Abstract
Background
The aim of this study was to evaluate the efficacy of superselective renal artery embolization in patients with bleeding into the urinary system.
Material/Methods
From 2007 to 2012, 20 patients were treated with superselective renal artery embolization for bleeding after percutaneous nephrolithotomy (PCNL), nephron-sparing surgery (NSS), including 1 patient with AVF after PCNL. During the procedure, embolization material was injected through a microcatheter to stop the bleeding. Embolization materials included a mixture of cyanoacrylate and lipiodol, embolization coils, and Spongostan. Clinical evaluation included remission of hematuria and normalization of blood morphotic elements.
Results
The cause of bleeding into the urinary tract was damage to vessels (all cases): with coexisting false aneurism (8 cases); with coexisting arterio-venus fistula (1 case); and with coexisting intrarenal hematoma (3 cases). The bleeding occurred 2–5 days after PCNL and NSS, and 10 days after PCNL with AVF. The mean hematocrit level was 22%–24%. Technical success was achieved in 20 cases. Clinical success was achieved in 19 cases. One patient with hematuria after PCNL with AVF needed a second endovascular treatment to stop bleeding.
Conclusions
Superselective renal artery embolization is an effective procedure in the treatment of iatrogenic bleeding into the urinary tract after PCNL and NSS.
MeSH Keywords: Embolization, Therapeutic; Lithotripsy; Renal Artery
Background
Embolization is a well-known and accepted form of treatment for iatrogenic bleeding and hematuria which occur after percutaneous nephrolithotomy (PCNL), nephron-sparing surgery (NSS), vascular defects, and neoplasms. Therapeutic embolization is defined as the voluntary occlusion of 1 or several vessels, which is achieved by inserting material into the lumen for obtaining transient or permanent thrombosis in the downstream vascular bed. There are a number of indications for this approach in urological practice, in particular in patients with parenchymatous or vascular kidney disease [1].
Voluntary embolization of the renal parenchyma has been used to treat kidney cancer since the 1970s. Many other applications in very different clinical situations have also been developed during this period [1].
Before embolization, angiography must be performed to determine the origin of the vascular pathology and its progression. This enables clinicians to make decisions on the embolization material and instruments necessary to perform the procedure [2].
Embolization can be divided into the following types [2]:
Central: occlusion of the main trunk artery with patency of its branches (causing possible development of collateral circulation, preventing necrosis of tissues);
Peripheral: occlusion of all arteries of 100–200 μm (collateral circulation is considerably restricted, causing possible blood flow in capillary vessels); and
Capillary: occlusion of the whole artery bed, leading to complete ischemia and development of acute necrosis.
Superselective renal artery embolization is performed in emergency cases of iatrogenic complications such as bleeding into the urinary system after PCNL and NSS.
The advantages of embolization are minimal invasiveness, few early or late complications, and lack of necessity for general or intrathecal anaesthesia. Compared with surgical treatment, there is a lower risk of intraoperative blood loss, fewer local complications such as suppuration, and a lower rate of urine extravasation or ureterocutaneostomies; moreover, there is no need for nephrectomy associated with failure of surgical treatment. Embolization is only required if treatment with hemostatic agents (fresh-frozen plasma or tranexamic acid) fails or if a patient has only 1 well-functioning kidney.
The main criteria for selecting embolization are:
Rapid decrease of morphotic elements of the blood, determined by levels of hemoglobin, hematocrit, and thrombocytes;
Speed of accretion of perirenal hematoma;
Only 1 anatomical or active kidney; and
Relative and absolute contraindications for anaesthesia.
The remaining criteria are well described in other papers.
The possible adverse effects after embolization include lumbar pain, nausea, vomiting, fever, and spikes in arterial pressure [2]. The main contraindications are advanced sclerotic changes of the renal artery, secondary kidney failure, thrombosis of the lower limbs, and allergy to iodinated contrast agents [2].
The aim of this study was to evaluate the efficiency of superselective renal artery embolization in patients with bleeding into the urinary tract.
Material and Methods
This retrospective analysis included 20 patients treated in our hospital from 2007 to 2012 due to a life-threatening bleeding from urinary tract after PCNL or NSS with using superselective renal artery embolization. We evaluated time to bleeding after PCNL or NSS, causes of bleeding (abnormalities), hematocrit level as a main inclusion criteria to endovascular procedure, technical and clinical success of endovascular treatment, and adverse events after the procedure.
The patients were admitted to hospital for emergency massive bleeding into their urinary systems and a decrease of morphotic elements. The patient population consisted of patients referred from other health centers, which prohibited accurate preoperative analysis. Computed tomography angiography (CTA) was performed to clarify the cause of bleeding before endovascular procedure in all patients.
The abnormalities associated with bleeding were categorized as vasculature damage (i.e., rupture of artery), false aneurysm, arteriovenous fistula, or hematoma.
Technical success was defined as a periprocedural bleeding artery occlusion without any evidence of blood extravasation.
Clinical success was defined as lack of evidence of bleeding after 5 days after endovascular treatments (e.g., hematuria), normalization of morphotic elements, and improvement in clinical status.
The endovascular procedure consisted of aorto-nephrography and selective arteriography of renal arteries to locate the bleeding. The total duration of the procedure was 50–90 min, with a mean duration of 70 min. Only low-osmolality contrast media such as Omnipaque 350 and Visipaque 320 were used in the embolization. The mean quantity of the contrast medium used was 120 mL for both diagnostics and the procedure. The radiation exposure dose ranged from 900 to 1500 mGy.
The diagnostic catheters used were LEV1 (Cook Medical Incorporated, Bloomington, IN, USA) and Impress (Merit Medical, South Jordan, UT, USA) with an inside diameter of 0.038 inches, and the microcatheters used were MicroFerret (Cook Medical Incorporated, Bloomington, IN, USA) and Progreat (Terumo, Somerset, NJ, USA). The embolization material was a mixture of cyanoacrylate and lipiodol, mostly in a ratio of 1:3 (a mixture in the ratio of 1:1 for AVF was used) and polyvinyl alcohol with molecule of diameter 100–200 μm. In some cases, detachable coils (Cook Medical Incorporated, Bloomington, IN, USA) and Spongostan were used (especially for small arteries) with a high-risk mixture of cyanoacrylate and lipiodol migration (Figures 1–3).
Figure 1.
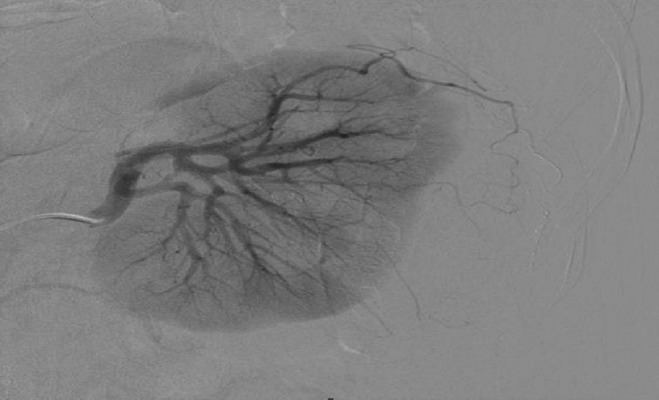
Selective arteriography of the left kidney with effective embolization of a branch of the subcapsular artery with a false microaneurysm following NSS by using Histoacryl with lipiodol.
Figure 3.

Selective arteriography of 1 of the left renal arteries for treating an AVF with bleeding into the pelvicaliceal system following PCNL by using Histoacryl with lipiodol.
During the procedure a superselective embolization was preferred; embolization material was injected through a microcatheter to stop bleeding. This material blocks the blood supply to the damaged vessels and arteries in the whole organ or to a large vascular area and causes infarction or local ischemia, or stops bleeding from the damaged vessel.
In each case an informed consent was obtained.
Results
The reason for bleeding into the urinary tract was damage of vessels (all cases): with coexisting false aneurism (8 cases); with coexisting arterio-venus fistula (1 case); and with coexisting intrarenal hematoma (3 cases).
The bleeding occurred after PCNL and NSS in 2–5 days, and after PCNL with AVF in 10 days.
The mean hematocrit level was 22–24%.
Technical success was achieved in 20 cases and clinical success was achieved in 19 cases. One patient with hematuria after PCNL with AVF needed a second endovascular treatment to stop bleeding.
In the 1 patient experiencing adverse events, part of the embolization material (1:1 mixture of cyanoacrylate and lipiodol) was dislocated by fast blood flow from the large AVF to the lungs, but it did not cause any obstructive thrombus complications and no remaining cyanoacrylate-lipiodol mixture was seen on computed tomography scans 1 month later (Figure 4).
Figure 4.
Superselective embolization performed after PCNL, NSS, or treatment of an AVF.
Other complications were mostly minor, such as nausea and vomiting, a feeling of warmth, and a pain in the side. Other mild complications (e.g., fainting, generic urticaria, or bronchospasms) and serious complications (e.g., pulmonary edema, convulsion, or shock) were not observed.
Discussion
The main reason for bleeding into the urinary tract observed in our study was renal vasculature damage secondary to PCNL or NSS, with coexisting intrarenal hematoma, false aneurysms, and AVF (in 1 patient). The main factor that determined selection of endovascular treatment was hematocrit level. A very high efficacy of endovascular treatment was observed with technical success in 20 patients. In 1 case of bleeding with AVF, a second treatment was performed. The failure of embolization was caused by large AVFs with very fast blood flow and damage to the vessels of the renal hilus. Nonetheless, some of the blood passed into the collecting system, resulting in bleeding into the urinary tract. We did not notice extrarenal bleeding in this case. Only 1 potentially severe adverse event was observed, with asymptomatic displacement mixture of cyanoacrylate with lipiodol into pulmonary arteries, despite the fact that for improving safety the 1:1 mixture was used in this patient. In other patients the 1:3 mixture was preferred. In some patients coils were first used to prevent dislocation of embolic agents in smaller arteries with fast blood flow, but in patients with large AVF there was a high probability of coil migration into pulmonary arteries.
The use of selective arteriography of the renal arteries majorly influences kidney efficiency and decreases the amount of contrast medium administered. The risk of renal failure or decline in kidney function after administration of a contrast medium increases the risk of comorbid conditions such as diabetes, acute or persistent circulatory insufficiency, and atherosclerosis. The total rate of renal failure in this study was <0.5% and renal replacement therapy was not required in any patient at hospitalization or during recovery. Temporary periodic spikes in renal parameters following embolization most often occur in patients with only 1 active kidney; however, these were not observed in our study population. To reduce or even to avoid the next contrast media injections in case of suspicion of selective renal embolization, we plan to design a study using novel magnetic resonance angiography techniques to evaluate renal vasculature [3].
Vessel embolization procedures performed before surgery are organ-sparing and are most often used in urology for treating bleeding into the urinary tract in emergency cases after PCNL and NSS. This is a reliable treatment and preoperative therapy [4,5].
Embolization is also used for treating urological tumors. Previous studies have reported that a combination of chemoembolization with microcapsules (including chemotherapeutic agents and immunotherapy) prolongs survival in patients with inoperable renal or urinary bladder tumors [6].
Highly selective embolization of the renal artery branches is a minimally invasive procedure and does not considerably influence kidney function [7]. This procedure is, therefore, often used preoperatively to reduce postoperative complications [7].
According to the results of other studies and this study, superselective embolization appears to be a minimally invasive and organ-sparing procedure.
After the coaxial catheter was introduced, catheterization of subsegmental vessels and more distal embolization became possible, allowing a more precise access to bleeding locations and thus avoiding surrounding parenchyma [8]. In long-term observations monitored by creatinine level in plasma, dynamic scintigraphy using technetium and urography, arteriography and computed tomography, considerable improvement in kidneys function was proven. This is possible because of collateral circulation and reperfusion of the ischemic area and the tendency to shrink the infarct area in the kidney with the use of this superselective technique with microcatheters. In some patients, the infarct area completely disappeared, probably due to early recanalization without rebleeding [9].
According to the EAU (European Association of Urology), most iatrogenic bleeding due to renal injuries (after PCNL or NSS), particularly in patients with chronic nephropathy, may be treated conservatively by placing the patient in the supine position, clamping the nephrostomy catheter, and forcing diuresis [10]. Superselective embolization is required in <1% cases and has shown over 90% efficiency. Laparotomy is often performed for treating bleeding outside the urinary tract after NSS. However, embolization remains the criterion standard in diagnostics and treatment of bleeding complications after NSS and PCNL.
Conclusions
Superselective renal artery embolization used in emergency cases of bleeding into the urinary tract after PCNL or NSS is a minimally invasive, safe, and effective procedure. Because of the difficult operating conditions and problematic bleeding locations, treatment attempts often lead to nephrectomy. Moreover, there is often an increased risk of intraoperative and postoperative complications. In these cases, embolization is an organ-sparing treatment that maintains renal function.
Figure 2.

Selective arteriography of 1 of the right renal arteries after effective embolization with a coil and Histoacryl with lipiodol mixture following PCNL.
Footnotes
Conflict of interest
There is no conflict of interest.
Source of support: Departmental sources
References
- 1.Loffroy R, Rao P, Kwak BK, et al. Transcatheter Arterial Embolization in Patients with Kidney Diseases: an Oveview of the Technical Aspects and Clinical Indications. Korean J Radiol. 2010;11:257–68. doi: 10.3348/kjr.2010.11.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zieliński H. [Renal artery embolization in patients with renal carcinoma]. Współcz Onkol. 2004;4:203. [in Polish] [Google Scholar]
- 3.Serafin Z, Strześniewski P, Lasek W, Beuth W. Time-resolved imaging of contrast kinetics does not improve performance of follow-up MRA of embolized intracranial aneurysms. Med Sci Monit. 2012;18(7):MT60–65. doi: 10.12659/MSM.883199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Igarashi A, Masuyama T, Watanabe K, et al. Long-term result of the transcatheter arterial embolization for ruptured renal angiomyolipoma. Nippon Hinyokika Gakkai Zasshi. 2002;93:702–6. doi: 10.5980/jpnjurol1989.93.702. [DOI] [PubMed] [Google Scholar]
- 5.Simmons JL, Hussain SA, Riley P, Wallace DMA. Management of renal angiomyolipoma in patients with tuberous sclerosis complex. Oncol Rep. 2003;10:237–41. [PubMed] [Google Scholar]
- 6.Mitsumori K, Sato K, Kato I. Intra-arterial chemotherapy in urological cancer. Gan To Kagaku Ryoho. 2002;29:197–203. [PubMed] [Google Scholar]
- 7.El-Nahas AR, Shokeir AA, Mohsen T, et al. Functional and morphological effects of postpercutaneous nephrolithotomy superselective renal angiographic embolization. J Urol. 2008;71:408–12. doi: 10.1016/j.urology.2007.10.033. [DOI] [PubMed] [Google Scholar]
- 8.Lazarov R, de Kort GA, van Moorselaar RJ. Persistent renal bleeding treated with selective vascular embolisation with preservation of renal function. Ned Tijdschr Geneeskd. 2002;146:994–98. [PubMed] [Google Scholar]
- 9.Summerton DJ, Kitrey ND, Lumen N, et al. Eau Guidelines on iatrogenic trauma. Eur Urol. 2012;62:628–39. doi: 10.1016/j.eururo.2012.05.058. [DOI] [PubMed] [Google Scholar]
- 10.Jain V, Ganpule A, Vyas J, et al. Management of non-neoplasmatic renal hemorrhage by transarterial embolization. J Urol. 2009;74:522–26. doi: 10.1016/j.urology.2008.11.062. [DOI] [PubMed] [Google Scholar]



