contribute to these disparities, including complex interactions of recipient-donor match, etiology of transplant, access to care, socioeconomic status, and cultural differences affecting health care delivery. However, some outcome disparity may be attributed to biological factors conferred by genetic differences. In comparison with Caucasian PCTx recipients, African-American patients have a higher burden of genetic variants predisposing to inflammation, which may require more intensive maintenance immunosuppression.61 In addition, African-American patients are more likely to be carriers of pharmacogenotypes associated with reduced drug availability and efficacy.61 Thus, it seems rational to prescribe tacrolimus dosing to African-American transplant recipients based on CYP3A genotype rather than an assumed ‘most likely’ genotype based on recipient race. Refinement of predictive models that incorporate clinical and genetic risk factors in order to personalize therapy holds particular promise to improve outcomes for at-risk populations.
Future Directions
Many of the limitations to the evidence base for pharmacogenomics in PCTx are due to small sample size. Multicenter consortia such as the Pediatric Heart Transplant Study have the potential to assemble and follow a large, diverse cohort to determine the impact of genetic variants on pharmacokinetics and pharmacodynamics through investigation of endpoints such as drug levels, drug toxicity, transplant rejection and mortality. Coupling such resources with modern statistical analyses (e.g. implementation of propensity score techniques), sophisticated biomarker analyses, and precise phenotyping for covariates and outcomes including adverse drug events will enable incorporation of dense clinical and genetic data to determine independent predictors of drug response and ultimate transplant outcomes. Evaluation of the impact of pharmacogenetic variants in the context of current therapeutic regimens, including induction agents, is ongoing. A key component for these studies is inclusion and study of patients at the highest risk for poor outcomes, including African-Americans. Validation of the effects of known variants and discovery of additional pharmacogenes may enable not only personalized care for these at-risk patients, but also lead to better understanding of the mechanisms of rejection and drug action, leading to novel therapies.
Conclusions
We have the technology to perform rapid, low-cost genotyping, sophisticated data analyses, and personalized CDS based on patient data, clinical parameters, and genotypes. The wide variability in PCTx outcomes, not predicted by clinical factors alone, provides the opportunity for implementation of pharmacogenomic testing and continued research. There is consistent evidence that genetic variation in CYP3A5 and TPMT alter the pharmacokinetics of tacrolimus and azathioprine, respectively, and the impact of preemptive pharmacogenomic testing for these genes on clinical outcomes in PCTx can only be established through application and evaluation of pharmacogenomic programs. There are many additional genes encoding drug metabolism enzymes, drug transporters, drug targets and regulatory genes relevant to PCTx recipients. We envision use of clinical and pharmacogenomic factors for personalizing therapeutic decisions for PCTx. This approach has the potential to minimize rejection events while avoiding the serious, and sometimes fatal, complications of immunosuppressive therapy.
Supplementary Material
Acknowledgments
Funding Sources: SLV is supported by a Pharmaceutical Research and Manufacturers of America Foundation Faculty Development Award in Clinical Pharmacology and by Clinical and Translational Science Award (CTSA) KL2 TR 000446 from the National Center for Advancing Translational Sciences, NIH. SAW is supported by 5P50 HL 074732 from the National Heart Lung and Blood Institute, NIH.
Footnotes
Conflict of Interest Disclosures: None
References
- 1.Dipchand AI, Kirk R, Mahle WT, Tresler MA, Naftel DC, Pahl E, Miyamoto SD, Blume E, Guleserian KJ, White-Williams C, Kirklin JK. Ten yr of pediatric heart transplantation: A report from the Pediatric Heart Transplant Study. Pediatr Transplant. 2013;17:99–111. doi: 10.1111/petr.12038. [DOI] [PubMed] [Google Scholar]
- 2.Manickaraj AK, Mital S. Personalized medicine in pediatric cardiology: do little changes make a big difference? Curr Opin Pediatr. 2012;24:584–591. doi: 10.1097/MOP.0b013e328357a4ea. [DOI] [PubMed] [Google Scholar]
- 3.Xie H-G. Personalized immunosuppressive therapy in pediatric heart transplantation: Progress, pitfalls and promises. Pharmacol Ther. 2010;126:146–158. doi: 10.1016/j.pharmthera.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 4.Burckart GJ, Amur S. Update on the clinical pharmacogenomics of organ transplantation. Pharmacogenomics. 2010;11:227–236. doi: 10.2217/pgs.09.177. [DOI] [PubMed] [Google Scholar]
- 5.Crews KR, Gaedigk A, Dunnenberger HM, Klein TE, Shen DD, Callaghan JT, Kharasch ED, Skaar TC. Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for codeine therapy in the context of cytochrome P450 2D6 (CYP2D6) genotype. Clin Pharmacol Ther. 2012;91:321–326. doi: 10.1038/clpt.2011.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crews KR, Gaedigk A, Dunnenberger HM, Leeder JS, Klein TE, Caudle KE, Haidar CE, Shen DD, Callaghan JT, Sadhasivam S, Prows CA, Kharasch ED, Skaar TC Clinical Pharmacogenetics Implementation Consortium. Clinical Pharmacogenetics Implementation Consortium guidelines for cytochrome P450 2D6 genotype and codeine therapy: 2014 update. Clin Pharmacol Ther. 2014;95:376–382. doi: 10.1038/clpt.2013.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson JA, Gong L, Whirl-Carrillo M, Gage BF, Scott SA, Stein CM, Anderson JL, Kimmel SE, Lee MTM, Pirmohamed M, Wadelius M, Klein TE, Altman RB. Clinical Pharmacogenetics Implementation Consortium Guidelines for CYP2C9 and VKORC1 genotypes and warfarin dosing. Clin Pharmacol Ther. 2011;90:625–629. doi: 10.1038/clpt.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilke RA, Ramsey LB, Johnson SG, Maxwell WD, McLeod HL, Voora D, Krauss RM, Roden DM, Feng Q, Cooper-Dehoff RM, Gong L, Klein TE, Wadelius M, Niemi M. The clinical pharmacogenomics implementation consortium: CPIC guideline for SLCO1B1 and simvastatin-induced myopathy. Clin Pharmacol Ther. 2012;92:112–117. doi: 10.1038/clpt.2012.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dipchand AI, Kirk R, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, Dobbels F, Lund LH, Rahmel AO, Yusen RD, Stehlik J International Society for Heart and Lung Transplantation. The Registry of the International Society for Heart and Lung Transplantation: sixteenth official pediatric heart transplantation report--2013; focus theme: age. J Heart Lung Transplant. 2013;32:979–988. doi: 10.1016/j.healun.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Zheng H, Webber S, Zeevi A, Schuetz E, Zhang J, Lamba J, Bowman P, Burckart GJ. The MDR1 polymorphisms at exons 21 and 26 predict steroid weaning in pediatric heart transplant patients. Hum Immunol. 2002;63:765–770. doi: 10.1016/s0198-8859(02)00426-3. [DOI] [PubMed] [Google Scholar]
- 11.Zheng H, Webber S, Zeevi A, Schuetz E, Zhang J, Bowman P, Boyle G, Law Y, Miller S, Lamba J, Burckart GJ. Tacrolimus dosing in pediatric heart transplant patients is related to CYP3A5 and MDR1 gene polymorphisms. Am J Transplant. 2003;3:477–483. doi: 10.1034/j.1600-6143.2003.00077.x. [DOI] [PubMed] [Google Scholar]
- 12.Zheng HX, Webber SA, Zeevi A, Schuetz E, Zhang J, Lamba J, Boyle GJ, Wilson JW, Burckart GJ. The impact of pharmacogenomic factors on steroid dependency in pediatric heart transplant patients using logistic regression analysis. Pediatr Transplant. 2004;8:551–557. doi: 10.1111/j.1399-3046.2004.00223.x. [DOI] [PubMed] [Google Scholar]
- 13.Ohmann EL, Burckart GJ, Brooks MM, Chen Y, Pravica V, Girnita DM, Zeevi A, Webber SA. Genetic polymorphisms influence mycophenolate mofetil–related adverse events in pediatric heart transplant patients. J Heart Lung Transplant. 2010;29:509–516. doi: 10.1016/j.healun.2009.11.602. [DOI] [PubMed] [Google Scholar]
- 14.Gijsen VM, van Schaik RH, Elens L, Soldin OP, Soldin SJ, Koren G, de Wildt SN. CYP3A4*22 and CYP3A combined genotypes both correlate with tacrolimus disposition in pediatric heart transplant recipients. Pharmacogenomics. 2013;14:1027–1036. doi: 10.2217/pgs.13.80. [DOI] [PubMed] [Google Scholar]
- 15.Gijsen V, Mital S, van Schaik RH, Soldin OP, Soldin SJ, van der Heiden IP, Nulman I, Koren G, de Wildt SN. Age and CYP3A5 genotype affect tacrolimus dosing requirements after transplant in pediatric heart recipients. J Heart Lung Transplant. 2011;30:1352–1359. doi: 10.1016/j.healun.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Wildt SN, van Schaik RHN, Soldin OP, Soldin SJ, Brojeni PY, van der Heiden IP, Parshuram C, Nulman I, Koren G. The interactions of age, genetics, and disease severity on tacrolimus dosing requirements after pediatric kidney and liver transplantation. Eur J Clin Pharmacol. 2011;67:1231–1241. doi: 10.1007/s00228-011-1083-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferraresso M, Tirelli A, Ghio L, Grillo P, Martina V, Torresani E, Edefonti A. Influence of the CYP3A5 genotype on tacrolimus pharmacokinetics and pharmacodynamics in young kidney transplant recipients. Pediatr Transplant. 2007;11:296–300. doi: 10.1111/j.1399-3046.2006.00662.x. [DOI] [PubMed] [Google Scholar]
- 18.Tirelli S, Ferraresso M, Ghio L, Meregalli E, Martina V, Belingheri M, Mattiello C, Torresani E, Edefonti A. The effect of CYP3A5 polymorphisms on the pharmacokinetics of tacrolimus in adolescent kidney transplant recipients. Med Sci Monit. 2008;14:CR251–254. [PubMed] [Google Scholar]
- 19.Turolo S, Tirelli AS, Ferraresso M, Ghio L, Belingheri M, Groppali E, Torresani E, Edefonti A. Frequencies and roles of CYP3A5, CYP3A4 and ABCB1 single nucleotide polymorphisms in Italian teenagers after kidney transplantation. Pharmacol Rep. 2010;62:1159–1169. doi: 10.1016/s1734-1140(10)70378-9. [DOI] [PubMed] [Google Scholar]
- 20.Zhao W, Fakhoury M, Baudouin V, Storme T, Maisin A, Deschênes G, Jacqz-Aigrain E. Population pharmacokinetics and pharmacogenetics of once daily prolonged-release formulation of tacrolimus in pediatric and adolescent kidney transplant recipients. Eur J Clin Pharmacol. 2013;69:189–195. doi: 10.1007/s00228-012-1330-6. [DOI] [PubMed] [Google Scholar]
- 21.Zhao W, Elie V, Roussey G, Brochard K, Niaudet P, Leroy V, Loirat C, Cochat P, Cloarec S, André JL, Garaix F, Bensman A, Fakhoury M, Jacqz-Aigrain E. Population pharmacokinetics and pharmacogenetics of tacrolimus in de novo pediatric kidney transplant recipients. Clin Pharmacol Ther. 2009;86:609–618. doi: 10.1038/clpt.2009.210. [DOI] [PubMed] [Google Scholar]
- 22.Jordán de Luna C, Herrero Cervera MJ, Sánchez Lázaro I, Almenar Bonet L, Poveda Andrés JL, Aliño Pellicer SF. Pharmacogenetic study of ABCB1 and CYP3A5 genes during the first year following heart transplantation regarding tacrolimus or cyclosporine levels. Transplant Proc. 2011;43:2241–2243. doi: 10.1016/j.transproceed.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 23.Kniepeiss D, Renner W, Trummer O, Wagner D, Wasler A, Khoschsorur GA, Truschnig-Wilders M, Tscheliessnigg K-H. The role of CYP3A5 genotypes in dose requirements of tacrolimus and everolimus after heart transplantation. Clin Transplant. 2011;25:146–150. doi: 10.1111/j.1399-0012.2009.01198.x. [DOI] [PubMed] [Google Scholar]
- 24.Shilbayeh S, Zmeili R, Almardini RI. The impact of CYP3A5 and MDR1 polymorphisms on tacrolimus dosage requirements and trough concentrations in pediatric renal transplant recipients. Saudi J Kidney Dis Transplant. 2013;24:1125–1136. doi: 10.4103/1319-2442.121268. [DOI] [PubMed] [Google Scholar]
- 25.Grimm M, Rinaldi M, Yonan NA, Arpesella G, Arizón Del Prado JM, Pulpón LA, Villemot JP, Frigerio M, Rodriguez Lambert JL, Crespo-Leiro MG, Almenar L, Duveau D, Ordonez-Fernandez A, Gandjbakhch J, Maccherini M, Laufer G. Superior prevention of acute rejection by tacrolimus vs. cyclosporine in heart transplant recipients--a large European trial. Am J Transplant. 2006;6:1387–1397. doi: 10.1111/j.1600-6143.2006.01300.x. [DOI] [PubMed] [Google Scholar]
- 26.Irving CA, Webber SA. Immunosuppression therapy for pediatric heart transplantation. Curr Treat Options Cardiovasc Med. 2010;12:489–502. doi: 10.1007/s11936-010-0085-6. [DOI] [PubMed] [Google Scholar]
- 27.Fanta S, Niemi M, Jönsson S, Karlsson MO, Holmberg C, Neuvonen PJ, Hoppu K, Backman JT. Pharmacogenetics of cyclosporine in children suggests an age-dependent influence of ABCB1 polymorphisms. Pharmacogenet Genomics. 2008;18:77–90. doi: 10.1097/FPC.0b013e3282f3ef72. [DOI] [PubMed] [Google Scholar]
- 28.Ferraresso M, Belingheri M, Turolo S, Ghio L, Tirelli AS, Grillo P, Lepore M, Edefonti A. Long-term effects of ABCB1 and SXR SNPs on the systemic exposure to cyclosporine in pediatric kidney transplant patients. Pharmacogenomics. 2013;14:1605–1613. doi: 10.2217/pgs.13.148. [DOI] [PubMed] [Google Scholar]
- 29.Herrero MJ, Almenar L, Jordán C, Sánchez I, Poveda JL, Aliño SF. Clinical interest of pharmacogenetic polymorphisms in the immunosuppressive treatment after heart transplantation. Transplant Proc. 2010;42:3181–3182. doi: 10.1016/j.transproceed.2010.05.129. [DOI] [PubMed] [Google Scholar]
- 30.Taegtmeyer AB, Breen JB, Smith J, Burke M, Leaver N, Pantelidis P, Lyster H, Yacoub MH, Barton PJR, Banner NR. ATP-binding cassette subfamily B member 1 polymorphisms do not determine cyclosporin exposure, acute rejection or nephrotoxicity after heart transplantation. Transplantation. 2010;89:75–82. doi: 10.1097/TP.0b013e3181c342fd. [DOI] [PubMed] [Google Scholar]
- 31.Fanta S, Jönsson S, Karlsson MO, Niemi M, Holmberg C, Hoppu K, Backman JT. Long-term changes in cyclosporine pharmacokinetics after renal transplantation in children: evidence for saturable presystemic metabolism and effect of NR1I2 polymorphism. J Clin Pharmacol. 2010;50:581–597. doi: 10.1177/0091270009348223. [DOI] [PubMed] [Google Scholar]
- 32.Ferraresso M, Turolo S, Belinghieri M, Tirelli AS, Grillo P, Groppali E, Edefonti A, Ghio L. The potential of steroids and xenobiotic receptor polymorphisms in forecasting cyclosporine pharmacokinetic variability in young kidney transplant recipients. Pediatr Transplant. 2012;16:658–663. doi: 10.1111/j.1399-3046.2012.01751.x. [DOI] [PubMed] [Google Scholar]
- 33.Ohmann EL, Burckart GJ, Chen Y, Pravica V, Brooks MM, Zeevi A, Webber SA. Inosine 5′-monophosphate dehydrogenase 1 haplotypes and association with mycophenolate mofetil gastrointestinal intolerance in pediatric heart transplant patients. Pediatr Transplant. 2010;14:891–895. doi: 10.1111/j.1399-3046.2010.01367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burckart GJ, Figg WD, 2nd, Brooks MM, Green DJ, Troutman SM, Ferrell R, Chinnock R, Canter C, Addonizio L, Bernstein D, Kirklin JK, Naftel D, Price DK, Sissung TM, Girnita DM, Zeevi A, Webber SA. Multi-institutional Study of Outcomes After Pediatric Heart Transplantation: Candidate Gene Polymorphism Analysis of ABCC2. J Pediatr Pharmacol Ther. 2014;19:16–24. doi: 10.5863/1551-6776-19.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao W, Fakhoury M, Deschênes G, Roussey G, Brochard K, Niaudet P, Tsimaratos M, André JL, Cloarec S, Cochat P, Bensman A, Azougagh S, Jacqz-Aigrain E. Population pharmacokinetics and pharmacogenetics of mycophenolic acid following administration of mycophenolate mofetil in de novo pediatric renal-transplant patients. J Clin Pharmacol. 2010;50:1280–1291. doi: 10.1177/0091270009357429. [DOI] [PubMed] [Google Scholar]
- 36.Ting LSL, Benoit-Biancamano M-O, Bernard O, Riggs KW, Guillemette C, Ensom MHH. Pharmacogenetic impact of UDP-glucuronosyltransferase metabolic pathway and multidrug resistance-associated protein 2 transport pathway on mycophenolic acid in thoracic transplant recipients: an exploratory study. Pharmacotherapy. 2010;30:1097–1108. doi: 10.1592/phco.30.11.1097. [DOI] [PubMed] [Google Scholar]
- 37.Woillard J-B, Rerolle J-P, Picard N, Rousseau A, Drouet M, Munteanu E, Essig M, Marquet P, Le Meur Y. Risk of diarrhoea in a long-term cohort of renal transplant patients given mycophenolate mofetil: the significant role of the UGT1A8 2 variant allele. Br J Clin Pharmacol. 2010;69:675–683. doi: 10.1111/j.1365-2125.2010.03625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grenda R, Prokurat S, Ciechanowicz A, Piatosa B, Kalici ski P. Evaluation of the genetic background of standard-immunosuppressant-related toxicity in a cohort of 200 paediatric renal allograft recipients--a retrospective study. Ann Transplant. 2009;14:18–24. [PubMed] [Google Scholar]
- 39.Dervieux T, Médard Y, Baudouin V, Maisin A, Zhang D, Broly F, Loirat C, Jacqz-Aigrain E. Thiopurine methyltransferase activity and its relationship to the occurrence of rejection episodes in paediatric renal transplant recipients treated with azathioprine. Br J Clin Pharmacol. 1999;48:793–800. doi: 10.1046/j.1365-2125.1999.00087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lennard L, Lilleyman JS, Van Loon J, Weinshilboum RM. Genetic variation in response to 6-mercaptopurine for childhood acute lymphoblastic leukaemia. Lancet. 1990;336:225–229. doi: 10.1016/0140-6736(90)91745-v. [DOI] [PubMed] [Google Scholar]
- 41.Schütz E, Gummert J, Armstrong VW, Mohr FW, Oellerich M. Azathioprine pharmacogenetics: the relationship between 6-thioguanine nucleotides and thiopurine methyltransferase in patients after heart and kidney transplantation. Eur J Clin Chem Clin Biochem. 1996;34:199–205. doi: 10.1515/cclm.1996.34.3.199. [DOI] [PubMed] [Google Scholar]
- 42.Liang JJ, Geske JR, Boilson BA, Frantz RP, Edwards BS, Kushwaha SS, Kremers WK, Weinshilboum RM, Pereira NL. TPMT genetic variants are associated with increased rejection with azathioprine use in heart transplantation. Pharmacogenet Genomics. 2013;23:658–665. doi: 10.1097/FPC.0000000000000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Relling MV, Gardner EE, Sandborn WJ, Schmiegelow K, Pui C-H, Yee SW, Stein CM, Carrillo M, Evans WE, Hicks JK, Schwab M, Klein TE. Clinical pharmacogenetics implementation consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing: 2013 update. Clin Pharmacol Ther. 2013;93:324–325. doi: 10.1038/clpt.2013.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lemaitre F, Bezian E, Goldwirt L, Fernandez C, Farinotti R, Varnous S, Urien S, Antignac M. Population pharmacokinetics of everolimus in cardiac recipients: comedications, ABCB1, and CYP3A5 polymorphisms. Ther Drug Monit. 2012;34:686–694. doi: 10.1097/FTD.0b013e318273c899. [DOI] [PubMed] [Google Scholar]
- 45.Pulley JM, Denny JC, Peterson JF, Bernard GR, Vnencak-Jones CL, Ramirez AH, Delaney JT, Bowton E, Brothers K, Johnson K, Crawford DC, Schildcrout J, Masys DR, Dilks HH, Wilke RA, Clayton EW, Shultz E, Laposata M, McPherson J, Jirjis JN, Roden DM. Operational Implementation of Prospective Genotyping for Personalized Medicine: The Design of the Vanderbilt PREDICT Project. Clin Pharmacol Ther. 2012;92:87–95. doi: 10.1038/clpt.2011.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Driest SL, Shi Y, Bowton EA, Schildcrout JS, Peterson JF, Pulley J, Denny JC, Roden DM. Clinically actionable genotypes among 10,000 patients with preemptive pharmacogenomic testing. Clin Pharmacol Ther. 2014;95:423–431. doi: 10.1038/clpt.2013.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.The Pharmacogenomics Knowledge Base [PharmGKB] [Internet] [cited 2014 Feb 26];Available from: http://www.pharmgkb.org/index.jsp.
- 48.Relling MV, Gardner EE, Sandborn WJ, Schmiegelow K, Pui C-H, Yee SW, Stein CM, Carrillo M, Evans WE, Klein TE. Clinical Pharmacogenetics Implementation Consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing. Clin Pharmacol Ther. 2011;89:387–391. doi: 10.1038/clpt.2010.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Teml A, Schaeffeler E, Herrlinger KR, Klotz U, Schwab M. Thiopurine treatment in inflammatory bowel disease: clinical pharmacology and implication of pharmacogenetically guided dosing. Clin Pharmacokinet. 2007;46:187–208. doi: 10.2165/00003088-200746030-00001. [DOI] [PubMed] [Google Scholar]
- 50.Gurwitz D, Rodríguez-Antona C, Payne K, Newman W, Gisbert JP, de Mesa EG, Ibarreta D. Improving pharmacovigilance in Europe: TPMT genotyping and phenotyping in the UK and Spain. Eur J Hum Genet. 2009;17:991–998. doi: 10.1038/ejhg.2009.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fargher EA, Tricker K, Newman W, Elliott R, Roberts SA, Shaffer JL, Bruce I, Payne K. Current use of pharmacogenetic testing: a national survey of thiopurine methyltransferase testing prior to azathioprine prescription. J Clin Pharm Ther. 2007;32:187–195. doi: 10.1111/j.1365-2710.2007.00805.x. [DOI] [PubMed] [Google Scholar]
- 52.Schmiegelow K, Forestier E, Kristinsson J, Söderhäll S, Vettenranta K, Weinshilboum R, Wesenberg F Nordic Society of Paediatric Haematology and Oncology. Thiopurine methyltransferase activity is related to the risk of relapse of childhood acute lymphoblastic leukemia: results from the NOPHO ALL-92 study. Leukemia. 2009;23:557–564. doi: 10.1038/leu.2008.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Relling MV, Altman RB, Goetz MP, Evans WE. Clinical implementation of pharmacogenomics: overcoming genetic exceptionalism. Lancet Oncol. 2010;11:507–509. doi: 10.1016/S1470-2045(10)70097-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.MyDrugGenome.org [Internet] [cited 2014 Aug 26]; Available from: http://www.mydruggenome.org/overview.php.
- 55.Birdwell KA, Grady B, Choi L, Xu H, Bian A, Denny JC, Jiang M, Vranic G, Basford M, Cowan JD, Richardson DM, Robinson MP, Ikizler TA, Ritchie MD, Stein CM, Haas DW. The use of a DNA biobank linked to electronic medical records to characterize pharmacogenomic predictors of tacrolimus dose requirement in kidney transplant recipients. Pharmacogenet Genomics. 2012;22:32–42. doi: 10.1097/FPC.0b013e32834e1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Singh TP, Almond CS, Gauvreau K. Improved survival in pediatric heart transplant recipients: have white, black and Hispanic children benefited equally? Am J Transplant. 2011;11:120–128. doi: 10.1111/j.1600-6143.2010.03357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Singh TP, Naftel DC, Addonizio L, Mahle W, Foushee MT, Zangwill S, Blume ED, Kirklin JK, Singh R, Johnston JK, Chinnock R. Association of race and socioeconomic position with outcomes in pediatric heart transplant recipients. Am J Transplant. 2010;10:2116–2123. doi: 10.1111/j.1600-6143.2010.03241.x. [DOI] [PubMed] [Google Scholar]
- 58.Singh TP, Almond C, Givertz MM, Piercey G, Gauvreau K. Improved survival in heart transplant recipients in the United States: racial differences in era effect. Circ Heart Fail. 2011;4:153–160. doi: 10.1161/CIRCHEARTFAILURE.110.957829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cohen O, De La Zerda D, Beygui RE, Hekmat D, Laks H. Ethnicity as a predictor of graft longevity and recipient mortality in heart transplantation. Transplant Proc. 2007;39:3297–3302. doi: 10.1016/j.transproceed.2007.06.086. [DOI] [PubMed] [Google Scholar]
- 60.Flattery MP, Baker KM. Evidence for racial disparity in cardiac transplantation survival rates. J Cult Divers. 2004;11:25–30. [PubMed] [Google Scholar]
- 61.Girnita DM, Webber SA, Ferrell R, Burckart GJ, Brooks MM, McDade KK, Chinnock R, Canter C, Addonizio L, Bernstein D, Kirklin JK, Girnita AL, Zeevi A. Disparate distribution of 16 candidate single nucleotide polymorphisms among racial and ethnic groups of pediatric heart transplant patients. Transplantation. 2006;82:1774–1780. doi: 10.1097/01.tp.0000250656.33731.08. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


