Abstract
Background
The invasive Asian tiger mosquito Aedes albopictus has dramatically expanded its distribution range, being catalogued as one of the world’s 100 worst invasive alien species. As vectors of pathogens, Ae. albopictus may create novel epidemiological scenarios in the invaded areas.
Methods
Here, the frequency of encounters of Ae. albopictus with the avian malaria parasite Plasmodium and the related Haemoproteus was studied in an area with established populations in northeastern Italy and compared with those from four native mosquito species, Anopheles maculipennis s.l., Culex hortensis, Culex pipiens, and Ochlerotatus caspius. The abdomens of mosquitoes with a recent blood meal were used to identify both the blood meal source and the parasites harboured.
Results
Aedes albopictus had a clear antropophilic behaviour while An. maculipennis and Oc. caspius fed mainly on non-human mammals. Birds were the most common hosts of Cx. pipiens and reptiles of Cx. hortensis. Parasites were isolated from three mosquito species, with Cx. pipiens (30%) showing the highest parasite prevalence followed by Cx. hortensis (9%) and Ae. albopictus (5%).
Conclusions
These results are the first identifying the avian malaria parasites harboured by mosquitoes in Italy and represent the first evidence supporting that, although Ae. albopictus could be involved in the transmission of avian malaria parasites, the risk of avian malaria parasite spread by this invasive mosquito in Europe would be minimal.
Keywords: Aedes albopictus, Avian diseases, Culex pipiens, Haemoproteus, Mosquitoes, Plasmodium, Vector, West Nile virus
Background
Establishment of exotic mosquitoes to new areas may create novel epidemiological scenarios with potential dramatic consequences for wildlife and human health [1]. The invasive Asian tiger mosquito Aedes albopictus, indigenous to Southeast Asia, islands of the Western Pacific and Indian Ocean, has expanded its distribution range to Africa, Europe and the Americas [2,3]. In Europe, this species was first recorded in 1979 in Albania [4] and subsequently in Italy, France and other countries of the Mediterranean region and northern Europe [1,3]. Aedes albopictus is vector of a diversity of pathogens including flaviviruses (e.g., West Nile virus, Dengue virus), alphaviruses (e.g., Chikungunya virus), and other viruses and filarial worms [5,6]. In Europe, Ae. albopictus has been incriminated in the transmission of both introduced (Chikungunya and Dengue viruses) and endemic (Dirofilaria nematodes) pathogens [5,7].
Avian malaria parasites of the genus Plasmodium, and the relative haemosporidian Haemoproteus, produce pathogenic effects on both vertebrate and invertebrate hosts [8]. Plasmodium parasites require the intervention of a mosquito vector to be transmitted from an infected bird to another individual. Haemoproteus parasites have a similar life cycle, requiring a biting midge Culicoides or louse flies instead of mosquitoes to be transmitted between birds [8]. During a bite event a mosquito feeding on an infected bird is able to acquire the parasites contained in the blood. A number of mosquito species belonging to different genera such as Aedes, Anopheles and Culex, have been reported as potential vectors of avian malaria parasites [9]. Although different factors may influence the subsequent development of parasites in the mosquito after blood ingestion [10], pathogen isolation of recent blood meals may provide valuable information on parasite-mosquito encounters and potential parasite transmission [11,12]. In this respect, the blood ingested by potential vectors can be used as a source of host DNA to identify both the feeding sources of mosquitoes [13] and the blood parasites reaching these potential vectors [12].
In spite of their importance on parasite transmission, only a handful of studies have identified the blood parasites interacting with wild mosquito populations in Europe [14-18], and, no previous study has tested for the presence of avian malaria parasites in invasive populations of the tiger mosquito Ae. albopictus. Here, two molecular approaches were used to identify both host and avian malaria parasites from blood contained in the mosquito’s abdomen following protocols described by Alcaide et al. [13] and Hellgren et al. [19], respectively. Samples from five different mosquito species collected in northeastern Italy were included in this study: the invasive Ae. albopictus, and the native Anopheles maculipennis s.l., Culex hortensis, Culex pipiens, and Ochlerotatus caspius.
Methods
Mosquito sampling and morphological identification
Mosquitoes were collected from May to October 2012 using BG-sentinel traps baited with BG-lure and dry ice. Twenty traps in Veneto and ten in Trentino provinces operated once a week or two weeks for 24 hr, respectively. In Trentino mosquitoes were also collected using a motor-powered aspirator. Mosquitoes were morphologically identified following the keys of the Italian Culicidae adults [20] and preserved frozen (−20 or −80°C) until examination.
Blood meal source and parasite identification
DNA from the abdomen of blood-fed mosquitoes was individually isolated using the DNeasy Blood and Tissue® kit (QIAGEN, Hilden, Germany) following company specifications. This DNA extraction approach resulted in a higher efficacy of host identification than other protocols such as Hotshot [21]. Vertebrate blood meal origin was identified using a nested-PCR approach [13] to amplify a 758-base pairs fragment of the mitochondrial cytochrome oxidase 1 (COI) gene. Both negative controls for PCR reactions (at least one per plate) and DNA extraction were included in the analysis. DNA was also used to identify the presence of Plasmodium and Haemoproteus parasites based on the amplification of a fragment of the mitochondrial Cytochrome b gene [19].
Positive amplifications were sequenced using the Big Dye 1.1 technology (Applied Biosystems). Labelled DNA fragments of positive PCR products were resolved with an ABI 3130xl automated sequencer (Applied Biosystems) using the same forward and reverse primers used in the nested-PCR amplification for the case of blood parasite identifications. For blood meal identifications, amplicons were sequenced in one direction using the primer BCRV2, except for the case of Ae. albopictus mosquitoes that were sequenced using the primer BCVINT-RV (see [22]). Sequences were edited using the software Sequencher™ v 4.9 (Gene Codes Corp., © 1991–2009, Ann Arbor, MI 48108, USA). Blood meal sequences were assigned to particular vertebrate species when agreement was ≥98% to sequences of known species in GenBank DNA sequence database (National Center for Biotechnology Information Blast) or the Barcode of Life Data Systems (BOLD). Parasite lineages were identified by comparison with sequences deposited in GenBank database. Statistical significance of differences in parasite prevalence was tested with statistical software JMP (version 9.0.1).
Results
Overall, 348 blood-fed mosquitoes belonging to five different species were included in this study. The most extensively species sampled was Cx. pipiens (n = 264), followed by Ae. albopictus (n = 41), An. maculipennis (n = 16), Oc. caspius (n = 16) and Cx. hortensis (n = 11). The blood meal source of 290 (83.3%) of them was successfully identified, compromising, at least, 36 vertebrate species including 11 mammals, 23 birds and two reptiles (Table 1). Blackbirds Turdus merula was the most common host species of mosquitoes compromising 73 blood meals. Clear differences in mosquito feeding sources were found among mosquito species (Figure 1). Three mosquitoes showed evidence of mixed blood meals tentatively identified: H. sapiens + Columba livia, H. sapiens + Gallus gallus and H. sapiens + Felis silvestris/catus.
Table 1.
Blood meal source of mosquitoes in Italy
| Mosquito species | Mammal | Bird | Reptile |
|---|---|---|---|
| Ae. albopictus | Homo sapiens (31) | Passer montanus (1) | |
| Erinaceus europaeus (1) | Turdus merula (1) | ||
| An. maculipennis | Canis lupus familiares (3) | Gallus gallus (1) | |
| Equus asinus (2) | |||
| Equus caballus (2) | |||
| Lepus europaeus (2) | |||
| Bos taurus (1) | |||
| Capra hircus (1) | |||
| Felis silvestris/catus (1) | |||
| Homo sapiens (1) | |||
| Vulpes vulpes (1) | |||
| Cx. hortensis | Homo sapiens (1) | Podarcis muralis (7) | |
| Cx. pipiens | Homo sapiens (14) | Turdus merula (72) | Podarcis muralis (4) |
| Felis silvestris/catus (9) | Passer domesticus (26) | Lacerta spp. (1) | |
| Canis lupus familiaris (5) | Gallus gallus (21) | ||
| Equus caballus (3) | Streptopelia decaocto (16) | ||
| Sus scrofa (3) | Columba livia (5) | ||
| Bos taurus (2) | Passer montanus (5) | ||
| Erinaceus europaeus (1) | Athene noctua (4) | ||
| Meleagris gallopavo (3) | |||
| Columba palumbus (3) | |||
| Pica pica (3) | |||
| Anas platyrhychos (2) | |||
| Sturnus vulgaris (2) | |||
| Accipiter nisus (1) | |||
| Cairina moschata (1) | |||
| Carduelis carduelis (1) | |||
| Gallinula chloropus (1) | |||
| Jynx torquilla (1) | |||
| Numida meleagris (1) | |||
| Nycticorax nycticorax (1) | |||
| Oriolus oriolus (1) | |||
| Parus major (1) | |||
| Phasianus colchinus (1) | |||
| Sylvia atricapilla (1) | |||
| Oc. caspius | Felis silvestris/catus (6) | Gallus gallus (1) | |
| Equus asinus (3) | |||
| Equus caballus (2) | |||
| Bos taurus (1) | |||
| Canis lupus familiaris (1) | |||
| Homo sapiens (1) |
Mixed blood meals from more than one host species were excluded.
Figure 1.
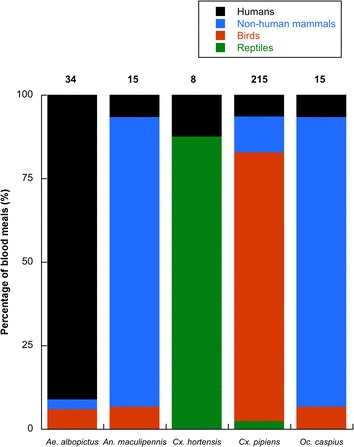
Source of mosquito blood meals of the five mosquito species analysed. Numbers above bars indicate the number of blood meals per mosquito species identified. Mixed blood meals from more than one host species were excluded.
Parasite infection status differed among mosquito species (χ2 = 35.78, d.f. = 4, p < 0.001) with Cx. pipiens showing higher prevalence of infection (30%, 80 infected out of 264 tested) than Cx. hortensis (9%, 1/11) and Ae. albopictus (5%, 2/41) (Table 2). Blood parasites were not found in An. maculipennis nor Oc. caspius. With the exception of two mosquitoes with blood meals from reptiles, the rest of the parasites detected corresponded to mosquitoes containing an avian-derived blood meal (Table 3). Seven out of 58 mosquito abdomens with too degraded blood to allow blood meal origin identification showed parasite positive amplifications. Sequences with double peaks in the chromatogram were obtained from four mosquitoes, probably reflecting the presence of more than one parasite lineages. Six Plasmodium and four Haemoproteus lineages were isolated from mosquitoes (Tables 2,3). The lineages identified were the Plasmodium lineages: SGS1 (also called Rinshi-1, belonging to Plasmodium relictum, n = 9), LINN1 (also called pSPHUjJ, n = 10), SYAT05 (also called Rinshi-11, belonging to Plasmodium vaughani, n = 47), Delurb4 (n = 2), GRW11 (also called Rinshi-7, belonging to Plasmodium relictum, n = 1) and Aftru5 (n = 1). The Haemoproteus lineages isolated were TURDUS2 (also called Bolin1, belonging to Haemoproteus minutus, n = 3), Padom3 (n = 2), hItCxpip01 (n = 2) and hCIRCUM05 (n = 1). The Haemoproteus lineage hItCxpip01 [GenBank: KP120693], described here for the first time, was isolated from two mosquitoes with blood from magpies Pica pica.
Table 2.
Blood parasite lineages isolated from mosquito blood meals
| Plasmodium | Haemoproteus | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AFTRU5 | LINN1 | Delurb4 | SGS1 | GRW11 | SYAT05 | TURDUS2 | hCIRCUM05 | hItCxpip01 | Padom3 | |
| Cx. pipiens | 1 | 9 | 2 | 9 | 1 | 46 | 4 | 1 | 2 | 2 |
| Cx. hortensis | 1 | |||||||||
| Ae. albopictus | 1 | |||||||||
Table 3.
Blood meal source of mosquitoes harbouring identified blood parasite lineages
| Parasite genus | Lineage | Mosquito species | Hosts | Times isolated | Known distribution |
|---|---|---|---|---|---|
| Plasmodium | AFTRU5 | Cx. pipiens | Turdus merula | 1 | Africa, Asia, Europe* |
| Delurb4 | Cx. pipiens | Numida meleagris | 1 | Asia, Europe | |
| Passer domesticus | 1 | ||||
| GRW11 | Cx. pipiens | Passer domesticus | 1 | Africa, Asia, Europe | |
| LINN1 | Cx. pipiens | Turdus merula | 6 | Asia, Europe, Oceanía** | |
| Passer montanus | 1 | ||||
| Athene noctua | 1 | ||||
| Cx. hortensis | Podarcis muralis | 1 | |||
| SGS1 | Cx. pipiens | Passer domesticus | 4 | Africa, America, Asia, Europe, Oceania | |
| Passer montanus | 1 | ||||
| Turdus merula | 1 | ||||
| Gallus gallus | 1 | ||||
| SYAT05 | Cx. pipiens | Turdus merula | 41 | Africa, America, Asia, Europe, Oceanía | |
| Meleagris gallopavo | 1 | ||||
| Passer domesticus | 2 | ||||
| Podarcis muralis | 1 | ||||
| Ae. albopictus | Turdus merula | 1 | |||
| Haemoproteus | TURDUS2 | Cx. pipiens | Turdus merula | 3 | America, Asia, Europe |
| hCircum05 | Cx. pipiens | Pica pica | 1 | Europe | |
| hItCxpip01 | Cx. pipiens | Pica pica | 2 | Europe | |
| Padom3 | Cx. pipiens | Passer montanus | 1 | Europe |
Discussion
Avian Plasmodium and Haemoproteus parasites were isolated from three different mosquito species with clear differences in parasite prevalence. Culex pipiens showed, by far, the highest parasite prevalence, suggesting that this species probably play a central role in the transmission of blood parasites in the studied area. Molecular isolation of parasites from mosquitoes could be used to identify the occurrence of encounters between parasites and mosquitoes, although this not necessarily implies that insects are real vectors of the parasite lineages isolated, because the parasites can be unable to replicate in the salivary glands, that may be the case of Haemoproteus parasites [23]. Although there is no previous information on the role of wild mosquitoes in the transmission of avian malaria parasites in Italy, recent studies have isolated Plasmodium parasites from Cx. pipiens mosquitoes captured in Czech Republic [16], Portugal [18], Switzerland [15,17], and Spain [14]. Also, sporozoites of both Plasmodium relictum and Plasmodium vaughani, parasite lineages found in this study, have been previously isolated from Cx. pipiens mosquitoes [9,24]. Furthermore, the Plasmodium lineage LINN1 was isolated from whole un-engorged Cx. pipiens mosquitoes [25]. Curiously, we isolated avian blood parasites from two mosquito abdomens containing a non-avian derived blood meal. This could be due to the occurrence of undetected mixed blood meals in the mosquito, the presence of avian parasites in the blood on these vertebrate hosts (see [12]) or simply the fact that parasites isolated were in the mosquito tissue but not in the blood meal [26]. This last possibility is supported by the detection of parasite DNA in mosquitoes with no host identification due to degraded blood, and consequently where it is unlikely that the parasites detected come from the vertebrate blood.
The invasive mosquito Ae. albopictus preferably bites on mammals, especially on humans [27-29], which is clearly supported by results from this study. However, this species is able to feed on non-mammal species including birds, with birds compromising between 0.8 to 73.0% of the total blood meals identified in previous studies [30], potentially playing a role on the transmission of avian malaria parasites. Under laboratory conditions, development of avian Plasmodium sporozoites occurs in Ae. albopictus [31], see also [9]. Furthermore, avian malaria parasites have been isolated from wild Ae. albopictus mosquitoes, but usually showing low prevalence of infection [32-34]. Also, some studies have reported the absence of Plasmodium in Ae. albopictus mosquitoes from Japan [27,35]. Overall, results from these studies together with those reported here support that Ae. albopictus could be involved in the transmission of avian malaria parasites, although the risk of parasite spread by this mosquito species in Europe would be minimal due to its low biting rate on birds.
The use of molecular tools on avian malaria studies has allowed the identification of a broad diversity of parasite lineages infecting birds in different areas and, as a result, it is possible to infer their current geographical distribution. Most of the parasite lineages isolated in this study are widespread, being isolated from birds from different countries of the old world (Table 3, see Malavi database [36]). However, although the same parasite cyt b lineage could be found in birds from both Europe and Africa, as in the case of the widespread Plasmodium SGS1, the characterization of highly variable genes (i.e. merozoite surface protein 1 gene, MSP1) provided strong evidence of parasite differentiation among continents, and consequently parasites with the same sequence of cytb may in fact correspond to different lineages with more reduced distribution areas affecting the inference of geographical areas of parasite transmission [37].
In conclusion, our results support that, the risk of spread of avian malaria parasites by the invasive mosquito Ae. albopictus in Europe would be minimal. However, its ability to transmit other pathogens of sanitary importance including viruses and nematodes support the necessity of setting up an active surveillance and control programme on this species [1]. Further studies are necessary in order to identify those factors affecting infections by different avian pathogens (i.e., WNV and avian malaria parasites) in vertebrate [38] and invertebrate [39] hosts, which may determine parasite epidemiology.
Acknowledgements
This study was funded by EU grants FP7-261391 EuroWestNile and FP7-261504 EDENext and the project CGL2012-30759 from the Spanish Ministry of Science and Innovation. This study is catalogued by the EDENext Steering Committee as EDENext301 (http://www.edenext.eu). The contents of this publication are the sole responsibility of the authors and don't necessarily reflect the views of the European Commission. JMP is currently supported by a Juan de la Cierva contract. JM was supported by an International Outgoing Fellowship (FP7-PEOPLE-2010; ADAPT-ENVGENOME; Grant Number 271485). AR, DA and GC were partially supported by the Italian Ministry of Health under the grant Aedespread (RF-2010-2318965). We thank Isabel Martín for their help in the laboratory.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
JMP, AR, GC, RS, and JF conceived and designed the experiments. GC, FM, AR, and DA collected and identified mosquitoes. JMP, JM, RS, and JF conducted molecular analysis. All authors have read and approved the final manuscript.
Contributor Information
Josué Martínez-de la Puente, Email: jmp@ebd.csic.es.
Joaquín Muñoz, Email: quini@ebd.csic.es.
Gioia Capelli, Email: gcapelli@izsvenezie.it.
Fabrizio Montarsi, Email: fmontarsi@izsvenezie.it.
Ramón Soriguer, Email: soriguer@ebd.csic.es.
Daniele Arnoldi, Email: daniele.arnoldi@fmach.it.
Annapaola Rizzoli, Email: annapaola.rizzoli@fmach.it.
Jordi Figuerola, Email: jordi@ebd.csic.es.
References
- 1.Medlock JM, Hansford KM, Schaffner F, Versteirt V, Hendrickx G, Zeller H, et al. A review of the invasive mosquitoes in Europe: ecology, public health risks, and control options. Vector Borne Zoonotic Dis. 2012;12:435–47. doi: 10.1089/vbz.2011.0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gratz NG. Critical review of the vector status of Aedes albopictus. Med Vet Entomol. 2004;18:215–27. doi: 10.1111/j.0269-283X.2004.00513.x. [DOI] [PubMed] [Google Scholar]
- 3.Bonizzoni M, Gasperi G, Chen X, James AA. The invasive mosquito species Aedes albopictus: current knowledge and future perspectives. Trends Parasitol. 2013;29:460–8. doi: 10.1016/j.pt.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adhami J, Reiter P. Introduction and establishment of Aedes (Stegomyia) albopictus Skuse (Diptera: Culicidae) in Albania. J Am Mosq Control Assoc. 1998;14:340–3. [PubMed] [Google Scholar]
- 5.Cancrini G, Frangipane di Regalbono A, Ricci I, Tessarin C, Gabrielli S, Pietrobelli M. Aedes albopictus is a natural vector of Dirofilaria immitis in Italy. Vet Parasitol. 2003;118:195–202. doi: 10.1016/j.vetpar.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 6.Paupy C, Delatte H, Bagny L, Corbel V, Fontenille D. Aedes albopictus, an arbovirus vector: from the darkness to the light. Microbes Infect. 2009;11:1177–85. doi: 10.1016/j.micinf.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Schaffner F, Medlock JM, Van Bortel W. Public health significance of invasive mosquitoes in Europe. Clin Microbiol Infect. 2013;19:685–92. doi: 10.1111/1469-0691.12189. [DOI] [PubMed] [Google Scholar]
- 8.Valkiūnas G. Avian malaria parasites and other haemosporidia. Boca Ratón: CRC Press; 2005. [Google Scholar]
- 9.Santiago-Alarcon D, Palinauskas V, Schaefer HM. Diptera vectors of avian Haemosporidian parasites: untangling parasite life cycles and their taxonomy. Biol Rev Camb Philos Soc. 2012;87:928–64. doi: 10.1111/j.1469-185X.2012.00234.x. [DOI] [PubMed] [Google Scholar]
- 10.Lefèvre T, Vantaux A, Dabiré KR, Mouline K, Cohuet A. Non-genetic determinants of mosquito competence for malaria parasites. PLoS Pathog. 2013;9:e1003365. doi: 10.1371/journal.ppat.1003365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hellgren O, Bensch S, Malmqvist B. Bird hosts, blood parasites and their vectors—associations uncovered by molecular analyses of blackfly blood meals. Mol Ecol. 2008;17:1605–13. doi: 10.1111/j.1365-294X.2007.03680.x. [DOI] [PubMed] [Google Scholar]
- 12.Santiago-Alarcon D, Havelka P, Schaefer HM, Segelbacher G. Bloodmeal analysis reveals avian Plasmodium infections and broad host preferences of Culicoides (Diptera: Ceratopogonidae) vectors. PLoS ONE. 2012;7:e31098. doi: 10.1371/journal.pone.0031098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alcaide M, Rico C, Ruiz S, Soriguer R, Muñoz J, Figuerola J. Disentangling vector-borne transmission networks: a universal DNA barcoding method to identify vertebrate hosts from arthropod bloodmeals. PLoS ONE. 2009;4:e7092. doi: 10.1371/journal.pone.0007092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferraguti M, Martínez-de la Puente J, Muñoz J, Roiz D, Ruiz S, Soriguer R, et al. Avian Plasmodium in Culex and Ochlerotatus mosquitoes from Southern Spain: effects of season and host-feeding source on parasite dynamics. PLoS ONE. 2013;8:e66237. doi: 10.1371/journal.pone.0066237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glaizot O, Fumagalli L, Iritano K, Lalubin F, Van Rooyen J, Christe P. High prevalence and lineage diversity of avian malaria in wild populations of great tits (Parus major) and mosquitoes (Culex pipiens) PLoS ONE. 2012;7:e34964. doi: 10.1371/journal.pone.0034964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Synek P, Munclinger P, Albrecht T, Votýpka J. Avian haemosporidians in haematophagous insects in the Czech Republic. Parasitol Res. 2013;112:839–45. doi: 10.1007/s00436-012-3204-3. [DOI] [PubMed] [Google Scholar]
- 17.Lalubin F, Delédevant A, Glaizot O, Christe P. Temporal changes in mosquito abundance (Culex pipiens), avian malaria prevalence and lineage composition. Parasit Vectors. 2013;6:307. doi: 10.1186/1756-3305-6-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ventim R, Ramos JA, Osorio H, Lopes RJ, Pérez-Tris J, Mendes L. Avian malaria infections in western European mosquitoes. Parasitol Res. 2012;111:637–45. doi: 10.1007/s00436-012-2880-3. [DOI] [PubMed] [Google Scholar]
- 19.Hellgren O, Waldenstrom J, Bensch S. A new PCR assay for simultaneous studies of Leucocytozoon, Plasmodium and Haemoproteus from avian blood. J Parasitol. 2004;90:797–802. doi: 10.1645/GE-184R1. [DOI] [PubMed] [Google Scholar]
- 20.Severini F, Toma L, Luca MD, Romi R. Identification of the adult stages of the Italian mosquitoes (Diptera, Culicidae) Fragm Entomol. 2009;41:213–372. [Google Scholar]
- 21.Martínez-de la Puente J, Ruiz S, Soriguer R, Figuerola J. Effect of blood meal digestion and DNA extraction protocol on the success of blood meal source determination in the malaria vector Anopheles atroparvus. Malar J. 2013;12:109. doi: 10.1186/1475-2875-12-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muñoz J, Eritja R, Alcaide M, Montalvo T, Soriguer RC, Figuerola J. Host-feeding patterns of native Culex pipiens and invasive Aedes albopictus mosquitoes (Diptera: Culicidae) in urban zones from Barcelona, Spain. J Med Entomol. 2011;48:956–60. doi: 10.1603/ME11016. [DOI] [PubMed] [Google Scholar]
- 23.Valkiūnas G. Haemosporidian vector research: marriage of molecular and microscopical approaches is essential. Mol Ecol. 2011;20:3084–6. doi: 10.1111/j.1365-294X.2011.05187.x. [DOI] [PubMed] [Google Scholar]
- 24.Ziegyte R, Bernotienė R, Bukauskaitė D, Palinauskas V, Iezhova T, Valkiūnas G. Complete sporogony of Plasmodium relictum (lineages pSGS1 and pGRW11) in mosquito Culex pipiens pipiens form molestus, with implications to avian malaria epidemiology. J Parasitol. 2014;100:878–82. doi: 10.1645/13-469.1. [DOI] [PubMed] [Google Scholar]
- 25.Kimura M, Darbro JM, Harrington LC. Avian malaria parasites share congeneric mosquito vectors. J Parasitol. 2010;96:144–51. doi: 10.1645/GE-2060.1. [DOI] [PubMed] [Google Scholar]
- 26.Valkiūnas G, Kazlauskienė R, Bernotienė R, Palinauskas V, Iezhova TA. Abortive long-lasting sporogony of two Haemoproteus species (Haemosporida, Haemoproteidae) in the mosquito Ochlerotatus cantans, with perspectives on haemosporidian vector research. Parasitol Res. 2013;112:2159–69. doi: 10.1007/s00436-013-3375-6. [DOI] [PubMed] [Google Scholar]
- 27.Kim KS, Tsuda Y, Yamada A. Bloodmeal identification and detection of avian malaria parasite from mosquitoes (Diptera: Culicidae) inhabiting coastal areas of Tokyo Bay, Japan. J Med Entomol. 2009;46:1230–4. doi: 10.1603/033.046.0535. [DOI] [PubMed] [Google Scholar]
- 28.Sawabe K, Isawa H, Hoshino K, Sasaki T, Roychoudhury S, Higa Y, et al. Host-feeding habits of Culex pipiens and Aedes albopictus (Diptera: Culicidae) collected at the urban and suburban residential areas of Japan. Med Entomol. 2010;47:442–50. doi: 10.1093/jmedent/47.3.442. [DOI] [PubMed] [Google Scholar]
- 29.Egizi A, Healy SP, Fonseca DM. Rapid blood meal scoring in anthropophilic Aedes albopictus and application of PCR blocking to avoid pseudogenes. Infect Genet Evol. 2013;16:122–8. doi: 10.1016/j.meegid.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 30.Faraji A, Egizi A, Fonseca DM, Unlu I, Crepeau T, Healy SP, et al. Comparative host feeding patterns of the Asian tiger mosquito, Aedes albopictus, in urban and suburban Northeastern USA and implications for disease transmission. PLoS Negl Trop Dis. 2014;8:e3037. doi: 10.1371/journal.pntd.0003037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.LaPointe DA, Goff ML, Atkinson CT. Comparative susceptibility of introduced forest-dwelling mosquitoes in Hawaii to avian malaria, Plasmodium relictum. J Parasitol. 2005;91:843–9. doi: 10.1645/GE-3431.1. [DOI] [PubMed] [Google Scholar]
- 32.Ejiri H, Sato Y, Sasaki E, Sumiyama D, Tsuda Y, Sawabe K, et al. Detection of avian Plasmodium spp. DNA sequences from mosquitoes captured in Minami Daito Island of Japan. J Vet Med Sci. 2008;70:1205–10. doi: 10.1292/jvms.70.1205. [DOI] [PubMed] [Google Scholar]
- 33.Tanigawa M, Sato Y, Ejiri H, Imura T, Chiba R, Yamamoto H, et al. Molecular identification of avian haemosporidia in wild birds and mosquitoes on Tsushima Island, Japan. J Vet Med Sci. 2013;2013(75):319–26. doi: 10.1292/jvms.12-0359. [DOI] [PubMed] [Google Scholar]
- 34.Trout Fryxell R, Thompson Lewis T, Peace H, Hendricks BM, Paulsen D. Identification of avian malaria (Plasmodium sp.) and canine heartworm (Dirofilaria immitis) in the mosquitoes of Tennessee. J Parasitol. 2014;100:455–62. doi: 10.1645/13-443.1. [DOI] [PubMed] [Google Scholar]
- 35.Ejiri H, Sato Y, Sawai R, Sasaki E, Matsumoto R, Ueda M, et al. Prevalence of avian malaria parasite in mosquitoes collected at a zoological garden in Japan. Parasitol Res. 2009;105:629–33. doi: 10.1007/s00436-009-1434-9. [DOI] [PubMed] [Google Scholar]
- 36.Bensch S, Hellgren O, Pérez-Tris J. MalAvi: a public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Mol Ecol Res. 2009;9:1353–8. doi: 10.1111/j.1755-0998.2009.02692.x. [DOI] [PubMed] [Google Scholar]
- 37.Hellgren O, Atkinson CT, Bensch S, Albayrak T, Dimitrov D, Ewen JG, et al. Global phylogeography of the avian malaria pathogen Plasmodium relictum based on MSP1 allelic diversity. Ecography, in press.
- 38.Medeiros MC, Anderson TK, Higashiguchi JM, Kitron UD, Walker ED, Brawn JD, et al. An inverse association between West Nile virus serostatus and avian malaria infection status. Parasit Vectors. 2014;7:415. doi: 10.1186/1756-3305-7-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hughes T, Irwin P, Hofmeister E, Paskewitz SM. Occurrence of avian Plasmodium and West Nile Virus in Culex species in Wisconsin. J Am Mosq Control Assoc. 2010;26:24–31. doi: 10.2987/09-5893.1. [DOI] [PubMed] [Google Scholar]


