Abstract
Potassium 8-R1-9-R2-10-R3-3-R-2-oxo-2H-[1,2,4]triazino[2,3-c]quinazoline-6-thiolates 2.1–2.26 were synthesized via cyclocondensation of 6-R-3-(3-R1-4-R2-5-R3-aminophenyl)-1,2,4-triazin-5-ones 1.1–1.26 with carbon disulfide, potassium hydroxide, and ethanol or with potassium O-ethyl dithiocarbonate in 2-propanol. The corresponding thiones 3.1–3.26 were obtained by treatment of 2.1–2.26 with hydrochloric acid. It was found that the nature of the substituents in positions 3, 4, and 5 of the corresponding 6-R-3-(3-R1-4-R2-5-R3-aminophenyl)-1,2,4-triazin-5-ones were affected on the terms of the reaction. The structures of compounds were proven by a complex of physicochemical methods (1H, 13C NMR, LC–MS, and EI-MS). The results of the antibacterial and antifungal activity assay allowed the determination of the high sensitivity of Staphylococcus aureus ATCC 25923 (MIC 6.25–100 μg/mL, MBC 12.5–200 μg/mL) to the synthesized compounds.
Keywords: Synthesis; Potassium salt; 6-Thioxo-6,7-dihydro-2H-[1,2,4]triazino[2,3-c]quinazolin-2-one derivatives; Antimicrobial activity
Introduction
Native and synthetic quinazoline derivatives are some of the priority objects of investigation in current organic and pharmaceutical chemistry. A lot of attention over the mentioned class of compounds caused by the broad potential of its chemical modification is aimed at the synthesis of the novel perspective potential medications [1–4]. Recent publications describe the synthesis and biological activity of the [1, 2, 4]triazino[2, 3-c]-quinazoline series [5–13]. It is known that the introduction of a thio-group in position 6 of the [1, 2, 4]triazino[2, 3-c]quinazoline system allows the obtainment of compounds with significant cytotoxic action against Photobacterium leiognathi [9, 11–13] and antimicrobial action against Staphyloccocus aureus and Aspergillus niger [8–13]. The following structure modification of 6-thio-3-R-2H-[1, 2, 4]triazino[2, 3-c]quinazoline-2-ones by synthesis of S-substituted derivatives enabled the obtainment of compounds with anticancer action [9, –13]. Authors noted that the above-mentioned type of action depends substantially on the nature of the pharmacophore fragment in positions 3 and 6. Adhering to the previously developed strategies of the target synthesis of chemotherapeutic agents, we decided to realize further structure modifications of 6-thio-3-R-2H-[1, 2, 4]triazino[2, 3-c]-quinazoline-2-ones by the introduction of halogen and methyl substituents in positions 8, 9, 10 and study the antibacterial and antifungal activities of the synthesized compounds. Thus, this work aimed to study the influence of substituents (alkyl and halogen groups) in 6-R-3-(2-aminophenyl)-2H-[1, 2, 4]triazin-5-ones on the cyclocondensation process and on the antibacterial and antifungal activities of the synthesized compounds.
Results and Discussion
Chemistry
As starting compounds we used 6-R-3-(3-R1-4-R2-5-R3-2-aminophenyl)-1,2,4-triazin-5-ones (1.1–1.26), which were obtained according to known protocols, namely by nucleophilic cleavage of the pyrimidine fragment in 3-R-8-R1-9-R2-10-R3-2H-[1, 2, 4]triazino[2, 3-c]quinazolin-2-ones or hydrazinolysis of 2-aryl-[(3H-quinazolin-4-ylidene)hydrazono]acetic acids esters [14]. Synthesis of potassium thiolates 2.1–2.26 was performed by the interaction of initial compounds 1.1–1.26 with sulfur disulfide, ethanol, and potassium hydroxide in ethanol (Method A) or potassium O-ethyl dithiocarbonate in 2-propanol [8] 1.1–1.26 (Method B, Scheme 1). The products of the mentioned cyclocondensation were the individual compounds 2.1–2.26 with yields of 64-99%. Method B had some advantages: ease of execution, health safety, high yields, and purity of the final products. To prove the structure, the synthesized thiolates 2.1–2.26 were transformed in the corresponding thions 3.1–3.26 by acidifying the water solutions of the mentioned potassium salts with hydrochloric acid to pH 2–3. We noted that the substituents in positions 3, 4, and 5 in the corresponding 6-R-3-(2-aminophenyl)-1,2,4-triazino-5-ones (1.1–1.26) significantly affected the reaction process. So, according to LC-MS (APCI), the initial compounds, which contained the substituents chlorine, bromine, or iodine that were needed, increased the duration of heating to 8–10 hours.
Sch. 1.
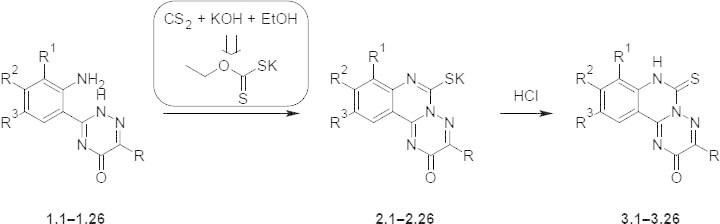
Synthesis of the potassium 3-R-8-R1-9-R2-10-R3-2-oxo-2H-[1, 2, 4]triazino[2, 3-c]-quinazoline-6-thiolates 2.1–2.26 and 3-R-8-R1-9-R2-10-R3-6-thioxo-6,7-dihydro-2H-[1, 2, 4]triazino[2, 3-c]quinazolin-2-ones 3.1–3.26
The purity of the synthesized compounds was confirmed by LC-MS (APCI) data, and the structure by IR-, 1H-, 13C NMR-, mass-spectra, and elemental analysis. In the LC-MS (APCI) spectra of 3.1–3.26, the positive ions [M+1] and [M+3] (sulfur isotope) were observed. The value of the molecular mass coincided with the expected for the synthesized compounds. The mass-spectra (EI) of thiones 3.1–3.4, 3.8 had some traits and substantial differences from the other heteroaromatic sulfides. In this case, molecular ion fragmentation under the electron impact pass on the C(2)–C(3) and N(4)–N(5) bond followed by the elimination of the nitrile radical and formation of an ion with m/z 203 had the highest intensity (96.7–100%). The mentioned ion underwent cleavage with the elimination of S, SH, CNS, CHNS, and CNO fragments and formation of an ion with m/z 171, 170, 145, 144, 161 with the corresponding intensity.
Our attempt to record the NMR spectral data for potassium 8-R1-9-R2-10-R3-3-R-2-oxo-2H-[1, 2, 4]triazino[2, 3-c]quinazoline-6-thiolates in DMSO-d6, CDCl3, and D2O failed. We considered this as a consequence of the exchange processes, tautomeric transformations, and insufficient solubility. However, for the evaluation of the mentioned compound structure, we converted them into thiones 3.1–3.26 and recorded all of the necessary spectral data to prove their structures. So, as we considered, the confirmation of the structures 3.1–3.26 in combination with the IR and elemental analysis data, proved the structures of compounds 2.1–2.26.
The 1H-NMR-spectra of compounds 3.1–3.26 are characterized by singlet signals of the thioamide group proton at 14.22–13.79 ppm and aromatic protons of the triazinoquinazoline system with corresponding chemical shifts [16]. The appearance at 171.05–168.79 ppm and 158.68-160.2 ppm of the characteristic signals of deshielded C-2 and C-6 carbons in the 13C NMR-spectra of compounds 3.1, 3.3, 3.4, and 3.8 confirm the formation of the new heterocyclic system. Also in the 13C NMR-spectra of the mentioned compounds, the signals of the corresponding aliphatic carbons are present. As we considered, the used physicochemical methods completely confirm the structure of the synthesized compounds.
The IR-spectra of compounds 3.1–3.26 are characterized by intensive absorption at 1767–1590 cm-1, which correspond to vibrations of the C=S and C=O groups and substantially differentiate them from their initial compounds. At the same time in the IR-spectra of thiolates (2.1–2.26), the mentioned signals were subjected to the hypsochromic shift which may be explained by the formation of an ion bond. The vibrations of C=C bonds of the aromatic fragment at 1589–1468 cm-1 of the non-plate deforming vibrations of the =C-H bond at 850–666 cm-1 and intensive signals at 2960–2850 cm-1, caused by the symmetric and asymmetric vibrations of CH2 and CH3 groups, are also present in the IR-spectra of the compounds 2.1–2.26 and 3.1–3.26. The IR-spectra of compounds, which contain halogen intensive signals of vC-F, vC-CI, vC-Br, vC-l, are also present.
Tab. 1.
Antimicrobial activities of potassium 8-R1-9-R2-10-R3-3-R-2-oxo-2H-[1, 2, 4]triazino[2, 3-c]quinazoline-6-thiolates
| Comp. | R | R1 = R2 = R3 = H (if not specified) | Investigated strains | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| E. coli | S. aureus | P. aeruginosa | C. albicans | |||||||
| MIC μg/mL | MBC μg/mL | MIC μg/mL | MBC μg/mL | MIC μg/mL | MBC μg/mL | MIC μg/mL | MBC μg/mL | |||
| 2.1 | CH3 | 100 | 100 | 50 | 200 | 50 | 200 | 100 | 100 | |
| 2.2 | C6H5 | 100 | 200 | 25 | 100 | 100 | 200 | 50 | 100 | |
| 2.3 | C6H4(CH3)-p | 50 | 100 | 12.5 | 100 | 100 | 200 | 100 | 100 | |
| 2.4 | C6H4(CH3)2-3,4 | 100 | 100 | 100 | 200 | 50 | 100 | 50 | 100 | |
| 2.5 | C6H4(C2H5)-p | 100 | 100 | 12.5 | 50 | 100 | 100 | 50 | 100 | |
| 2.6 | C6H4(i-C3H7)-p | 100 | 100 | 25 | 50 | 50 | 100 | 50 | 100 | |
| 2.7 | C6H4(tert-C4H9)-p | 50 | 100 | 6.25 | 12.5 | 50 | 100 | 100 | 100 | |
| 2.8 | C6H4(OCH3)-p | 100 | 200 | 100 | 200 | 50 | 200 | 50 | 100 | |
| 2.9 | C6H4(OC2H5)-p | 100 | 100 | 25 | 100 | 50 | 100 | 50 | 100 | |
| 2.10 | C6H4F-p | 50 | 50 | 12.5 | 50 | 50 | 100 | 50 | 100 | |
| 2.11 | C6H5 | R1 = CH3 | 100 | 100 | 25 | 100 | 50 | 200 | 50 | 100 |
| 2.12 | C6H5 | R2= F | 100 | 200 | 6.25 | 25 | 100 | 200 | 50 | 50 |
| 2.13 | C6H4F-p | R2= F | 100 | 200 | 6.25 | 25 | 50 | 100 | 50 | 100 |
| 2.15 | C6H5 | R3 = Cl | 100 | 200 | 6.25 | 100 | 50 | 100 | 50 | 50 |
| 2.16 | C6H4(CH3)-p | R3 = Cl | 100 | 200 | 6.25 | 100 | 100 | 100 | 50 | 100 |
| 2.17 | C6H4(OCH3)-p | R3 = Cl | 100 | 200 | 12.5 | 100 | 50 | 100 | 100 | 100 |
| 2.18 | C6H4F-p | R3 = Cl | 100 | 200 | 12.5 | 50 | 100 | 200 | 50 | 50 |
| 2.19 | C6H4F-p | R1 = Br | 100 | 200 | 12.5 | 25 | 50 | 100 | 50 | 100 |
| 2.20 | C6H4F-p | R2 = Br | 100 | 200 | 6.25 | 25 | 100 | 200 | 50 | 100 |
| 2.21 | C6H5 | R3 = Br | 100 | 200 | 6.25 | 50 | 50 | 200 | 50 | 50 |
| 2.22 | C6H4(CH3)-p | R3 = Br | 100 | 200 | 12.5 | 50 | 50 | 100 | 50 | 50 |
| 2.23 | C6H4F-p | R3 = Br | 100 | 200 | 6.25 | 25 | 50 | 200 | 50 | 50 |
| 2.24 | C6H4(OCH3)-p | R3 = Br | 100 | 200 | 12.5 | 200 | 100 | 100 | 100 | 100 |
| 2.25 | C6H4F-p | R3 = 1 | 100 | 200 | 6.25 | 200 | 100 | 200 | 50 | 50 |
| 2.26 | C6H4(OCH3)-p | R3 = l | 100 | 200 | 50 | 200 | 100 | 100 | 50 | 50 |
| Trimethoprim | – | 50 | 50 | 31.2 | 62.5 | 62.5 | 125 | 62.5 | 125 | |
| Nitrofural | – | 1.5 | – | 6.25 | – | 6.25 | – | 25.0 | – | |
Antimicrobial Activities
Results of the conducted microbiological screening showed that 3-R-8-R1-9-R2-10-R3-6-thioxo-6,7-dihydro-2H-[1, 2, 4]triazino[2, 3-c]quinazolin-2-ones (3.1–3.26) exhibit moderate inhibitory activity (MIC 50–100 μg/mL, Tab. 1) on the Escherichia coli strain (lower than Nitrofural (MIC 1.5 μg/mL), similar to Trimetoprim (MIC 50 μg/mL)).
It is important to note that potassium thiolates 2.1–2.26, as more water-soluble in most cases, are less active (MIC 100 μg/mL, Table 2). The antimicrobial activity of compounds 2.1–2.26 and 3.1–3.26 towards the strain of Pseudomonas aeruginosa is also moderate (MIC 50–100 μg/mL) and substantially inferior to the inhibitory action of Nitrofural (MIC 6.25 μg/mL, tables 1 and 2).
Tab. 2.
Antimicrobial activities 8-R1-9-R2-10-R3-3-R-6-thio-6,7-dihydro-2H-[1, 2, 4]triazino[2, 3-c]quinazoline-2-ones (3.1–3.26)
| Comp. | R | R1 = R2 = R3 = H (if not specified) | Investigated strains | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| E. coli | S. aureus | P. aeruginosa | C. albicans | |||||||
| MIC μg/mL | MBC μg/mL | MIC μg/mL | MBC μg/mL | MIC μg/mL | MBC μg/mL | MIC μg/mL | MBC μg/mL | |||
| 3.1 | CH3 | 100 | 100 | 100 | 200 | 50 | 200 | 100 | 100 | |
| 3.2 | C6H5 | 50 | 100 | 12.5 | 50 | 100 | 100 | 100 | 100 | |
| 3.3 | C6H4(CH3)-p | 50 | 50 | 12.5 | 100 | 50 | 100 | 50 | 50 | |
| 3.4 | C6H4(CH3)2-3,4 | 50 | 100 | 100 | 200 | 50 | 100 | 50 | 100 | |
| 3.5 | C6H4(C2H5)-p | 50 | 50 | 12.5 | 25 | 50 | 100 | 100 | 100 | |
| 3.6 | C6H4(i-C3H7)-p | 50 | 100 | 12.5 | 50 | 50 | 100 | 100 | 100 | |
| 3.7 | C6H4(tert-C4H9)-p | 50 | 100 | 6.25 | 12.5 | 50 | 200 | 50 | 100 | |
| 3.9 | C6H4(OC2H5)-p | 100 | 100 | 25 | 100 | 50 | 100 | 100 | 100 | |
| 3.10 | C6H4F-p | 50 | 100 | 12.5 | 50 | 50 | 200 | 50 | 100 | |
| 3.11 | C6H5 | R1 = CH3 | 50 | 100 | 25 | 50 | 100 | 100 | 50 | 100 |
| 2.11 | C6H5 | R3= F | 100 | 200 | 6.25 | 50 | 100 | 200 | 50 | 50 |
| 2.15 | C6H5 | R3= F | 100 | 200 | 6.25 | 50 | 100 | 200 | 50 | 50 |
| 3.16 | C6H5(CH3)-p | R3 = Cl | 100 | 200 | 12.5 | 100 | 100 | 200 | 50 | 100 |
| 3.18 | C6H4F-p | R3 = Cl | 100 | 200 | 25 | 25 | 50 | 100 | 100 | 100 |
| 3.19 | C6H4F-p | R1 = Br | 50 | 100 | 100 | 200 | 100 | 100 | 100 | 100 |
| 3.20 | C6H4F-p | R2 = Br | 100 | 200 | 25 | 50 | 50 | 200 | 50 | 50 |
| 3.21 | C6H5 | R3 = Br | 100 | 200 | 25 | 25 | 100 | 200 | 50 | 50 |
| 3.22 | C6H4(CH3)-p | R3 = Br | 50 | 100 | 50 | 200 | 100 | 200 | 50 | 50 |
| 3.23 | C6H4F-p | R3 = Br | 100 | 200 | 12.5 | 50 | 50 | 200 | 50 | 50 |
| 3.24 | C6H4(OCH3)-p | R3 = Br | 100 | 200 | 12.5 | 200 | 100 | 200 | 50 | 50 |
| Trimethoprim | – | 50 | 50 | 31.2 | 62,5 | 62.5 | 125 | 62.5 | 125 | |
| Nitrofural | – | 1,5 | – | 6.25 | – | 6.25 | – | 25.0 | – | |
At the same time, compounds 3.1–3.26 revealed high activity against Staphylococcus aureus (MIC 6.25–100 μg/mL), comparable to Nitrofural (MIC 6.25 μg/mL, Table 2). For potassium thiolates 2.1–2.26, increasing antimicrobial activity was observed. It is important to note that the inhibitory action in lower concentrations was higher for derivatives with halogen substituents in the quinazoline cycle. Compounds 2.1–2.26 and 3.1–3.26 moderately inhibited Candida albicans (MIC 50-100 μg/mL), and revealed antifungal action comparable to Nitrofural (MIC 25 μg/mL, tables 1 and 2). Analysis of the structure–antimicrobial activity relationships showed the absence of obvious correlations. Antimicrobial activities, within the scope of each strain, were on similar levels.
Experimental
Chemistry
General Methods
Melting points were determined in open capillary tubes and were uncorrected. The elemental analyses (C, H, N, S) were performed using the ELEMENTAR vario EL Cube analyzer (USA). Analyses were indicated by the symbols of the elements or functions within ±0.3% of the theoretical values. The IR spectra (4000–600 cm-1) were recorded on a Bruker ALPHA FT-IR spectrometer (Bruker Bioscience, Germany) using a module for measuring attenuated total reflection (ATR). The 1H NMR spectra (400 MHz) and 13C NMR spectra (100 MHz) were recorded on a Varian-Mercury 400 (Varian Inc., Palo Alto, CA, USA) spectrometer with TMS as the internal standard in DMSO-cf6 solution. The LC-MS were recorded using a chromato-mass spectrometric system which consisted of a highperformance liquid chromatograph «Agilent 1100 Series» (Agilent, Palo Alto, CA, USA) equipped with a diode-matrix and mass-selective detector «Agilent LC/MSD SL» (atmospheric pressure chemical ionization – APCI). The electron impact mass spectra (EI-MS) were recorded on a Varian 1200 L instrument at 70 eV (Varian, USA). The purity of all obtained compounds was checked by 1H-NMR and LC-MS.
Substances 1.1–1.26 were synthesized according to the reported procedures [14]. Other starting materials and solvents were obtained from commercially available sources and used without additional purification.
General procedures for the synthesis of potassium 8-R1-9-R2-10-R3-3-R-2-oxo-2H-[1, 2, 4]triazino[2, 3-c]quinazolin-6-thiolates (2.1-2.26) were:
Method A
0.76 g (10 mmol) of carbon disulfide was added with stirring to a solution of 0.56 g (10 mmol) of potassium hydroxide in 20 mL of ethanol. Proper 6-R-3-(3-R1-4-R2-5-R3-2-aminophenyl)-2H-[1, 2, 4]triazin-5-one (1) (10 mmol) was added to the obtained solution and refluxed for 4-10 hours. The resulting mixture was cooled, and the solid was filtered and dried.
Method B
1.60 g (10 mmol) of potassium O-ethyl dithiocarbonate was added to the suspension of proper 6-R-3-(3-R1-4-R2-5-R3-2-aminophenyl)-2H-[1, 2, 4]triazin-5-one (1) (10 mmol) in 20 mL of 2-propanol and refluxed for 4–10 hours. The resulting mixture was cooled, and the solid was filtered and dried.
Potassium 3-methyl-2-oxo-2H-[1, 2, 4]triazino[2, 3-c]quinazoline-6-thiolate (2.1)
Yield: 73.5% (Method A), 84% (Method B), mp >320°C; IR (cm-1): 3407, 3290, 3063, 2984, 2909, 2842, 1621, 1566, 1520, 1472, 1430, 1379, 1337, 1298, 1264, 1239, 1210, 1166, 1150, 1030, 944, 862, 766, 735, 688, 661, 636, 614; Anal. Calcd for C11H7N4OSK: C, 46.79; H, 2.50; N, 19.84; S, 11.35; Found: C, 46.78; H, 2.50; N, 19.83; S, 11.35.
Potassium 2-oxo-3-phenyl-2H-[1, 2, 4]triazino[2, 3-c]quinazoline-6-thiolate (2.2)
Yield: 75% (Method A), 93% (Method B), mp >320°C; IR (cm-1): 1620, 1602, 1570, 1524, 1493, 1474, 1463, 1432, 1371, 1345, 1319, 1296, 1277, 1253, 1232, 1171, 1155, 1077, 1034, 1001, 985, 939, 856, 818, 755, 695, 656, 606; Anal. Calcd for C16H9N4OSK: C, 55.79; H, 2.63; N, 16.27; S, 9.31; Found: C, 55.78; H, 2.65; N, 16.27; S, 9.31.
Potassium 3-(4-methylphenyl)-2-oxo-2H-[1, 2, 4]triazino[2, 3-c]quinazoline-6-thiolate (2.3)
Yield: 77% (Method A), 77% (Method B), mp >320°C; IR (cm-1): 3079, 1620, 1602, 1570, 1524, 1477, 1463, 1433, 1405, 1371, 1346, 1320, 1300, 1278, 1246, 1233, 1172, 1112, 1075, 1035, 1023, 985, 939, 874, 855, 835, 798, 783, 755, 715, 695, 684, 660, 641, 629, 611; Anal. Calcd for C17H11N4OSK: C, 56.96; H, 3.09; N, 15.64; S, 8.95; Found: C, 56.94; H, 3.09; N, 15.65; S, 8.94.
Potassium 3-(3,4-dimethylphenyl)-2-oxo-2H-[1, 2, 4]triazino[2,3-c]quinazoline-6-thiolate (2.4)
Yield: 67.2% (Method A), 92.1% (Method B), mp >320°C; IR (cm-1): 3438, 3394, 3292, 3054, 3021, 2962, 2916, 1660, 1644, 1626, 1602, 1568, 1537, 1524, 1475, 1432, 1393, 1369, 1347, 1295, 1274, 1254, 1232, 1185, 1167, 1126, 1078, 1013, 982, 950, 903, 890, 868, 849, 833, 756, 736, 713, 704, 688, 659, 634; Anal. Calcd for C18H14N4OSK: C, 58.04; H, 3.52; N, 15.04; S, 8.61; Found: C, 58.06; H, 3.58; N, 15.02; S, 8.59.
Potassium 3-(4-ethylphenyl)-2-oxo-2H-[1, 2, 4]triazino[2, 3-c]quinazoline-6-thiolate (2.5)
Yield: 79% (Method B), mp >320°C; IR (cm-1): 3389, 3238, 2955, 2927, 1620, 1602, 1571, 1520, 1478, 1464, 1435, 1412, 1370, 1348, 1318, 1303, 1280, 1254, 1233, 1175, 1161, 1120, 1075, 1045, 1019, 984, 940, 881, 846, 761, 735, 697, 688, 674, 657, 641, 630, 610; Anal. calcd. for C18H13N4OSK: C, 58.04; H, 3.52; N, 15.04; S, 8.61; Found: C, 58.06; H, 3.51; N, 15.05; S, 8.60.
Potassium 3-(4-isopropylphenyl)-2-oxo-2H-[1, 2, 4]triazino[2, 3-c]quinazoline-6-thiolate (2.6)
Yield: 90% (Method B), mp >320°C; IR (cm-1): 3411, 3056, 2954, 2926, 2867, 1621, 1604, 1570, 1538, 1505, 1477, 1463, 1440, 1415, 1369, 1343, 1302, 1279, 1245, 1234, 1174, 1117, 1074, 1051, 986, 942, 870, 847, 780, 754, 694, 686, 674, 658, 643, 627, 612; Anal. calcd. for C19H15N4OSK: C, 59.04; H, 3.91; N, 14.50; S, 8.30; Found: C, 59.05; H, 3.90; N, 14.52; S, 8.28.
Potassium 3-(4-tert-butylphenyl)-2-oxo-2H-[1, 2, 4]triazino[2, 3-c]quinazoline-6-thiolate (2.7)
Yield: 67% (Method A), 78% (Method B), mp >320°C; IR (cm-1): 3563, 3405, 2963, 2867, 1627, 1607, 1574, 1539, 1509, 1478, 1444, 1412, 1345, 1304, 1280, 1269, 1255, 1233, 1198, 1179, 1135, 1114, 1075, 986, 943, 847, 756, 694, 685, 662, 611; Anal. calcd. for C20H17N4OSK: C, 59.97; H, 4.28; N, 13.99; S, 8.01; Found: C, 59.95; H, 4.28; N, 13.99; S, 8.03.
Potassium 3-(4-methoxyphenyl)-2-oxo-2H-[1, 2, 4]triazino[2, 3-c]quinazoline-6-thiolate (2.8)
Yield: 83% (Method A), 99% (Method B), mp >320°C; IR (cm-1): 2963, 2904, 2830, 1650, 1601, 1570, 1536, 1505, 1477, 1439, 1417, 1368, 1343, 1315, 1297, 1280, 1261, 1254, 1232, 1168, 1133, 1075, 1029, 1018, 1008, 984, 939, 857, 837, 819, 800, 754, 724, 705, 689, 657, 635, 625, 611; Anal. Calcd for C17H11N4O2SK: C, 54.53; H, 2.96; N, 14.96; S, 8.56; Found: C, 54.53; H, 2.97; N, 14.95; S, 8.55.
Potassium 3-(4-ethoxyphenyl)-2-oxo-2H-[1, 2, 4]triazino[2, 3-c]quinazoline-2-thiolate (2.9)
Yield: 91% (Method B), mp >320°C; IR (cm-1): 2980, 1636, 1602, 1571, 1532, 1512, 1477, 1435, 1418, 1368, 1344, 1298, 1233, 1172, 1155, 1123, 1075, 1039, 986, 942, 921, 896, 835, 825, 799, 781, 762, 722, 704, 692, 672, 645, 625; Anal. calcd. for C18H13N402SK: C, 55.65; H, 3.37; N, 14.42; S, 8.25; Found: C, 55.67; H, 3.36; N, 14.42; S, 8.24.
Potassium 3-(4-fluorophenyl)-2-oxo-2H-[1, 2, 4]triazino[2, 3-c]quinazoline-6-thiolate (2.10)
Yield: 86% (Method A), 94% (Method B), mp >320°C; IR (cm-1): 3352, 2965, 1635, 1599, 1572, 1532, 1505, 1474, 1435, 1408, 1370, 1344, 1294, 1280, 1231, 1173, 1153, 1131, 1095, 1071, 1015, 982, 954, 939, 844, 818, 801, 783, 767, 719, 695, 659, 635, 626, 611; Anal. calcd. for C16H8FN4OSK: C, 53.02; H, 2.22; N, 15.46; S, 8.85; Found: C, 53.05; H, 2.21; N, 15.45; S, 8.84.
Potassium 8-methyl-2-oxo-3-phenyl-2H-[1, 2, 4]triazino[2, 3-c]quinazoline-6-thiolate (2.11)
Yield: 91% (Method A), 94% (Method B), mp >320°C; IR (cm-1): 3350, 3049, 2965, 2915, 1636, 1614, 1599, 1575, 1532, 1480, 1443, 1412, 1364, 1350, 1291, 1260, 1241, 1192, 1179, 1132, 1097, 1023, 1001, 975, 833, 813, 750, 706, 686, 658, 619; Anal. calcd. for C17H11N4OSK: C, 56.96; H, 3.09; N, 15.63; S, 8.95; Found: C, 56.97; H, 3.09; N, 15.64; S, 8.95.
Potassium 9-fluoro-2-oxo-3-phenyl-2H-[1, 2, 4]triazino[2, 3-c]quinazoline-6-thiolate (2.12)
Yield: 81% (Method B); mp >320°C; IR (cm-1): 3187, 3124, 3079, 3059, 3010, 2967, 2927, 1665, 1618, 1556, 1516, 1490, 1444, 1393, 1355, 1315, 1295, 1276, 1261, 1190, 1171, 1143, 1104, 1085, 1034, 1018, 1001, 977, 939, 860, 831, 814, 788, 776, 747, 709, 681, 660, 638, 618; Anal. calcd. for C16H8FN4OSK: C, 53.02; H, 2.22; N, 15.46; S, 8.85; Found: C, 53.06; H, 2.22; N, 15.44; S, 8.83.
Potassium 9-fluoro-3-(4-fluorophenyl)-2-oxo-2H-[1, 2, 4]triazino[2, 3-c]quinazoline-6-thiolate (2.13)
Yield: 83% (Method A), 87% (Method B); mp >320°C; IR (cm-1): 3099, 3058, 3014, 2961, 2925, 1650, 1625, 1599, 1557, 1518, 1503, 1433, 1413, 1392, 1354, 1294, 1273, 1237, 1184, 1166, 1143, 1103, 1017, 977, 943, 867, 841, 826, 804, 772, 752, 718, 702, 682, 672, 640, 620; Anal. calcd. for C16H7F2N4OSK: C, 50.52; H, 1.85; N, 14.73; S, 8.43; Found: C, 50.56; H, 1.85; N, 14.72; S, 8.41
Potassium 9-fluoro-3-(4-methoxyphenyl)-2-oxo-2H-[1, 2, 4]triazino[2, 3-c]quinazoline-6-thiolate (2.14)
Yield: 83% (Method B); mp >320°C; IR (cm-1): 3082, 3010, 2963, 2879, 2838, 2774, 1658, 1600, 1574, 1552, 1538, 1504, 1487, 1435, 1398, 1354, 1304, 1267, 1188, 1168, 1107, 1081, 1027, 1014, 976, 938, 862, 837, 808, 798, 776, 765, 750, 708, 676, 641, 617; Anal. calcd. for C17H10FN4O2SK: C, 52.03; H, 2.57; N, 14.28; S, 8.17; Found: C, 52.02; H, 2.56; N, 14.29; S, 8.18.
Potassium 10-chloro-2-oxo-3-phenyl-2H-[1, 2, 4]triazino[2, 3-c]quinazoline-6-thiolate (2.15)
Yield: 75% (Method A), 82% (Method B), mp >320°C; IR (cm-1): 3899, 3851, 3645, 3626, 3389, 1640, 1625, 1599, 1558, 1538, 1493, 1474, 1449, 1410, 1368, 1346, 1319, 1278, 1251, 1237, 1174, 1130, 1094, 1038, 1004, 991, 959, 885, 863, 833, 818, 756, 700, 688, 674, 652, 624; Anal. calcd. for C16H8CIN4OSK: C, 50.72; H, 2.13; N, 14.79; S, 8.46; Found: C, 50.74; H, 2.13; N, 14.78; S, 8.45.
Potassium 10-chloro-3-(4-methylphenyl)-2-oxo-2H-[1, 2, 4]triazino[2, 3-c]quinazoline-6-thiolate (2.16)
Yield: 90% (Method B); mp >320°C; IR (cm-1): 1624, 1556, 1537, 1475, 1447, 1407, 1368, 1342, 1278, 1237, 1186, 1174, 1133, 1091, 1059, 1021, 994, 959, 882, 864, 836, 828, 770, 746, 713, 700, 673, 656, 642, 631; Anal. calcd. for C17H10CIKN4OS: C, 51.97; H, 2.57; N, 14.26; S, 8.16; Found: C, 51.94; H, 2.57; N, 14.28; S, 8.18.
Potassium 10-chloro-3-(4-methoxyphenyl)-2-oxo-2H-[1, 2, 4]triazino[2, 3-c]quinazoline-6-thiolate (2.17)
Yield: 82% (Method B); mp >320°C; IR (cm-1): 3056, 2981, 2942, 2915, 1649, 1608, 1596, 1574, 1552, 1541, 1521, 1503, 1478, 1456, 1428, 1394, 1344, 1305, 1271, 1237, 1188, 1175, 1150, 1124, 1114, 1096, 1017, 966, 902, 873, 839, 822, 769, 760, 728, 698, 673, 629; Anal. calcd. for C17H10CIN4O2SK: C, 49.93; H, 2.46; N, 13.70; S, 7.84; Found: C, 49.97; H, 2.46; N, 13.68; S, 7.82.
Potassium 10-chloro-3-(4-fluorophenyl)-2-oxo-2H-[1, 2, 4]triazino[2, 3-c]quinazoline-6-thiolate (2.18)
Yield: 66% (Method A), 79% (Method B), mp >320°C; IR (cm-1): 1628, 1598, 1557, 1539, 1504, 1474, 1446, 1403, 1367, 1341, 1320, 1299, 1278, 1237, 1172, 1157, 1130, 1100, 1089, 1065, 1009, 994, 958, 884, 867, 847, 831, 771, 747, 717, 700, 673, 657, 639, 627; Anal. calcd. for C16H7CIFKN4OS: C, 48.42; H, 1.78; N, 14.12; S, 8.08; Found: C, 48.46; H, 1.78; N, 14.13; S, 8.10.
Potassium 8-bromo-3-(4-fluorophenyl)-2-oxo-2H-[1, 2, 4]triazino[2, 3-c]quinazoline-6-thiolate (2.19)
Yield: 86% (Method B), mp >320°C; IR (cm-1): 2954, 1621, 1593, 1556, 1532, 1504, 1472, 1446, 1411, 1383, 1365, 1345, 1311, 1285, 1271, 1250, 1233, 1218, 1160, 1147, 1112, 1074, 991, 964, 922, 847, 817, 751, 717, 707, 692, 664, 627, 609; Anal. calcd. for C16H7BrFN4OSK: C, 43.54; H, 1.60; N, 12.70; S, 7.27; Found: C, 43.52; H, 1.61; N, 12.71; S, 7.28.
Potassium 9-bromo-3-(4-fluorophenyl)-2-oxo-2H-[1, 2, 4]triazino[2, 3-c]quinazoline-6-thiolate (2.20)
Yield: 99% (Method B); mp >320°C; IR (cm-1): 3390, 1632, 1591, 1557, 1538, 1505, 1455, 1406, 1372, 1337, 1288, 1263, 1232, 1159, 1102, 1070, 990, 941, 894, 845, 818, 763, 715, 669, 655, 621; Anal. calcd. for C16H7BrFN4OSK: C, 43.54; H, 1.60; N, 12.70; S, 7.27; Found: C, 43.50; H, 1.60; N, 12.72; S, 7.22.
Potassium 10-bromo-2-oxo-3-phenyl-2H-[1, 2, 4]triazino[2, 3-c]quinazoline-6-thiolate (2.21)
Yield: 65% (Method B); mp >320°C; IR (cm-1): 3149, 3097, 3057, 2979, 2909, 1657, 1630, 1614, 1595, 1555, 1507, 1474, 1445, 1425, 1393, 1342, 1283, 1260, 1232, 1190, 1152, 1120, 1088, 1034, 1014, 957, 922, 907, 869, 837, 813, 794, 751, 735, 688, 669, 645, 624; Anal. calcd. for C16H8BrN4OSK: C, 45.40; H, 1.90; N, 13.23; S, 7.57; Found: C, 45.44; H, 1.90; N, 13.22; S, 7.55.
Potassium 10-bromo-3-(4-methylphenyl)-2-oxo-2H-[1, 2, 4]triazino[2, 3-c]quinazoline-6-thiolate (2.22)
Yield: 91% (Method B); mp >320°C; IR (cm-1): 1623, 1597, 1556, 1537, 1472, 1444, 1404 1369, 1339, 1322, 1276, 1242, 1185, 1173, 1134, 1080, 1021, 993, 953, 881, 860, 836 826, 800, 769, 730, 713, 699, 659, 630; Anal. calcd. for C17H10BrN4OSK: C, 46.69; H 2.30; N, 12.81; S, 7.33; Found: C, 46.65; H, 2.30; N, 12.83; S, 7.35.
Potassium 10-bromo-3-(4-fluorophenyl)-2-oxo-2H-[1, 2, 4]triazino[2, 3-c]quinazoline-6-thiolate (2.23)
Yield: 88% (Method B); mp >320°C; IR (cm-1): 1627, 1596, 1556, 1538, 1504, 1471, 1444 1401, 1369, 1339, 1277, 1241, 1173, 1158, 1132, 1101, 1077, 1009, 993, 953, 882, 846 830, 771, 732, 717, 700, 673, 660, 638, 626; Anal. calcd. for C16H7BrFN4OSK: C, 43.54 H, 1.60; N, 12.70; S, 7.27; Found: C, 43.58; H, 1.60; N, 12.68; S, 7.25.
Potassium 10-bromo-3-(4-methoxyphenyl)-2-oxo-2H-[1, 2, 4]triazino[2, 3-c]quinazoline-6-thiolate (2.24)
Yield: 89% (Method B); mp >320°C; IR (cm-1): 3079, 3050, 3018, 2988, 2930, 2833, 1622 1606, 1571, 1555, 1533, 1511, 1472, 1444, 1434, 1409, 1371, 1339, 1306, 1284, 1271 1242, 1173, 1133, 1123, 1112, 1079, 1030, 1009, 992, 953, 862, 837, 830, 805, 769, 730 702, 673, 659, 628; Anal. calcd. for C17H10BrN4O2SK: C, 45.04; H, 2.22; N, 12.36; S, 7.07 Found: C, 45.00; H, 2.22; N, 12.37; S, 7.10
Potassium 3-(4-fluorophenyl)-10-iodo-2-oxo-2H-[1, 2, 4]triazino[2, 3-c]quinazoline-6-thiolate (2.25)
Yield: 89% (Method B); mp >320°C; IR (cm-1): 3499, 3359, 1678, 1628, 1609, 1590, 1555 1538, 1521, 1505, 1487, 1472, 1444, 1402, 1374, 1337, 1327, 1296, 1276, 1241, 1215 1172, 1158, 1126, 1100, 1074, 1011, 992, 950, 875, 844, 831, 824, 815, 772, 759, 747 718, 700, 687, 672, 648, 637, 621; Anal. calcd. for C16H7FIN4OSK: C, 39.35; H, 1.44; N 11.47; S, 6.57; Found: C, 39.39; H, 1.44; N, 11.45; S, 6.56.
Potassium 10-iodo-3-(4-methoxyphenyl)-2-oxo-2H-[1, 2, 4]triazino[2, 3-c]quinazoline-6-thiolate (2.26)
Yield: 90% (Method B); mp >320°C; IR (cm-1): 3080, 3051, 3020, 2971, 2930, 2834, 1622 1606, 1590, 1571, 1552, 1532, 1511, 1472, 1445, 1435, 1421, 1405, 1368, 1338, 1306 1284, 1270, 1243, 1173, 1139, 1124, 1112, 1078, 1030, 1010, 992, 951, 880, 861, 837 830, 805, 769, 723, 702, 652, 628; Anal. calcd. for C17H10IN4O2SK: C, 40.81; H, 2.01; N 11.20; S, 6.41; Found: C, 40.84; H, 2.01; N, 11.18; S, 6.40.
The general procedure for the synthesis of 8-R1-9-R2-10-R3-3-R-6-thio-6,7-dihydro-2H-[1, 2, 4]triazino[2, 3-c]quinazolin-2-ones (3.1–3.26) was the following:
Proper potassium 8-R1-9-R2-10-R3-3-R-2-oxo-2H-[1, 2, 4]triazino[2, 3-c]quinazolin-2-one (2) (10 mmol) was dissolved in 20 mL of water and acidified by the addition of hydrochlori acid to pH 2–3. The formed precipitate was filtered and dried.
3-Methyl-6-thioxo-6,7-dihydro-2H-[1, 2, 4]triazino[2, 3-c]quinazolin-2-one (3.1)
Yield: 99%, mp 254-256°C; IR (CM-1): 3118, 3056, 3029, 2954, 2917, 2849, 1767, 1743 1687, 1637, 1616, 1594, 1564, 1519, 1471, 1455, 1421, 1381, 1360, 1322, 1303, 1277 1257, 1225, 1169, 1128, 1111, 1039, 1025, 988, 949, 862, 771, 752, 716, 671, 655, 623 1H NMR: δ=2.34 (s, 3H, CH3), 7.48-7.43 (m, 2H, H-8, 10), 7.82 (t, 1H, J3 = 7.9, J4 = 1.4, H-9), 8.29 (d, 1H, J = 7.9, H-11), 13.83 (s, 1H, NH); 13C NMR: δ =20.04 (CH3), 118.29 (11a), 127.49 (8), 130.30 (10), 136.24 (11), 141.07 (9), 144.04 (3), 150.25 (11b), 154.72 (7a), 160.16 (2), 170,1 (6); EI-MS, m/z (Irel, %) = 246 (5.8), 245 (11.4), 244 (M+», 65.5), 205 (2.1), 204 (13.4), 203 (100.0), 198 (10.2), 174 (10.2), 171 (7.4), 170 (12.4), 163 (4.0), 161 (35.4), 160 (6.7), 146 (2.8), 145 (76.7), 144 (21.1), 143 (22.5), 142 (5.8), 134 (13.6), 117 (8.6), 116 (9.0), 108 (6.9), 107 (7.7), 105 (8.8), 103 (11.3), 102 (35.9), 91 (6.1), 90 (42.7), 89 (5.3), 88 (5.1), 86 (11.1), 78 (5.0), 77 (8.9), 76 (15.5), 75 (23.4), 74 (5.1), 70 (10.7), 69 (10.5), 65 (8.5), 64 (27.5), 63 (19.8), 62 (5.9); LC-MS, m/z = 245 [M+1], 247 [M+3]; Anal. Calcd for C11H8N4OS: C, 54.09; H, 3.30; N, 22.94; S, 13.13; Found: C, 54.07; H, 3.31; N, 22.93; S, 13.14.
3-Phenyl-6-thioxo-6, 7-dihydro-2H-[1, 2, 4]triazino[2, 3-c]quinazolin-2-one (3.2)
Yield: 96%, mp >300°C; IR (cm-1): 3358, 3182, 3014, 1654, 1608, 1589, 1546, 1512, 1497, 1456, 1398, 1356, 1337, 1302, 1278, 1243, 1205, 1178, 1156, 1128, 1091, 1046, 1024, 981, 943, 869, 833, 810, 776, 753, 721, 686, 632; 1H NMR: δ=7.61-7.42 (m, 5H, H-3', 4', 5', 8, 10), 7.82 (t, 1H, J3 = 7.9, J4 = 1.4, H-9), 8.36-8.20 (m, 3H, H-2', 6', 11,), 13.92 (s, 1H, NH); EI-MS, m/z (Irel, %) = 308 (7.2), 307 (25.8), 306 (M+, 69.9), 229 (5.1), 205 (35.2), 204 (74.2), 203 (96.7), 187 (11.1), 176 (5.8), 175 (6.3), 174 (19.1), 171 (9.6), 170 (59.5), 163 (6.4), 162 (13.9), 161 (100.0), 160 (36.6), 159 (7.7), 146 (27.2), 145 (98.6), 144 (43.2), 143 (82.0), 142 (13.8), 135 (8.0), 134 (54.3), 129 (7.3), 122 (7.3), 118 (8.0), 117 (49.2), 116 (13.9), 108 (5.0), 107 (5.4), 104 (7.4), 103 (37.1), 102 (68.7), 91 (6.8), 90 (56.3), 89 (23.1), 88 (5.7), 86 (7.1), 77 (21.6), 76 (35.4), 75 (24.1), 69 (5.9), 64 (13.2), 63 (28.6), 62 (8.6), 52 (8.3), 51 (18.2), 50 (14.9); LC-MS, m/z = 307 [M+1]; Anal. Calcd for C16H10N4OS: C, 62.73; H, 3.29; N, 18.29; S, 10.47; Found: C, 62.70; H, 3.30; N, 18.27; S, 10.46.
3-(4-Methylphenyl)-6-thioxo-6, 7-dihydro-2H-[1, 2, 4]triazino[2, 3-c]quinazolin-2-one (3.3)
Yield: 86%, mp >300°C; IR (cm-1): 3560, 3171, 3112, 3065, 3014, 2975, 2927, 1647, 1618, 1603, 1573, 1548, 1518, 1506, 1483, 1454, 1395, 1364, 1345, 1307, 1268, 1248, 1195, 1182, 1160, 1149, 1108, 1081, 1026, 1014, 961, 943, 886, 869, 833, 810, 776, 753, 721, 686, 632,616; 1H NMR: δ=2.39 (s, 3H, CH3), 7.35 (d, 2H, J = 8.2, H-3', 5'), 7.52-7.43 (m, 2H, H-8, 10), 7.82 (t, 1H, J3 = 7.9, J4 = 1.4, H-9), 8.24 (d, 2H, J = 8.2, H-2‗, 6‗), 8.32 (d, 1H, J = 7.9, H-11), 13.88 (s, 1H, NH); 13C NMR: δ=21.50 (CH3), 110.01 (11a), 115.92 (8), 117.69 (10), 128.79 (2',6'-Ph), 128.93 (11), 129.06 (9, 1'-Ph), 129.19 (3',5'-Ph), 130.73 (3), 133.53 (4'-Ph), 140.24 (11-b), 150.20 (7a), 158.68 (2), 168.79 (6); EI-MS, m/z (Irel, %) = 320 (M+», 4.1), 205 (6.1), 204 (12.9), 203 (100.0), 171 (8.2), 170 (10.8), 163 (3.3), 161 (24.3), 160 (8.8), 149 (15.0), 146 (6.4), 145 (69.1), 144 (11.4), 143 (22.4), 134 (16.0), 129 (8.3), 119 (6.7), 118 (9.8), 117 (49.6), 116 (32.2), 103 (8.1), 102 (22.1), 97 (7.0), 91 (9.4), 90 (29.1), 89 (14.1), 85 (6.3), 83 (10.0), 77 (8.4), 76 (7.7), 75 (7.4), 73 (5.2), 71 (6.0), 69 (8.7), 64 (5.6), 63 (8.9), 60 (6.5), 57 (14.6), 56 (7.0), 55 (12.8), 51 (7.9), 50 (5.3), 45 (7.9), 43 (14.9), 41 (14.0); LC-MS, m/z = 321 [M+1], 322 [M+2]; Anal. Calcd for C17H12N4OS: C, 63.73; H, 3.78; N, 17.49; S, 10.01; Found: C, 63.74; H, 3.79; N, 17.48; S, 10.03.
3-(3,4-Dimethylphenyl)-6-thioxo-6, 7-dihydro-2H-[1, 2, 4]triazino[2, 3-c]quinazolin-2-one (3.4)
Yield: 93%, mp >310°C; IR (cm-1): 3246, 3192, 3119, 3070, 3033, 2969, 2923, 1674, 1656, 1618, 1551, 1535, 1516, 1499, 1483, 1447, 1389, 1343, 1305, 1258, 1220, 1188, 1144, 1131, 1107, 1083, 1030, 993, 958, 906, 868, 854, 834, 773, 756, 747, 711, 692, 682, 618; 1H NMR: δ=2.28 (s, 6H, 3,4-(CH3)2), 7.27 (d, 1H, J = 8.1, H-5'), 7.44 (m, 2H, H-8, 10), 7.81 (t, 1H, J3 = 7.9, J4 = 1.4, H-9), 8.08 (m, 2H, H-6‗, 2'), 8.29 (d, 1H, J = 7.9, H-11), 13.9 (s, 1H, NH); 13C NMR: δ=20.00 (3-CH3), 20.15 (4-CH3), 115.83 (11a), 116.15 (8), 125.80 (5'-Ph), 126.73 (10), 127.38 (11), 129.95 (6'-Ph), 130.05 (3), 130.52 (9), 136.22 (2'-Ph), 136.57 (3'-Ph), 137.87 (1'-Ph), 140.55 (4'-Ph), 149.37 (11-b), 151.02 (7a), 159.98 (2), 171.05 (6); EI-MS, m/z (Irel, %) = 334 (M+», 2.8), 205 (5.7), 204 (12.0), 203 (100.0), 171 (9.4), 170 (10.2), 163 (2.1), 161 (26.5), 160 (7.2), 149 (18.0), 146 (9.1), 145 (77.9), 144 (14.5), 143 (28.3), 134 (19.7), 132 (7.3), 131 (41.0), 130 (19.7), 129 (14.9), 123 (6.6), 119 (7.0), 118 (10.4), 117 (33.3), 116 (93.0), 115 (11.5), 105 (5.9), 104 (7.9), 103 (22.3), 102 (25.2), 97 (9.3), 91 (8.7), 90 (19.5), 89 (15.3), 85 (5.8), 84 (5.1), 83 (11.3), 77 (16.1), 76 (10.5), 75 (9.9), 74 (5.2), 73 (13.1), 69 (6.9), 64 (7.0), 63 (10.9), 60 (13.5), 57 (22.2), 56 (8.0), 55 (16.1), 51 (11.4), 50 (6.1), 45 (18.7), 44 (8.4), 43 (23.7); LC-MS, m/z = 335 [M+1], 337 [M+3]; Anal. Calcd for C18H14N4OS: C, 64.65; H, 4.22; N, 16.75; S, 9.59; Found: C, 64.67; H, 4.21; N, 16.76; S, 9.61.
3-(4-Ethylphenyl)-6-thioxo-6, 7-dihydro-2H-[1, 2, 4]triazino[2, 3-c]quinazolin-2-one (3.5)
Yield: 92%, mp >300°C; IR (cm-1): 3176, 3112, 3059, 3022, 2961, 2927, 1668, 1619, 1605, 1572, 1545, 1518, 1505, 1483, 1429, 1414, 1390, 1360, 1345, 1304, 1255, 1191, 1182, 1149, 1108, 1079, 1051, 1010, 976, 941, 868, 848, 834, 802, 748, 714, 701, 683, 629, 616; 1H NMR: δ=1.26 (t, J = 7.4 Hz, 3H, CH2CH3), 2.73 (q, J = 14.7, 7.4 Hz, 2H, CH2CH3), 7.28 (d, 2H, J = 8.2, H-3', 5'), 7.52-7.48 (m, 2H, H-8, 10), 7.82 (t, 1H, J = 7.8, H-9), 8.19 (d, 2H, J = 8.2, H-2‗, 6‗), 8.30 (d, 1H, J = 7.9, H-11), 13.90 (s, 1H, NH); LC-MS, m/z = 335 [M+1], 337 [M+3]; Anal. Calcd for C18H14N4OS: C, 64.65; H, 4.22; N, 16.75; S, 9.59; Found: C, 64.67; H, 4.22; N, 16.74; S, 9.58.
3-(4-Isopropylphenyl)-6-thioxo-6,7-dihydro-2H-[1, 2, 4]triazino[2, 3-c]quinazolin-2-one (3.6)
Yield: 89%, mp >300°C; IR (cm-1): 3180, 3126, 3056, 3014, 2959, 2925, 2869, 1764, 1643, 1615, 1548, 1495, 1456, 1404, 1366, 1346, 1308, 1266, 1247, 1194, 1159, 1109, 1083, 1055, 1011, 946, 873, 846, 819, 793, 749, 694, 684, 628, 617; 1H NMR: δ= 1.25 (d, J = 6.7 Hz, 6H, -CH(CH3)2), 2.98 (quin, J = 6.7 Hz, 1H, -CH(CH3)2, 7.26 (d, 2H, J = 8.2, H-3', 5'), 7.50-7.45 (m, 2H, H-8, 10), 7.81 (t, 1H, J = 7.8, H-9), 8.17 (d, 2H, J = 8.2, H-2‗, 6‗), 8.28 (d, 1H, J = 7.7, H-11), 13.92 (s, 1H, NH); LC-MS, m/z = 349 [M +1], 351 [M +3]; Anal. calcd. for C19H16N4OS: C, 65.50; H, 4.63; N, 16.08; S, 9.20; Found: C, 65.51; H, 4.63; N, 16.07; S, 9.20.
3-(4-tert-Butylphenyl)-6-thioxo-6, 7-dihydro-2H-[1, 2, 4]triazino[2, 3-c]quinazolin-2-one (3.7)
Yield: 91%, mp >300°C; IR (cm-1): 3201, 3126, 3077, 3061, 3016, 2961, 2933, 2903, 1766, 1643, 1617, 1549, 1513, 1496, 1485, 1405, 1370, 1345, 1307, 1266, 1250, 1192, 1158, 1147, 1124, 1107, 1083, 1011, 947, 875, 842, 798, 779, 751, 722, 687, 628, 616; LC-MS, m/z = 363 [M +1]; 1H NMR: δ= 1.39 (s, 9H, C(CH3)3), 7.17 (d, 2H, J = 8.2, H-3', 5'), 7.47-7.41 (m, 2H, H-8, 10), 7.77 (t, 1H, J = 7.8, H-9), 8.13 (d, 2H, J = 8.2, H-2‗, 6‗), 8.24 (d, 1H, J = 7.7, H-11), 13.81 (s, 1H, NH); Anal. calcd. for C20H18N4OS: C, 66.28; H, 5.01; N, 15.46; S, 8.85; Found: C, 66.29; H, 5.01; N, 15.45; S, 8.85.
3-(4-Methoxyphenyl)-6-thioxo-6, 7-dihydro-2H-[1, 2, 4]triazino[2, 3-c]quinazolin-2-one (3.8)
Yield: 93%, mp 280-282°C; IR (cm-1): 3180, 3117, 3065, 3021, 2960, 2905, 2835, 1680, 1624, 1600, 1551, 1522, 1508, 1485, 1432, 1390, 1360, 1344, 1304, 1259, 1202, 1174, 1147, 1118, 1105, 1076, 1007, 941, 868, 851, 814, 786, 772, 752, 724, 706, 693, 680, 628, 614; 1H NMR: δ=3.83 (s, 3H, OCH3), 7.09 (d, 2H, J=8.8, H-3', 5'), 7.45 (m, 2H, H-8, 10), 7.81 (t, 1H, J3 = 7.9, J4 = 1.4, H-9), 8.33 (m, 3H, H-11, 2', 6'), 13.91 (s, 1H, NH); 13C NMR: δ =55.90 (OCH3), 114.35 (3',5'-Ph), 115.81 (11a), 116.15 (8), 124.76 (10), 125.80 (11), 126.67 (1'-Ph), 131.56 (2',6'-Ph), 136.17 (9), 137.84 (3), 148.57 (11-b), 150.88 (7a), 160.07 (2), 162.26 (4'-Ph), 171.03 (6); EI-MS, m/z (Irel, %) = 336 (M+, 7.1), 205 (5.6), 204 (11.9), 203 (100.0), 170 (9.5), 163 (3.4), 161 (25.0), 160 (7.7), 149 (6.5), 146 (6.9), 145 (69.7), 144 (11.2), 143 (24.3), 134 (21.2), 133 (47.1), 129 (7.7), 119 (11.3), 118 (7.1), 117 (19.5), 116 (5.5), 104 (6.3), 103 (20.3), 102 (20.4), 91 (6.1), 90 (29.9), 76 (9.0), 75 (6.6), 64 (7.2), 63 (8.8), 57 (5.3), 55 (6.4), 51 (5.1), 45 (8.9), 41 (6.2); LC-MS, m/z = 337 [M+1], 339 [M+3]; Anal. Calcd for C17H12N4O2S: C, 60.70; H, 3.60; N, 16.66; S, 9.53; Found: C, 60.69; H, 3.59; N, 16.64; S, 9.54.
3-(4-Ethoxyphenyl)-6-thioxo-6, 7-dihydro-2H-[1, 2, 4]triazino[2, 3-c]quinazolin-2-one (3.9)
Yield: 91%, mp >300°C; IR (cm-1): 3177, 3114, 3061, 3016, 2979, 2926, 2901, 1659, 1618, 1601, 1546, 1504, 1484, 1421, 1395, 1365, 1344, 1308, 1261, 1241, 1190, 1175, 1150, 1121, 1109, 1088, 1034, 1014, 1004, 968, 944, 923, 898, 868, 843, 816, 799, 774, 753, 724, 685, 640, 627, 616; 1H NMR: 1.37 (t, J = 6.7 Hz, 3H, 0CH2CH3), 3.91 (quad, J = 6.7 Hz, 2H, -OCH2CH3), 7.01 (d, 2H, J=8.8, H-3', 5'), 7.38-7.46 (m, 2H, H-8, 10), 7.74 (t, 1H, J = 7.9, H-9), 8.27 (m, 3H, H-11, 2', 6'), 13.92 (s, 1H, NH); LC-MS, m/z = 351 [M+1]; Anal. calcd. for C18H14N4O2S: C, 61.70; H, 4.03; N, 15.99; S, 9.15; Found: C, 61.71; H, 4.03; N, 15.98; S, 9.15.
3-(4-Fluorophenyl)-6-thioxo-6, 7-dihydro-2H-[1, 2, 4]triazino[2, 3-c]quinazolin-2-one (3.10)
Yield: 92%, mp >300°C; IR (cm-1): 3564, 3487, 3111, 3070, 3014, 2926, 2889, 1755, 1656, 1620, 1601, 1549, 1520, 1504, 1484, 1398, 1368, 1345, 1310, 1295, 1267, 1250, 1226, 1196, 1160, 1103, 1013, 963, 944, 894, 853, 824, 805, 779, 754, 723, 702, 686, 633, 616; 1H NMR: δ=7.27 (t, J=8.0, 2H, H-3, 5), 7.45 (t, J=7.7, 1H, H-10), 7.56 (d, J=7.7, 1H, H-8), 7.79 (t, J=7.7, 1H, H-9), 8.40 (d, J=7.7, 1H, H-11), 8.50 (t, J=5.3, 2H, H-2, 6), 13.94 (bs, 1H, NH); Anal. calcd. for C16H9FN4OS: C, 59.25; H, 2.80; N, 17.27; S, 9.89; Found: C, 59.22; H, 2.81; N, 17.28; S, 9.89.
8-Methyl-3-phenyl-6-thioxo-6, 7-dihydro-2H-[1, 2, 4]triazino[2, 3-c]quinazolin-2-one (3.11)
Yield: 94%, mp >300°C; IR (cm-1): 3186, 3128, 3074, 3031, 2968, 1658, 1617, 1601, 1544, 1515, 1490, 1468, 1445, 1428, 1395, 1380, 1357, 1314, 1245, 1205, 1185, 1163, 1152, 1094, 1048, 1007, 997, 977, 933, 863, 838, 813, 800, 746, 689, 659, 619; 1H NMR δ= 3.07 (s, 1H, CH3), 7.57-7.40 (m, 4H, H-3', 4', 5', 10), 7.77 (d, 1H, J = 7.7, H-9), 8.33-8.17 (m, 3H, H-2', 6', 11,), 13.88 (s, 1H, NH); LC-MS, m/z = 321 [M+1], 323 [M+3] ; Anal. calcd. for C17H12N4OS: C, 63.73; H, 3.78; N, 17.49; S, 10.01; Found: C, 63.75; H, 3.79; N, 17.49; S, 10.02.
9-Fluoro-3-phenyl-6-thioxo-6, 7-dihydro-2H-[1, 2, 4]triazino[2, 3-c]quinazolin-2-one (3.12)
Yield: 95%, mp >300°C; IR (cm-1): 3077, 3019, 2893, 2838, 1665, 1618, 1555, 1516, 1490, 1444, 1393, 1355, 1314, 1296, 1276, 1262, 1191, 1171, 1143, 1104, 1085, 1035, 1017, 1001, 977, 939, 860, 832, 814, 788, 776, 747, 709, 682, 659, 638, 618, 609; 1H NMR, δ: 7.25 (m, 2H, H-8, 10), 7.62-7.46 (m, 3H, H-3', 4', 5'), 8.38 (d, J=7.1, 2H, H-2', 6'), 8.50-8.43 (m, 1H, H-11), 13.94 (s, 1H, NH); LC-MS, m/z =325 [M+1]; Anal. calcd. for C16H9FN4OS: C, 59.25; H, 2.80; N, 17.27; S, 9.89; Found: C, 59.27; H, 2.80; N, 17.25; S, 9.86.
9-Fluoro-3-(4-fluorophenyl)-6-thioxo-6, 7-dihydro-2H-[1, 2, 4]triazino[2, 3-c]quinazolin-2-one (3.13)
Yield: 92%, mp >300°C; IR (cm-1): 3096, 3057, 3016, 2894, 2809, 1651, 1625, 1598, 1558, 1502, 1471, 1411, 1391, 1354, 1335, 1295, 1272, 1234, 1184, 1166, 1143, 1103, 977, 942, 867, 841, 827, 802, 772, 752, 718, 703, 682, 672, 620; 1H NMR δ: 13.91 (bs, 1H, NH), 8.48 (t, J=5.3, 2H, H-2', 6'), 8.28 (t, J=7.0, 1H, H-11), 7.23 (m, 4H, H-8, 10, H-3',5'); LC-MS, m/z = 343 [M+1], 345 [M+3]; Anal. calcd. for C16H8F2N4OS: C, 56.14; H, 2.36; N, 16.37; S, 9.37; Found: C, 56.11; H, 2.36; N, 16.38; S, 9.38.
9-Fluoro-3-(4-methoxyphenyl)-6-thioxo-6,7-dihydro-2H-[1, 2, 4]triazino[2, 3-c]quinazolin-2-one (3.14)
Yield: 91%, mp >300°C; IR (cm-1): 3016, 2878, 2837, 2788, 1659, 1601, 1569, 1551, 1538, 1505, 1488, 1457, 1436, 1419, 1398, 1355, 1304, 1267, 1189, 1178, 1168, 1107, 1081, 1029, 1006, 976, 938, 862, 838, 808, 798, 776, 765, 751, 721, 708, 676, 640, 618; 1H NMR (400 MHz) δ: 3.89 (s, 3H, 0CH3), 7.03 (d, J=8.0, 2H, H-3', 5'), 7.33-7.16 (m, 2H, H-8, 10), 8.53-8.33 (m, 3H, H-11, H-2', 6'), 13.91 (s, 1H, NH); LC-MS, m/z =355 [M+1], 357 [M+3]; Anal. calcd. for C17H11FN4O2S: C, 57.62; H, 3.13; N, 15.81; S, 9.05; Found: C, 57.63; H, 3.13; N, 15.80; S, 9.08.
10-Chloro-3-phenyl-6-thioxo-6, 7-dihydro-2H-[1, 2, 4]triazino[2, 3-c]quinazolin-2-one (3.15)
Yield: 93%, mp >300°C; IR (cm-1): 3108, 3062, 2985, 2910, 1651, 1618, 1598, 1551, 1506, 1489, 1446, 1393, 1344, 1281, 1256, 1185, 1150, 1123, 1095, 1034, 1014, 1000, 963, 870, 827, 810, 792, 750, 689, 676, 651, 625; 1H NMR, δ: 7.62-7.40 (m, 4H, H-9, H-3', 4' 5'), 7.72 (d, J=7.3, 1H, H-8), 8.30 (s, 1H, H-11), 8.39 (d, J=6.9, 2H, H-2', 6'), 13.84 (s, 1H, NH); LC-MS, m/z =341 [M+1], 343 [M+3]; Anal. calcd. for C16H9CIN4OS: C, 56.39; H, 2.66; N, 16.44; S, 9.41; Found: C, 56.36; H, 2.66; N, 16.45; S, 9.44.
10-Chloro-3-(4-methylphenyl)-6-thioxo-6, 7-dihydro-2H-[1, 2, 4]triazino[2, 3-c]quinazolin-2-one (3.16)
Yield: 93%, mp >300°C; IR (cm-1): 3416, 3308, 3063, 2988, 2917, 1652, 1616, 1599, 1549, 1516, 1503, 1456, 1405, 1386, 1344, 1331, 1310, 1255, 1185, 1164, 1152, 1139, 1123, 1094, 1015, 964, 872, 830, 820, 758, 731, 711, 697, 671, 650, 621, 607; 1H NMR, δ 2.46 (s, 3H, CH3), 7.32 (d, J = 7.8 Hz, 2H, H-3', 5'), 7.54 (d, J=8.7, 1H, H-8), 8.11 (d, J=7.5, 1H, H-9), 8.44-8.24 (m, 3H, H-11, H-2',6'), 13.95 (bs, 1H, NH); LC-MS, m/z = 355 [M+1], 357 [M+3], 359 [M+5]; Anal. calcd. for C17H11CIN4OS: C, 57.55; H, 3.12; N, 15.79; S, 9.04; Found: C, 57.58; H, 3.12; N, 15.77; S, 9.02.
10-Chloro-3-(4-methoxyphenyl)-6-thioxo-6, 7-dihydro-2H-[1, 2, 4]triazino[2, 3-c]quinazolin-2-one (3.17)
Yield: 93%, mp >300°C; IR (cm-1): 3057, 2986, 2943, 2893, 2837, 1649, 1596, 1574, 1552, 1540, 1520, 1502, 1478, 1456, 1428, 1394, 1344, 1305, 1271, 1188, 1175, 1150, 1124, 1114, 1096, 1016, 1006, 965, 902, 872, 839, 821, 769, 759, 728, 699, 672, 629;1H NMR, δ: 3.89 (s, 3H, OCH3), 7.02 (d, J=8.6, 2H, H-3', 5'), 7.52 (d, J=8.7, 1H, H-8), 7.72 (d, J=8.6, 1H, H-9), 8.30 (s, 1H, H-11), 8.44 (d, J=8.6, 2H, H-2', 6'), 13.93 (s, 1H, NH); LC-MS, m/z =371 [M+1], 373 [M+3], 374 [M+4]; Anal. calcd. for C17H11CIN4S: C, 55.06; H, 2.99; N, 15.11; S, 8.65; Found: C, 55.09; H, 2.99; N, 15.10; S, 8.63.
10-Chloro-3-(4-fluorophenyl)-6-thioxo-6, 7-dihydro-2H-[1, 2, 4]triazino[2, 3-c]quinazolin-2-one (3.18)
Yield: 90%, mp >300°C; IR (cm-1): 3478, 3062, 2986, 2885, 2838, 1658, 1599, 1556, 1516, 1503, 1478, 1453, 1427, 1412, 1393, 1344, 1323, 1284, 1262, 1239, 1189, 1159, 1122, 1101, 1012, 965, 874, 843, 832, 815, 776, 758, 733, 720, 689, 669, 656, 626, 610; 1H NMR, δ: 7.27 (t, J=8.7, 2H, H-3', 5'), 7.53 (d, J=8.8, 1H, H-8), 7.74 (d, J=8.8, 1H, H-9), 8.32 (s, 1H, H-11), 8.51 (dd, J3=8.6, J4=5.7, 2H, H-2',6'), 14.22 (bs, 1H, NH); LC-MS, m/z =359 [M+1], 361 [M+3]; Anal. calcd. for C16H8CIFN4OS: C, 53.56; H, 2.25; N, 15.62; S, 8.94; Found: C, 53.56; H, 2.25; N, 15.62; S, 8.94.
8-Bromo-3-(4-fluorophenyl)-6-thioxo-6,7-dihydro-2H-[1, 2, 4]triazino[2, 3-c]quinazolin-2-one (3.19)
Yield: 92%, mp >300°C; IR (cm-1): 3473, 3327, 3065, 2974, 2787, 1597, 1587, 1550, 1530, 1514, 1473, 1428, 1413, 1379, 1334, 1320, 1287, 1259, 1230, 1161, 1101, 1089, 1055, 1024, 1015, 960, 924, 886, 843, 818, 787, 744,0 717, 699, 651, 626, 612; 1H NMR, δ: 6.64 (t, J=7.6, 1H, H-10), 7.23 (t, J=7.8, 2H, H-3', 5'), 7.62 (d, J=7.4, 1H, H-9), 7.76 (d, J=7.7, 1H, H-11), 8.30 (t, 2H, J=5.3, H-2',6'), 14.03 (bs, 1H, NH), LC-MS, m/z = 404 [M +1], 406 [M +3]; Anal. calcd. for C16H8BrN4OS: C, 47.66; H, 2.00; N, 13.89; S, 7.95; Found: C, 47.67; H, 2.01; N, 13.88; S, 7.94.
9-Bromo-3-(4-fluorophenyl)-6-thioxo-6, 7-dihydro-2H-[1, 2, 4]triazino[2, 3-c]quinazolin-2-one (3.20)
Yield: 92%, mp >300°C; IR (cm-1): 3063, 2892, 1658, 1591, 1553, 1515, 1498, 1474, 1410, 1385, 1340, 1289, 1233, 1184, 1157, 1115, 1101, 1082, 1068, 1013, 944, 901, 872, 843, 823, 773, 751, 715, 699, 676, 664, 634, 622; 1H NMR, δ: 7.26 (t, J=8.5, 2H, H-3', 5'), 7.54 (d, J=8.5, 1H, H-10), 7.67 (s, 1H, H-8), 8.29 (d, J=8.5, 1H, H-11), 8.50 (dd, J1=7.5, J2=6.1, 2H, H-2', 6'), 13.79 (s, 1H, NH); LC-MS, m/z = 403 [M]; Anal. calcd. for C16H8BrFN4OS: C, 47.66; H, 2.00; N, 13.89; S, 7.95; Found: C, 47.68; H, 2.00; N, 13.90; S, 7.96.
10-Bromo-3-phenyl-6-thioxo-6, 7-dihydro-2H-[1, 2, 4]triazino[2, 3-c]quinazolin-2-one (3.21)
Yield: 95%, mp >300°C; IR (cm-1): 1638, 1624, 1597, 1556, 1537, 1493, 1471, 1446, 1407, 1366, 1344, 1276, 1229, 1173, 1133, 1082, 1036, 1003, 990, 953, 859, 830, 817, 755, 699, 687, 669, 646; 1H NMR, δ: 7.63-7.37 (m, 4H, H-8, H-3', 4', 5'), 7.86 (d, J=8.7, 1H, H-9), 8.41 (d, J=6.9, 2H, H-2', 6'), 8.48 (s, 1H, H-11), 13.81 (s, 1H, NH), LC-MS, m/z = 387 [M+2]; Anal. calcd. for C16H8BrN4OS: C, 49.88; H, 2.35; N, 14.54; S, 8.32; Found: C, 49.85; H, 2.35; N, 14.56; S, 8.34.
10-Bromo-3-(4-methylphenyl)-6-thioxo-6, 7-dihydro-2H-[1, 2, 4]triazino[2, 3-c]quinazolin-2-one (3.22)
Yield: 91%, mp >300°C; IR (cm-1): 3173, 3093, 3068, 2985, 2918, 1657, 1619, 1538, 1504, 1473, 1386, 1340, 1310, 1255, 1185, 1124, 1086, 1015, 955, 868, 832, 770, 755, 739, 710, 698, 684, 659; 1H NMR, δ: 2.46 (s, 3H, CH3), 7.31 (d, J=7.7, 1H, H-3', 5'), 7.47 (d, J=8.7, 1H, H-8), 7.86 (d, J=8.7, 1H, H-9), 8.31 (d, J=7.6, 2H, H-2', 6'), 8.46 (s, 1H, H-11), 13.91 (bs, 1H, NH); LC-MS, m/z = 399 [M], 403 [M+4]; Anal. calcd. for C17H11BrN4OS: C, 51.14; H, 2.78; N, 14.03; S, 8.03; Found: C, 51.17; H, 2.78; N, 14.04; S, 8.05.
10-Bromo-3-(4-fluorophenyl)-6-thioxo-6, 7-dihydro-2H-[1, 2, 4]triazino[2, 3-c]quinazolin-2-one (3.23)
Yield: 95%, mp >300°C; IR (cm-1): 3478, 3173, 3065, 2985, 2888, 1650, 1598, 1555, 1516, 1501, 1475, 1453, 1411, 1384, 1340, 1323, 1299, 1281, 1257, 1236, 1186, 1158, 1124, 1101, 1085, 1015, 958, 882, 869, 841, 814, 776, 757, 738, 718, 695, 659, 625, 608; 1H NMR, δ: 7.27 (t, J=8.7, 2H, H-3', 5'), 7.48 (d, J=8.6, 1H, H-8), 7.87 (dd, J=8.7, J2=1.6, 1H, H-9), 8.47 (s, 1H, H-11), 8.55 (dd, J1=8.0, J2=6.1,2H, H-2', 6'), 13.87 (s, 1H, NH); LC-MS, m/z =403 [M]; Anal. calcd. for C16H8BrFN4OS: C, 47.66; H, 2.00; N, 13.89; S, 7.95; Found: C, 47.69; H, 2.00; N, 13.89; S, 7.97.
10-Bromo-3-(4-methoxyphenyl)-6-thioxo-6, 7-dihydro-2H-[1, 2, 4]triazino[2, 3-c]quinazolin-2-one (3.24)
Yield: 94%, mp >300°C; IR (cm-1): 3090, 3056, 2986, 2941, 2911, 1647, 1626, 1606, 1593, 1573, 1552, 1541, 1520, 1499, 1474, 1455, 1440, 1425, 1391, 1353, 1342, 1304, 1270, 1258, 1236, 1187, 1175, 1151, 1114, 1087, 1016, 959, 903, 870, 839, 820, 768, 759, 736, 698, 685, 659, 628; 1H NMR, δ: 3.89 (s, 3H, OCH3); 7.03 (d, J=8.7, H-3', 5'), 7.46 (d, J=8.8, 1H, H-8), 7.85 (d, J=8.6, 1H, H-9), 8.52-8.38 (m, 3H, H-11, H-2', 6'), 13.86 (bs, 1H, NHz); LC-MS, m/z = 415 [M]; Anal. calcd. for C17H11BrN4O2S: C, 49.17; H, 2.67; N, 13.49; S, 7.72; Found: C, 49.19; H, 2.67; N, 13.48; S, 7.74.
10-lodo-3-(4-fluorophenyl)-6-thioxo-6, 7-dihydro-2H-[1, 2, 4]triazino[2, 3-c]quinazolin-2-one (3.25)
Yield: 93,%, mp >300°C; IR (cm-1): 3496, 3358, 2886, 1678, 1628, 1609, 1590, 1554, 1538, 1521, 1505, 1488, 1472, 1444, 1403, 1375, 1337, 1328, 1297, 1276, 1241, 1215, 1172, 1158, 1126, 1100, 1075, 1009, 992, 951, 844, 831, 824, 815, 772, 759, 747, 718, 687, 622, 605; 1H NMR, δ: 6.69 (d, J=8.7, 1H, H-8); 7.18 (t, J=8.6, 2H, H-3', 5'), 7.41 (d, J=8.7, 1H, H-9), 8.07 (s, 1H, H-11), 8.31 (t, J=8.7, 2H, H-2', 6'), LC-MS, m/z =450 [M], 452 [M+2]; Anal. calcd. for C16H8FIN4OS: C, 42.68; H, 1.79; N, 12.44; S, 7.12; Found: C, 42.67; H, 1.79; N, 12.46; S, 7.14.
10-lodo-3-(4-methoxyphenyl)-6-thioxo-6, 7-dihydro-2H-[1, 2, 4]triazino[2, 3-c]quinazolin-2-one (3.26)
Yield: 93%, mp >300°C; IR (cm-1): 3078, 3019, 2974, 2896, 2836, 1622, 1606, 1590, 1571, 1552, 1533, 1511, 1472, 1445, 1435, 1421, 1405, 1368, 1338, 1306, 1284, 1270, 1243, 1173, 1139, 1124, 1112, 1078, 1030, 1010, 992, 951, 880, 861, 837, 830, 806, 770, 723, 702, 652, 628; 1H NMR: δ: 3.94 (s, 3H, OCH3); 7.14 (d, J=8.2, H-3', 5'), 7.51 (d, J=8.5, 1H, H-8), 7.91 (d, J=8.6, 1H, H-9), 8.59-8.42 (m, 3H, H-11, H-2', 6'), 13.92 (s, 1H,NH); LC-MS, m/z =463 [M+1], 465 [M+3]; Anal. calcd. for C17H11IN4O2S: C, 44.17; H, 2.40; N, 12.12;S, 6.94; Found: C, 44.19; H, 2.40; N, 12.11; S, 6.92.
Pharmacology
Antimicrobial Test
The sensitivity of the microorganisms to the synthesized compounds was evaluated according the described methods [8, 9]. The assay was conducted on Mueller-Hinton medium by two-fold serial dilution of the compound in 1 mL, after that 0.1 mL of microbial seeding (106 cells/mL) was added. The minimal inhibitory concentration of the compound was determined by the absence of visual growth in the test tube with a minimal concentration of the substance, then the minimal bactericide/fungicide concentration was determined by the absence of growth on agar after inoculation of the microorganism from the transparent test tubes. Dimethylsulfoxide was used as a solvent, with an initial solution concentration of 1 mg/mL. Preliminary screening was performed on Staphylococcus aureus ATCC 25923, Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, and Candida albicans ATCC 885-653 standard test cultures. All test strains were received from the bacteriological laboratory Zaporizhzhya Regional Laboratory Center of State Sanitary and Epidemiological Service of Ukraine. Nitrofual and Trimetoprim were used as the reference compound with proven antibacterial/antifungal activity. Additional quality control of the culture medium and solvents was conducted by commonly used methods [17].
Conclusion
In the present paper, 43 new 8-R1-9-R2-10-R3-3-R-6-thio-6, 7-dihydro-2H-[1, 2, 4]triazino-[2, 3-c]quinazoline-2-ones and their potassium salts were described. The synthesized compounds were tested for antibacterial and antifungal activity. As a result of the microbiological assay, the compounds with high inhibitory action against Staphylococcus aureus ATCC 25923 (MIC 6.25–100 μg/mL, MBC 12.5–200 μg/mL) were found.
Acknowledgement
The authors gratefully acknowledge “Enamine Ltd.” (Kiev, Ukraine) for the financial support of this work.
Authors’ Statement
Competing Interests
The authors declare no conflict of interest.
References
- [1].Michael JP. Quinoline, Quinazoline and Acridone Alkaloids. Nat Prod Rep. 2005; 22: 627–646. http://dx.doi.org/10.1039/b413750g [DOI] [PubMed] [Google Scholar]
- [2]. http://www.drugbank.ca/ [Google Scholar]
- [3].Armarego WLF. Chapter IV. Oxoquinazolines and 5-, 6-, 7-, and 8-Hydroxyquinazolines. Chem Heterocycl Comp. 2008; 24: 69–218. http://dx.doi.org/10.1002/9780470186916.ch4 [Google Scholar]
- [4].Marzaro G, Guiotto A, Chilin A. Quinazoline derivatives as potential anticancer agents: a patent review (2007-2010). Expert Opin Ther Pat. 2012; 22: 223–252. http://dx.doi.org/10.1517/13543776.2012.665876 [DOI] [PubMed] [Google Scholar]
- [5].Karpenko OV, Kovalenko SI, Chekotylo OlO, Shyshkyna SV. A new one-step synthesis of [1, 2, 4]triazino[2, 3-c]quinazolines. Heterocycles. 2007; 71: 619–626. http://dx.doi.org/10.3987/COM-06-10971 [Google Scholar]
- [6].Karpenko OV, Kovalenko SI, Shishkin OV. Synthesis of spiro-fused (C5)-pyrazolino-(C6)-triazinones, a new heterocyclic system. Tetrahedron. 2009; 65: 5964–5972. http://dx.doi.org/10.1016/j.tet.2009.05.091 [Google Scholar]
- [7].Voskoboynik OYu, Karpenko OV, Sergeieva TYu, Kovalenko SI, Okovytyy SI. [5+1]-Heterocyclisation in synthesis of as-triazino[2, 3-c]quinazoline systems. Zh Org Farm Khim [J Org Pharm Chem]. 2013; 11: 37–44. [Google Scholar]
- [8].Berest GG, Voskoboynic AY, Kovalenko SI, Sinyak RS, Omelchenko IV, Shishkin OV, Komarovska-Porokhnyavets EZ, Novikov VP. An Efficient Synthesis of 3-R-6-thio-6, 7-dihydro-2H-[1, 2, 4]triazino[2, 3-c]-quinazoline-2-ones and its Derivatives, Antimicrobial and Antifungal Activity. Zh Org Farm Khim [J Org Pharm Chem]. 2010; 8: 42–52. [Google Scholar]
- [9].Berest GG, Voskoboynik AY, Kovalenko SI, Antypenko AM, Nosulenko IS, Katsev AM, Shandrovskaya AS. Synthesis and biological activity of novel N-cycloalkyl-(cycloalkylaryl)-2-[(3-R-2-oxo-2H-[1, 2, 4]triazino[2, 3-c]quinazoline-6-yl)thio]acetamides. Eur J Med Chem. 2011; 46: 6066–6074. http://dx.doi.org/10.1016/j.ejmech.2011.10.022 [DOI] [PubMed] [Google Scholar]
- [10].Berest GG, Voskoboynik OY, Nosulenko IS, Rak IE, Sinyak RS. Synthesis of S-substituted 3-R-6-thio-6,7-dihydro-2H-[1, 2, 4]triazino[2, 3-c]quinazolin-2-ones. Clin Pharm Pharmacol Parmacother Med Stand. 2011;1–2: 197–205. [Google Scholar]
- [11].Berest GG, Voskoboynik OYu, Kovalenko SI, Nosulenko IS, Antypenko LM, Antypenko OM, Shvets VM, Katsev AM. Synthesis of new 6-{[3C9w-(dialkylamino(heterocyclyl)alkyl]thio}-3-R-2H-[1, 2, 4]triazino[2, 3-c]quinazoline-2-ones and evaluation of their anticancer and antimicrobial activities. Sci Pharm. 2012; 80: 37–65. http://dx.doi.org/10.3797/scipharm.1111-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kovalenko SI, Nosulenko IS, Voskoboynik AYu, Berest GG, Antypenko LM, Antipenko AN, Katsev AM. N-R-2-[(3-R-2-oxo-2H-[1, 2, 4]triazino[2, 3-c]quinazoline-6-yl)thio]acetamides with thiazole and thiadiazole fragments in a molecules. Synthesis, physico-chemical properties, cytotoxicity research by bioluminescence inhibition, anticancer activity. Sci Pharm. 2012; 80: 837–865. http://dx.doi.org/10.3797/scipharm.1208-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kovalenko SI, Nosulenko IS, Voskoboynik AYu, Berest GG, Antypenko LM, Antipenko AN, Katsev AM. Novel N-aryl(alkaryl)-2-[(3-R-2-oxo-2H-[1, 2, 4]triazino[2, 3-c]quinazoline-6-yl)thio]-acetamides: synthesis, cytotoxicity, anticancer activity, compare analysis and docking. Med Chem Res. 2013; 22: 2610–2632. http://dx.doi.org/10.1007/s00044-012-0257-x [Google Scholar]
- [14].Voskoboynik AYu, Berest GG, Skorina DYu, Karpenko AV, Kovalenko SI. Synthesis of 6-R-3-(2-aminophenyl)-2H-1,2,4-thriazin-5-ones: resources and limitations. Chem Chem Technol. 2011; 5: 129–132. [Google Scholar]
- [15].Wolfson NS, Zaikin VG, Mikaia AI. Mass spectrometry of Organic comounds. Moskow.:Chemistry, 1986: 312c. [Google Scholar]
- [16].Breitmaier E. Structure Elucidation By NMR In Organic Chemistry: A Practical Guide. John Wiley &Sons, Ltd. 2002: 258p. [Google Scholar]
- [17].Wayne PA. Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standard - 9th Edition. Clinical and Laboratory Standards Institute; CLSI M2-A9. [Google Scholar]


