Abstract
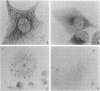
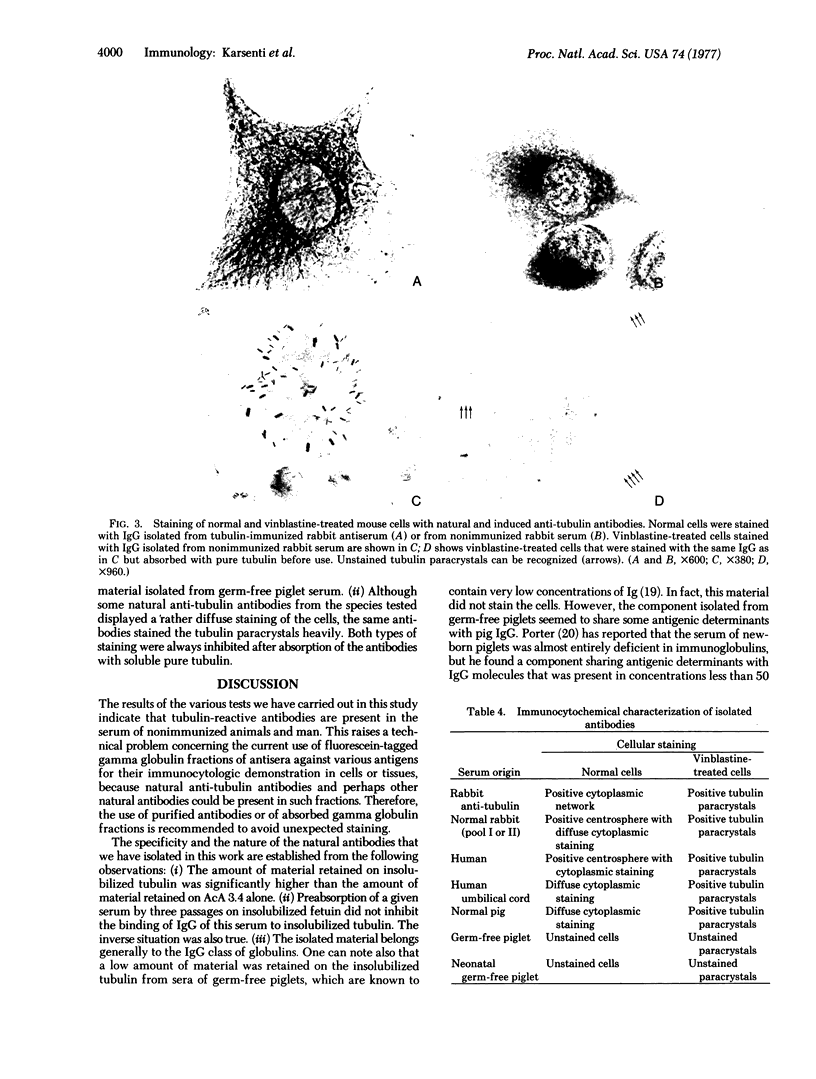
Sera of normal nonimmunized rabbits, pigs, calves, and humans contain tubulin-reactive antibodies. Usually, low amounts of antibodies against tubulin of the IgG class (2.5-4 mg/100 ml of serum from nonimmunized animals) were isolated. Anti-tubulin antibodies were also produced by injecting pig tubulin in complete Freund's adjuvant into rabbits. Slightly higher amounts of anti-tubulin antibody were isolated from sera of immunized rabbits (7 mg/100 ml of serum). The cytoplasmic network of microtubules of Tcc 36 mouse cells in culture was not clearly stained by natural anti-tubulin antibodies, but dense staining of the centrosphere was observed. In contrast, induced anti-tubulin antibodies densely stained cytoplasmic microtubular networks. Vinblastine-induced tubulin paracrystals were equally stained by natural and induced anti-tubulin antibodies.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avrameas S., Ternynck T. Biologically active water-insoluble protein polymers. I. Their use for isolation of antigens and antibodies. J Biol Chem. 1967 Apr 10;242(7):1651–1659. [PubMed] [Google Scholar]
- Avrameas S., Ternynck T. Peroxidase labelled antibody and Fab conjugates with enhanced intracellular penetration. Immunochemistry. 1971 Dec;8(12):1175–1179. doi: 10.1016/0019-2791(71)90395-8. [DOI] [PubMed] [Google Scholar]
- Bordenave G. R. A study of idiotypic suppression in adult rabbits immunized with Salmonella abortus-equi. Immunology. 1975 Apr;28(4):635–651. [PMC free article] [PubMed] [Google Scholar]
- Bussard A. E., Pages J. The peritoneal cell phenomenon: its general significance in cellular immunology. Ann Immunol (Paris) 1976 Jun-Jul;127(3-4):583–597. [PubMed] [Google Scholar]
- Fuller G. M., Brinkley B. R., Boughter J. M. Immunofluorescence of mitotic spindles by using monospecific antibody against bovine brain tubulin. Science. 1975 Mar 14;187(4180):948–950. doi: 10.1126/science.1096300. [DOI] [PubMed] [Google Scholar]
- GRABAR P., WILLIAMS C. A., Jr Méthode immuno-électrophorétique d'analyse de mélanges de substances antigéniques. Biochim Biophys Acta. 1955 May;17(1):67–74. doi: 10.1016/0006-3002(55)90320-6. [DOI] [PubMed] [Google Scholar]
- Grabar P. Hypothesis. Auto-antibodies and immunological theories: an analytical review. Clin Immunol Immunopathol. 1975 Nov;4(4):453–466. doi: 10.1016/0090-1229(75)90087-2. [DOI] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Hart D. A., Wang A. L., Pawlak L. L., Nisonoff A. Suppression of idiotypic specificities in adult mice by administration of antiidiotypic antibody. J Exp Med. 1972 Jun 1;135(6):1293–1300. doi: 10.1084/jem.135.6.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsenti E., Bornens M., Avrameas S. Control of density and microredistribution of concanavalin-A receptors in rat thymocytes at 4 degrees C. Eur J Biochem. 1977 May 2;75(1):251–256. doi: 10.1111/j.1432-1033.1977.tb11524.x. [DOI] [PubMed] [Google Scholar]
- Lidman K., Biberfeld G., Fagraeus A., Norberg R., Torstensson R., Utter G., Carlsson L., Luca J., Lindberg U. Anti-actin specificity of human smooth muscle antibodies in chronic active hepatitis. Clin Exp Immunol. 1976 May;24(2):266–272. [PMC free article] [PubMed] [Google Scholar]
- Luduena R. F., Woodward D. O. Alpha- and beta-tubulin: separation and partial sequence analysis. Ann N Y Acad Sci. 1975 Jun 30;253:272–283. doi: 10.1111/j.1749-6632.1975.tb19206.x. [DOI] [PubMed] [Google Scholar]
- Martin S. E., Martin W. J. Interspecies brain antigen detected by naturally occurring mouse anti-brain autoantibody. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1036–1040. doi: 10.1073/pnas.72.3.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W. J., Martin S. E. Thymus reactive IgM autoantibodies in normal mouse sera. Nature. 1975 Apr 24;254(5502):716–718. doi: 10.1038/254716a0. [DOI] [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. Prog Allergy. 1958;5:1–78. [PubMed] [Google Scholar]
- Porter P. Transfer of immunoglobulins IgG, IgA and IgM to lacteal secretions in the parturient sow and their absorption by the neonatal piglet. Biochim Biophys Acta. 1969 Jul 1;181(2):381–392. doi: 10.1016/0005-2795(69)90271-2. [DOI] [PubMed] [Google Scholar]
- Sela B. A., Wang J. L., Edelman G. M. Antibodies reactive with cell surface carbohydrates. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1127–1131. doi: 10.1073/pnas.72.3.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelanski M. L., Gaskin F., Cantor C. R. Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci U S A. 1973 Mar;70(3):765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Pollack R., Bibring T. Antibody against tuberlin: the specific visualization of cytoplasmic microtubules in tissue culture cells. Proc Natl Acad Sci U S A. 1975 Feb;72(2):459–463. doi: 10.1073/pnas.72.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingarten M. D., Lockwood A. H., Hwo S. Y., Kirschner M. W. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A. 1975 May;72(5):1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiche G., Cole R. D. An improved preparation of highly specific tublin antibodies. Exp Cell Res. 1976 Apr;99(1):15–22. doi: 10.1016/0014-4827(76)90674-1. [DOI] [PubMed] [Google Scholar]