Abstract
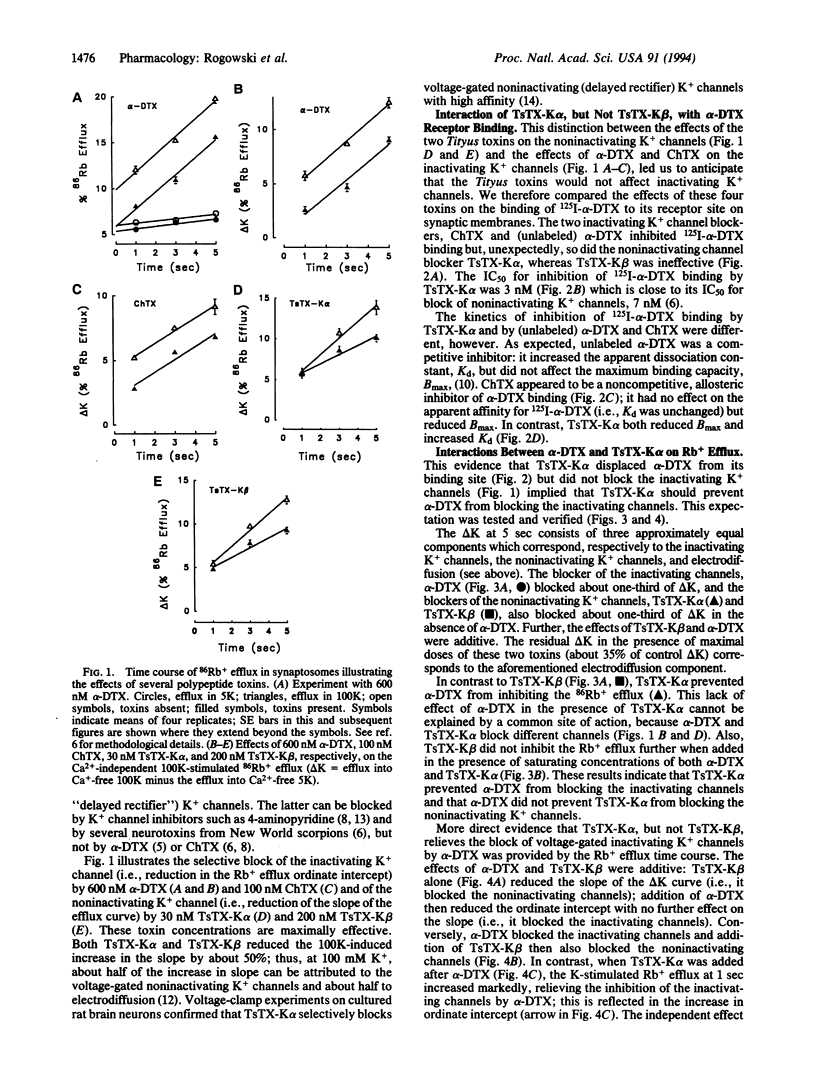
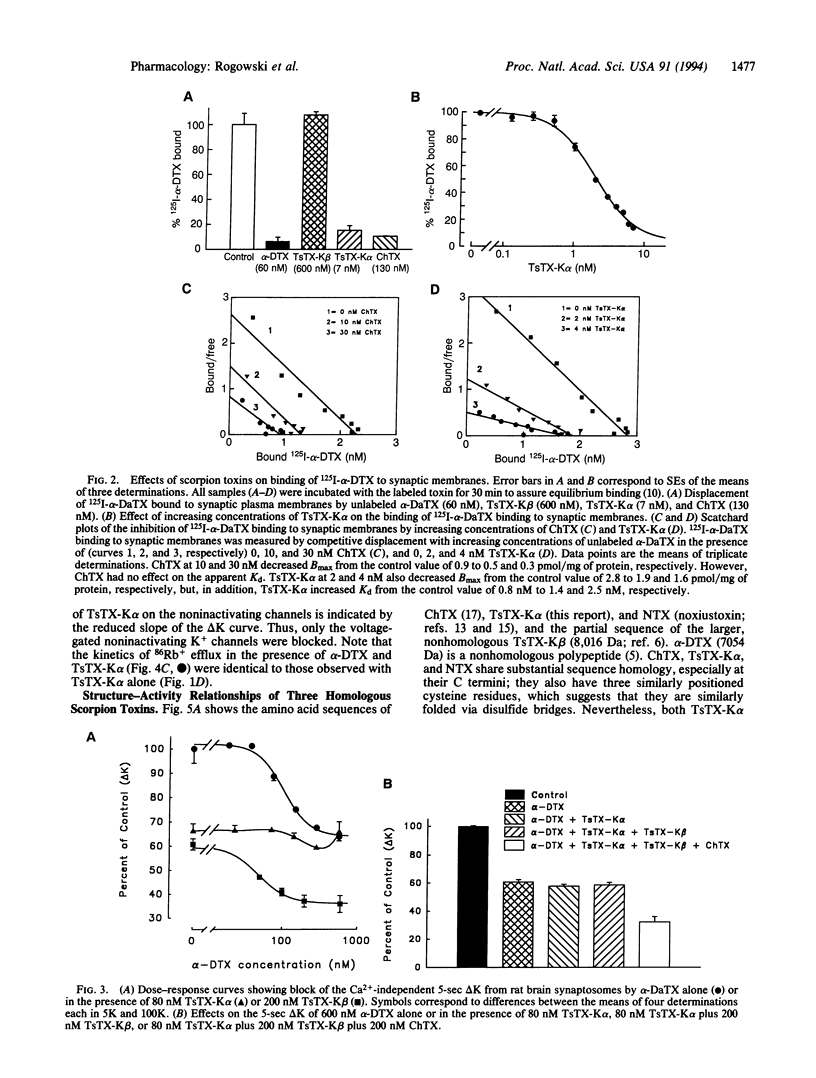
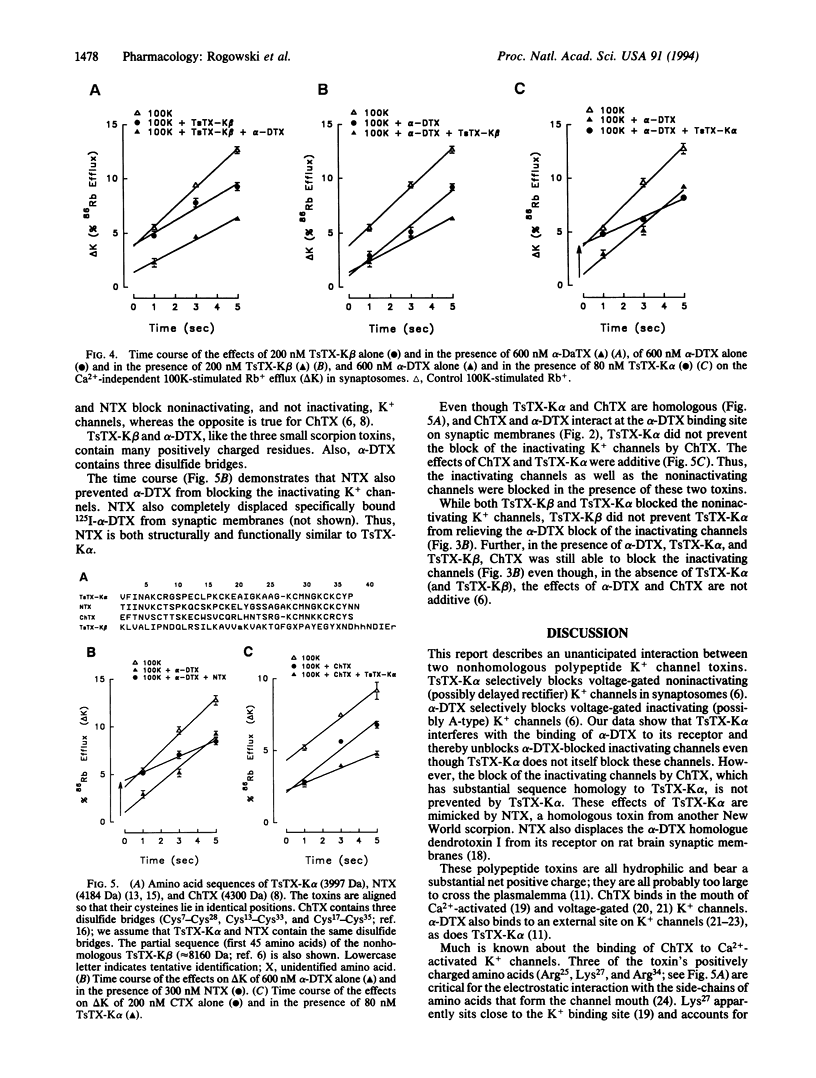
Two nonhomologous polypeptide toxins, tityustoxin K alpha (TsTX-K alpha) and tityustoxin K beta (TsTX-K beta), purified from the venom of the Brazilian scorpion Tityus serrulatus, selectively block voltage-gated noninactivating K+ channels in synaptosomes (IC50 values of 8 nM and 30 nM, respectively). In contrast, alpha-dendrotoxin (alpha-DTX) and charybdotoxin (ChTX) block voltage-gated inactivating K+ channels in synaptosomes (IC50 values of 90 nM and 40 nM, respectively). We studied interactions among these toxins in 125I-alpha-DTX binding and 86Rb efflux experiments. Both TsTX-K alpha and ChTX completely displaced specifically bound 125I-alpha-DTX from synaptic membranes, but TsTX-K beta had no effect on bound alpha-DTX. TsTX-K alpha and TsTX-K beta blocked the same noninactivating component of 100 mM K(+)-stimulated 86Rb efflux in synaptosomes. Both alpha-DTX and ChTX blocked the same inactivating component of the K(+)-stimulated 86Rb efflux in synaptosomes. Both the inactivating and the noninactivating components of the 100 mM K(+)-stimulated 86Rb efflux were completely blocked when 200 nM TsTX-K beta and either 600 nM alpha-DTX or 200 nM ChTX were present. The effects of TsTX-K alpha and ChTX on 86Rb efflux were also additive. When TsTX-K alpha was added in the presence of alpha-DTX, however, only the noninactivating component of the K(+)-stimulated efflux was blocked. The inactivating component could then be blocked by ChTX, which is structurally homologous to TsTX-K alpha. We conclude that TsTX-K alpha unblocks the voltage-gated inactivating K+ channels in synaptosomes when they are blocked by alpha-DTX, but not when they are blocked by ChTX. TsTX-K alpha binds to a site on the inactivating K+ channel that does not occlude the pore; its binding apparently prevents alpha-DTX (7054 Da), but not ChTX (4300 Da), from blocking the pore. The effects of TsTX-K alpha on 125I-alpha-DTX binding and 86Rb efflux are mimicked by noxiustoxin, which is homologous to TsTX-K alpha and ChTX.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auguste P., Hugues M., Mourre C., Moinier D., Tartar A., Lazdunski M. Scyllatoxin, a blocker of Ca(2+)-activated K+ channels: structure-function relationships and brain localization of the binding sites. Biochemistry. 1992 Jan 28;31(3):648–654. doi: 10.1021/bi00118a003. [DOI] [PubMed] [Google Scholar]
- Bartschat D. K., Blaustein M. P. Potassium channels in isolated presynaptic nerve terminals from rat brain. J Physiol. 1985 Apr;361:419–440. doi: 10.1113/jphysiol.1985.sp015653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benishin C. G., Sorensen R. G., Brown W. E., Krueger B. K., Blaustein M. P. Four polypeptide components of green mamba venom selectively block certain potassium channels in rat brain synaptosomes. Mol Pharmacol. 1988 Aug;34(2):152–159. [PubMed] [Google Scholar]
- Blaustein M. P., Rogowski R. S., Schneider M. J., Krueger B. K. Polypeptide toxins from the venoms of Old World and New World scorpions preferentially block different potassium channels. Mol Pharmacol. 1991 Dec;40(6):932–942. [PubMed] [Google Scholar]
- Chicchi G. G., Gimenez-Gallego G., Ber E., Garcia M. L., Winquist R., Cascieri M. A. Purification and characterization of a unique, potent inhibitor of apamin binding from Leiurus quinquestriatus hebraeus venom. J Biol Chem. 1988 Jul 25;263(21):10192–10197. [PubMed] [Google Scholar]
- Crest M., Jacquet G., Gola M., Zerrouk H., Benslimane A., Rochat H., Mansuelle P., Martin-Eauclaire M. F. Kaliotoxin, a novel peptidyl inhibitor of neuronal BK-type Ca(2+)-activated K+ channels characterized from Androctonus mauretanicus mauretanicus venom. J Biol Chem. 1992 Jan 25;267(3):1640–1647. [PubMed] [Google Scholar]
- Dreyer F. Peptide toxins and potassium channels. Rev Physiol Biochem Pharmacol. 1990;115:93–136. [PubMed] [Google Scholar]
- Galvez A., Gimenez-Gallego G., Reuben J. P., Roy-Contancin L., Feigenbaum P., Kaczorowski G. J., Garcia M. L. Purification and characterization of a unique, potent, peptidyl probe for the high conductance calcium-activated potassium channel from venom of the scorpion Buthus tamulus. J Biol Chem. 1990 Jul 5;265(19):11083–11090. [PubMed] [Google Scholar]
- Gimenez-Gallego G., Navia M. A., Reuben J. P., Katz G. M., Kaczorowski G. J., Garcia M. L. Purification, sequence, and model structure of charybdotoxin, a potent selective inhibitor of calcium-activated potassium channels. Proc Natl Acad Sci U S A. 1988 May;85(10):3329–3333. doi: 10.1073/pnas.85.10.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey A. L., Anderson A. J. Dendrotoxins: snake toxins that block potassium channels and facilitate neurotransmitter release. Pharmacol Ther. 1985;31(1-2):33–55. doi: 10.1016/0163-7258(85)90036-1. [DOI] [PubMed] [Google Scholar]
- Harvey A. L., Marshall D. L., Possani L. D. Dendrotoxin-like effects of noxiustoxin. Toxicon. 1992 Nov;30(11):1497–1500. doi: 10.1016/0041-0101(92)90528-d. [DOI] [PubMed] [Google Scholar]
- Hurst R. S., Busch A. E., Kavanaugh M. P., Osborne P. B., North R. A., Adelman J. P. Identification of amino acid residues involved in dendrotoxin block of rat voltage-dependent potassium channels. Mol Pharmacol. 1991 Oct;40(4):572–576. [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N. Structural elements involved in specific K+ channel functions. Annu Rev Physiol. 1992;54:537–555. doi: 10.1146/annurev.ph.54.030192.002541. [DOI] [PubMed] [Google Scholar]
- Lucchesi K., Ravindran A., Young H., Moczydlowski E. Analysis of the blocking activity of charybdotoxin homologs and iodinated derivatives against Ca2+-activated K+ channels. J Membr Biol. 1989 Aug;109(3):269–281. doi: 10.1007/BF01870284. [DOI] [PubMed] [Google Scholar]
- MacKinnon R., Heginbotham L., Abramson T. Mapping the receptor site for charybdotoxin, a pore-blocking potassium channel inhibitor. Neuron. 1990 Dec;5(6):767–771. doi: 10.1016/0896-6273(90)90335-d. [DOI] [PubMed] [Google Scholar]
- Miller C., Moczydlowski E., Latorre R., Phillips M. Charybdotoxin, a protein inhibitor of single Ca2+-activated K+ channels from mammalian skeletal muscle. Nature. 1985 Jan 24;313(6000):316–318. doi: 10.1038/313316a0. [DOI] [PubMed] [Google Scholar]
- Moczydlowski E., Lucchesi K., Ravindran A. An emerging pharmacology of peptide toxins targeted against potassium channels. J Membr Biol. 1988 Oct;105(2):95–111. doi: 10.1007/BF02009164. [DOI] [PubMed] [Google Scholar]
- Park C. S., Miller C. Interaction of charybdotoxin with permeant ions inside the pore of a K+ channel. Neuron. 1992 Aug;9(2):307–313. doi: 10.1016/0896-6273(92)90169-e. [DOI] [PubMed] [Google Scholar]
- Park C. S., Miller C. Mapping function to structure in a channel-blocking peptide: electrostatic mutants of charybdotoxin. Biochemistry. 1992 Sep 1;31(34):7749–7755. doi: 10.1021/bi00149a002. [DOI] [PubMed] [Google Scholar]
- Schneider M. J., Rogowski R. S., Krueger B. K., Blaustein M. P. Charybdotoxin blocks both Ca-activated K channels and Ca-independent voltage-gated K channels in rat brain synaptosomes. FEBS Lett. 1989 Jul 3;250(2):433–436. doi: 10.1016/0014-5793(89)80771-9. [DOI] [PubMed] [Google Scholar]
- Schweitz H., Stansfeld C. E., Bidard J. N., Fagni L., Maes P., Lazdunski M. Charybdotoxin blocks dendrotoxin-sensitive voltage-activated K+ channels. FEBS Lett. 1989 Jul 3;250(2):519–522. doi: 10.1016/0014-5793(89)80788-4. [DOI] [PubMed] [Google Scholar]
- Sorensen R. G., Blaustein M. P. Rat brain dendrotoxin receptors associated with voltage-gated potassium channels: dendrotoxin binding and receptor solubilization. Mol Pharmacol. 1989 Nov;36(5):689–698. [PubMed] [Google Scholar]
- Stocker M., Pongs O., Hoth M., Heinemann S. H., Stühmer W., Schröter K. H., Ruppersberg J. P. Swapping of functional domains in voltage-gated K+ channels. Proc Biol Sci. 1991 Aug 22;245(1313):101–107. doi: 10.1098/rspb.1991.0094. [DOI] [PubMed] [Google Scholar]
- Sugg E. E., Garcia M. L., Reuben J. P., Patchett A. A., Kaczorowski G. J. Synthesis and structural characterization of charybdotoxin, a potent peptidyl inhibitor of the high conductance Ca2(+)-activated K+ channel. J Biol Chem. 1990 Nov 5;265(31):18745–18748. [PubMed] [Google Scholar]
- Valdivia H. H., Smith J. S., Martin B. M., Coronado R., Possani L. D. Charybdotoxin and noxiustoxin, two homologous peptide inhibitors of the K+ (Ca2+) channel. FEBS Lett. 1988 Jan 4;226(2):280–284. doi: 10.1016/0014-5793(88)81439-x. [DOI] [PubMed] [Google Scholar]
- Werkman T. R., Gustafson T. A., Rogowski R. S., Blaustein M. P., Rogawski M. A. Tityustoxin-K alpha, a structurally novel and highly potent K+ channel peptide toxin, interacts with the alpha-dendrotoxin binding site on the cloned Kv1.2 K+ channel. Mol Pharmacol. 1993 Aug;44(2):430–436. [PubMed] [Google Scholar]
- Werkman T. R., Kawamura T., Yokoyama S., Higashida H., Rogawski M. A. Charybdotoxin, dendrotoxin and mast cell degranulating peptide block the voltage-activated K+ current of fibroblast cells stably transfected with NGK1 (Kv1.2) K+ channel complementary DNA. Neuroscience. 1992 Oct;50(4):935–946. doi: 10.1016/0306-4522(92)90216-o. [DOI] [PubMed] [Google Scholar]


