SUMMARY
Much recent attention has highlighted a subset of head and neck squamous cell carcinomas (HNSCCs) related to human papillomavirus (HPV) that has an epidemiologic, demographic, molecular and clinical profile which is distinct from non-HPV-related HNSCC. The clinical significance of detecting HPV in a HNSCC has resulted in a growing expectation for HPV testing of HNSCCs. Although the growing demand for routine testing is understandable and appropriate, it has impelled an undisciplined approach that has been largely unsystematic. The current state of the art has now arrived at a point where a better understanding of HPV-related tumorigenesis and a growing experience with HPV testing can now move wide scale, indiscriminant and non-standardized testing towards a more directed, clinically relevant and standardized approach. This review will address the current state of HPV detection; and will focus on why HPV testing is important, when HPV testing is appropriate, and how to test for the presence of HPV in various clinical samples. As no single test has been universally accepted as a best method, this review will consider the strengths and weaknesses of some of the more commonly used assays, and will emphasize some emerging techniques that may improve the efficiency of HPV testing of clinical samples including cytologic specimens.
Keywords: Oropharyngeal carcinoma, Head and neck squamous cell carcinoma, In situ hybridization, p16 immunohistochemistry, Hybrid Capture 2 (HC2) HPV DNA Test, Cervista® HPV HR test, cobas® HPV test
Introduction
High risk human papillomavirus (HPV), particularly type 16, has been established as a causative agent for a significant proportion of head and neck squamous cell carcinomas (HNSCC) [1,2], and the incidence of these HPV-related carcinomas is on the rise [3–6]. Given the distinctiveness of HPV-related carcinoma as a biological and clinical variant of HNSCC, the need for routine HPV testing of oropharyngeal carcinomas is compelling and urgent. The increasing incidence of HPV-associated HNSCC, along with the growing importance of HPV status as a versatile biomarker, is spurring a growing expectation for HPV testing and inclusion of HPV status as a parameter of emerging molecular staging systems. Indeed, the College of American Pathologists has recently recommended routine HPV testing as part of the standard pathologic evaluation of resected oropharyngeal squamous cell carcinomas (http://www.cap.org/apps/docs/committees/cancer/cancer_protocols/2013/Pharynx_13protocol_3300.pdf), and Cancer Care Ontario has published evidence based guidelines for routine testing of HNSCCs (https://www.cancercare.on.ca/common/pages/UserFile.aspx?fileId=279836).
Despite an escalating expectation for the reliable determination of HPV status, there is not yet a standard strategy or method for HPV detection in head and neck cancers. Even fundamental questions regarding when and why to test for HPV still bewilder pathologists and treating clinicians alike. As a result, HPV testing is either never requested or it is indiscriminately demanded without any contextual regard for anatomic site, microscopic findings, clinical relevance and other factors that may influence the likelihood and significance of detecting HPV in a clinical specimen. Moreover, methods of HPV testing across laboratories vary considerably reflecting the biases and tendencies of individual pathologists, and the cost to benefit ratio of each technique [7]. Detection strategies vary not just in design, but in their detection targets. These targets have included HPV DNA, HPV RNA, viral oncoproteins, cellular proteins and HPV-specific serum antibodies. In the ongoing effort to establish a consensus approach, the challenge for the oncologic community is to implement standardized HPV testing using a method that is highly accurate, technically feasible, cost effective and readily transferrable to the diagnostic pathology laboratory in a way that is prognostically relevant and supports clinical care.
Relevance of HPV testing
Clinicians have not been able to rely on prognostic markers other than tumor stage in their care of patients with HNSCC. Numerous studies have addressed the prognostic relevance of cell proliferation (e.g. Ki67), p53 immunohistochemical staining, apoptosis, aneuploidy, Epidermal growth factor receptor overexpression and other markers of biologic activity, but none have proved consistently reliable across multiple studies [8–11]. Even histologic grade does not perform well as a prognosticator. Into this void, HPV detection has stepped in as a powerful biomarker indicating a more favorable clinical outcome for patients with HNSCC. Compared with patients with HPV-negative tumors, those with HPV-positive tumors have a lower risk of tumor progression and death, reflecting in part an enhanced sensitivity to ionizing radiation with or without chemotherapy [2,12–15].
When it comes to patients with HNSCC, the value of HPV testing is by no means restricted to mere prognostication. Detection of HPV is emerging as a valid biomarker for discerning the presence and progress of disease encompassing all aspects of patient care. HPV testing is increasingly used for more refined tumor staging: HPV positivity can be used as evidence of oropharyngeal origin in patients with large and bulky tumors that involve multiple contiguous anatomic sites, and in those patients who present with cervical lymph node metastases. In the near future, HPV status will help guide a more individualized therapeutic approach for patients with HNSCC. In particular, the less aggressive behavior associated with HPV positivity may justify less toxic doses of chemotherapy and/or radiation therapy (i.e. therapeutic de-intensification) for patients with HPV-positive HNSCCs [16]. Knowledge of HPV status is now compulsory for meaningful comparison of treatment responses for patients enrolled in clinical trials. Indeed, the direction of current clinical trials, where patient selection for specific therapies is predicated on HPV tumor status, dramatically heightens the stakes for accurate HPV detection. Finally, HPV assessment may play some present or future role in comprehensive cancer care including early cancer detection [17], post-treatment tumor surveillance [18,19], and more informed discussions with patients and their partners.
HPV testing by anatomic sub-site
HPV infection is strongly correlated with oropharyngeal location, particularly the palatine and lingual tonsils [20]. This preferential targeting likely reflects multifaceted biological interactions between HPV and the highly specialized lymphoepithelium lining the tonsillar crypts [11]. As one important example, the PD-1:PD-L1 interaction mediates complex immunomodulatory pathways that render the tonsillar epithelium an “immune-privileged” site for initial viral infection, and enhances adaptive immune resistance once a tumors is established [21]. Although HPV positivity is sometimes reported in HNSCCs arising outside of the oropharynx such as the sinonasal tract [22–24] and nasopharynx [25,26], expanding the scope of routine HPV testing is not warranted until studies establish a clear relationship between HPV infection at these non-oropharyngeal sites and a distinct natural history including treatment responses. Based on this localization of HPV-related HNSCC to the oropharynx, directives for routine HPV testing is generally restricted to those carcinomas arising from this specific anatomic sub-site https://www.cancer-care.on.ca/common/pages/UserFile.aspx?fileId=279836). Current clinical practice appears to be out of step with clear directives for routine HPV detection restricted to oropharyngeal carcinomas. In one recent study, only 68% of North American head and neck practitioners routinely requested HPV testing of oropharyngeal carcinomas; and conversely, 32% routinely requested HPV testing of oral cavity carcinomas [27]. These findings underscore a need for further education to conform clinical practice with science-based guidelines.
In malignant transformation of the tonsillar epithelium, HPV does not act through a “hit and run” mechanism where its role is transient and limited to the initiation of tumorigenesis. Instead, the presence of HPV persists, and it is just as readily detected in metastatic implants as in the corresponding primary cancers [20,28]. Consequently, a lymph node metastasis is quite suitable as a substrate for HPV testing, obviating the need for additional tissue acquisition, particularly in those patients with small or even occult primary cancers. For those patients who present with neck metastases in the absence of an obvious primary tumor, HPV testing of lymph node metastases is an effective strategy for localizing the site of origin. In these patients, the detection of HPV in a lymph node metastasis is a reliable predictor of oropharyngeal origin [29,30]. Similarly, HPV status can be used to clarify tumor relationships in those patients with HNSCCs who go on to develop a squamous cell carcinoma at distant sites [28,31,32]. For example, the detection of HPV in a squamous cell carcinoma of the lung in a patient with a prior HNSCC helps identify the true nature of the lung cancer as a metastasis rather than a secondary primary [31,33].
Methods of HPV detection
There is currently no standard approach for HPV testing of clinical samples. Instead, methods of HPV testing across laboratories vary considerably reflecting the biases and tendencies of individual investigators, and the cost to benefit ratio of each technique [7,34,35]. Detection strategies vary not just in design, but in their detection targets. These targets have included HPV DNA, HPV RNA, viral oncoproteins, cellular proteins and HPV-specific serum antibodies. For widespread implementation in the clinical arena, detection methods must be accurate, cost effective and readily transferrable to the routine diagnostic laboratory.
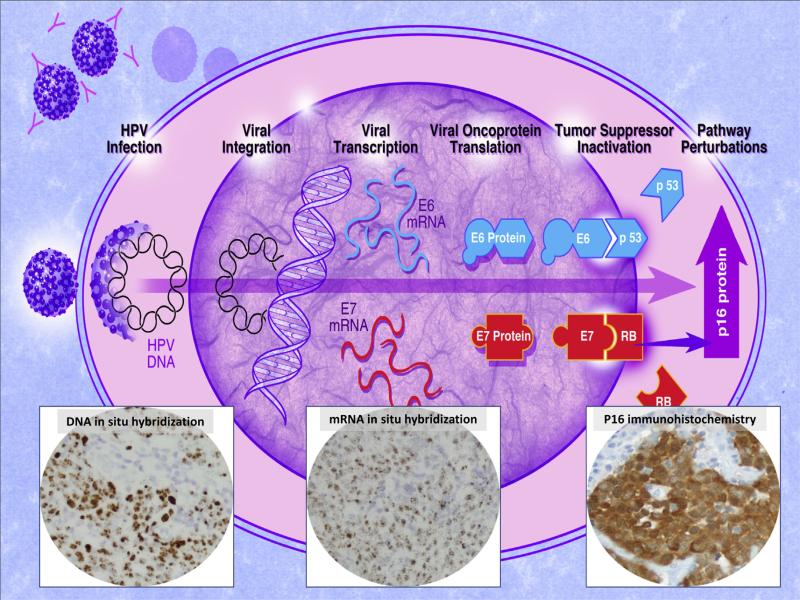
The various strategies that are currently available are guided by an understanding of HPV-induced malignant transformation of oropharyngeal epithelium, particularly its interaction with key components of the retinoblastoma (Rb) tumor suppressor gene pathway [36]. The p16 tumor suppressor gene is a member of the INK4 class of cell-cycle inhibitors and represents a key component of the Rb pathway. The binding of the p16 tumor suppressor gene product with the cyclin-dependant kinases 4 and 6 block its interaction with the D-type cyclins, maintains the retinoblastoma (Rb) gene in a hypophosphorylated state that binds E2F transcription factor and, in turn, prevents cell cycle progression. HPV integration results in the deletion of the viral E2 gene promoter causing transcription of E6 and E7. Binding of the E7 oncoprotein to the Rb protein leads to Rb protein degradation and presumably to the compensatory overexpression of both cytoplasmic and nuclear p16 protein in HPV infected tumor cells [37]. Given this capacity to target and disrupt the Rb tumor suppressor gene pathway, HPV detection strategies may look to detect: (1) HPV DNA, (2) post-integration transcription of viral E6 and/or E7 mRNA, (3) the viral oncoproteins E6 and E7, or (4) altered expression of cellular proteins such as overexpression of the p16 protein (Fig. 1).
Figure 1.
Schematic representation of progressive changes of HPV tumorigenesis from HPV infection, to viral integration into host genome, to transcription of viral E6/E7 mRNA, to translation into viral oncoproteins, to altered expression of cellular proteins including overexpression of the p16 tumor suppressor gene product. Advances in detection assays now permits visualization of these sequential steps using DNA in situ hybridization, RNA in situ hybridization, and p16 immunohistochemistry.
The ultimate value of any HPV detection strategy lays in its ability to both recognize the presence of HPV and discern its potential as a driving force of tumorigenesis. For example, a given assay may be highly sensitive in its ability to detect trace amounts of HPV, but it may have no clinical value if it cannot discern an incidental virus (e.g. viral contaminant) from an active oncologic agent. Evidence for transcriptional activation of the viral oncoproteins E6 and E7 is generally regarded as the gold standard method of clinically relevant HPV. In the absence of reliable immunohistochemical probes for E6 and E7 proteins, detection of E6/E7 messenger RNA (mRNA) is the current standard by which the sensitivities and specificities of other detection assays are measured. Until recently, detection of E6/E7 mRNA has required RNA extraction from fresh or frozen tissues followed by polymerase chain reaction (PCR) amplification of viral RNA – a technically challenging technique that is mainly restricted to the research laboratory. The ongoing challenge of HPV detection efforts has been to reproduce the accuracy and reliability of the PCR E6/E7 mRNA assay using techniques that are easier and transferrable to the diagnostic laboratory.
Routine microscopic evaluation
Lost in the dash to develop and implement diagnostic assays to detect the presence of HPV in HNSCCs is the unpretentious observation that these HPV-HNSCCs have a distinctive microscopic appearance, and that an awareness of these characteristic morphologic features can facilitate the diagnosis of HPV-related HNSCC ([38]. HPV-HNSCCs consistently arise from tonsillar crypts (Fig. 2). Involvement of the tonsillar surface, when it occurs, is generally a secondary phenomenon reflecting colonization of the surface epithelium as the carcinomas spill over from the tonsillar crypts. This transition between HPV-HNSCCs and the adjacent surface epithelium tends to be abrupt without transitional zones of epithelial precursor lesions. Indeed, the histologic progression through the sequential stages of dysplasia culminating in carcinoma in situ and invasive growth that characterize non-HPVHNSCCs is not generally evident for HPV-HNSCCs. As these carcinomas infiltrate, they tend to invade as sheets, lobules or ribbons of cells. Invasive growth often does not elicit a strong desmoplastic stromal reaction. Instead, the tumor nests are often surrounded by a zone of lymphoid cells. The degree to which these lymphoid cells permeate the tumor lobules as tumor infiltrating lymphocytes (TILS) is highly variable. When the TILS are numerous and disrupt the lobules into cords and individual cells, the HPV-HNSCC can take on a “lymphoepithelial” appearance [39]. At the cytologic level, the tumor cells display a high nuclear to cytoplasmic ratio, syncytial cytoplasm without intercellular bridges, and lack signifi-cant cytoplasmic keratinization [40]. These cellular features can impart a distinct basaloid appearance [41]. In lymph node metastases, the presence of cystic degeneration is a common finding that sometimes appears to recapitulate the formation of tonsillar crypts (Fig. 3) [42]. Its presence should warrant strong consideration of an HPV-related metastasis from the tonsil. These squamous lined cysts of the lateral neck are sometimes clinically and histologically mistaken for branchial cleft cysts.
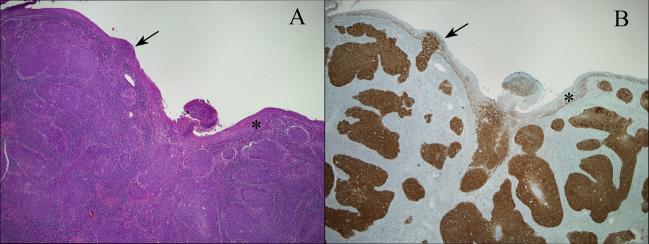
Figure 2.
HPV-related squamous cell carcinomas of the oropharynx typically target the tonsillar crypts (A, hematoxylin and eosin stain). The epithelium lining the surface of the tonsils (asterisks) is usually uninvolved by malignant or premalignant changes. When the surface epithelium is involved, it is usually be secondary extension from the crypts with an abrupt transition between tumor and normal epithelium (arrows). P16 immunohistochemistry allows visualization of HPV distribution in the tonsils (B, p16 immunohistochemical stain).
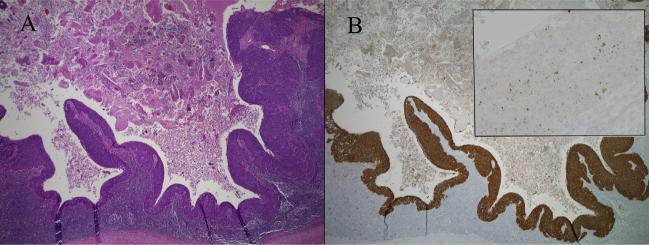
Figure 3.
When HPV-related squamous cell carcinomas metastasize to lymph nodes, they often undergo cystic degeneration (A, hematoxylin and eosin stain). Documenting the presence of HPV by p16 immunohistochemistry (B) and/or HPV DNA in situ hybridization (B inset showing dot-like in situ hybridization signals in tumor nuclei) provides strong support for oropharyngeal origin.
Awareness of the morphologic profile of HPV-HNSCC can help direct the initiation and interpretation of HPV detection assays. The morphology of HPV-related oropharyngeal carcinoma is consistently retained when these tumors metastasize to regional and distant sites. An appreciation for the morphologic features of HPV-HNSCC, whether encountered in a primary or metastatic site, should prompt definitive HPV testing. Moreover, HPV morphology may also facilitate the interpretation of HPV testing in those instances where there is a disparity between the morphologic findings and a test result. For example, absence of HPV detection in an oropharyngeal carcinoma exhibiting classic HPV-related changes should prompt consideration of repeat testing or the employment of some other detection strategy.
HPV DNA detection with polymerase chain reaction (PCR)
PCR amplification of HPV DNA is a target amplification technique that is capable of amplifying trace DNA sequences in a biological sample that contains heterogeneous cell types. The primer sets can be designed to target highly conserved consensus sequences shared by multiple HPV types allowing for the simultaneous identification of a wide range of HPV types, or they can target type-specific viral DNA sequences permitting HPV genotyping. Those who advocate PCR-based methods of HPV detection point to its incomparably sensitivity: these methods can detect HPV well below one viral copy genome per cell. The value of detecting HPV at very low levels, however, is offset by other factors that confound the biological and clinical relevance of viral detection [35]. First, clinical samples are very prone to cross contamination by other specimens. To minimize this adverse effect, surgical pathology facilities for processing oropharyngeal and gynecologic specimens should be physically separate, and diagnostic laboratories must use meticulous PCR precautions. Second, PCR-based methods do not permit the distinction between HPV that is acting as a driver of malignant transformation, and transcriptionally silent virus that is playing no role in the process of tumorigenesis (i.e. passenger virus). The problem is highlighted in those studies that have shown significant discordance between HPV DNA detection and the actual presence of E6/7 mRNA viral transcripts that define clinically relevant HPV infections [14,43].
The ability to distinguish HPV infections that are clinically relevant from those that are not may be supported by a real time PCR approach that can better measure viral load. Using this more quantitative approach, studies indicate that those tumors with a high viral load are much more likely to express E6/E7 mRNA and correlate with improved clinical outcomes [44–46]. One challenge in standardizing a quantitative PCR-based assessment for clinical application is the stipulation of the threshold separating low and high viral load.
HPV RNA detection with polymerase chain reaction (PCR)
The ultimate value of any HPV detection strategy lays in its ability to both recognize the presence of HPV and discern its potential as a driving force of tumorigenesis. In the absence of reliable immunohistochemical probes for the viral oncoproteins E6 and E7 proteins, detection of E6/E7 messenger RNA (mRNA) is the current gold standard for clinically relevant HPV and the benchmark by which the sensitivities and specificities of all other detection assays are measured. Until recently, detection of E6/E7 mRNA expression has required RNA extraction from fresh or frozen tissues followed by polymerase chain reaction (PCR) amplification of viral RNA. Although the transfer of this technique to formalinfixed and paraffin-embedded (FFPE) tissues has greatly expanded its application to clinical samples, it remains a technically challenging technique whose use is mainly restricted to the research laboratory [43]. The ongoing challenge of HPV detection efforts has been to reproduce the accuracy and reliability of the PCR E6/E7 mRNA assay using techniques that are easier and commonplace to the diagnostic pathology laboratory.
DNA in situ hybridization
DNA in situ hybridization (ISH) is a signal amplification technique that utilizes labeled DNA probes complementary to targeted viral DNA sequences. The DNA probes may hybridize to HPV type-specific DNA sequences, hybridize to a consensus sequence shared by multiple HPV types, or may be mixed in a single reaction to cover an extended range of HPV types (i.e. probe cocktail). Given the predominance of HPV16 in oropharyngeal carcinoma, a type 16-specific probe will detect the vast majority (greater than 90%) of HPV-associated carcinomas at this particular site. The widespread use of HPV probe cocktails (e.g. Inform® HPV-III probe cocktail, Ventana Medical systems, Tucson, AZ) now permits coverage across a broader range of high risk HPV types.
Direct comparison of DNA ISH and PCR-based methods suggests that DNA ISH may be a preferable HPV detection tool for both practical and biological considerations (Table 1): (1) HPV DNA ISH has been optimized for formalin fixed and paraffin embedded tissues. In contrast, PCR amplification is more efficient when the clinical samples are available as fresh frozen tissue. (2) Adaptation of ISH to formalin-fixed and paraffin-embedded tissues, together with recent advances in DNA ISH automated instrument systems, has made this technique compatible with standard tissue processing procedures and thus widely transferrable to most surgical pathology laboratories. (3) The introduction of various signal amplification steps has improved the sensitivity of ISH to the point where it can now detect as few as one viral copy per host genome [47]. The ability of non-quantitative PCR-based detection to detect HPV at a much lower threshold (less than 1 viral copy per host genome) may be of little oncologic significance. (4) The development of non-fluorescent chromogens allows visualization of hybridization using conventional light microscopy that, in turn, permits detection of the presence and distribution of HPV in the tissues. For PCR-based detection, the absence of a tissue context has significant drawbacks. It is not possible to determine if viral DNA arises from the population of cancer cells or the surrounding non-neoplastic tissues; and it does not allow for the recognition of a tumor free sample and thus the identification of a false negative result.
Table 1.
Comparison of PCR-based techniques and in situ hybridization for detection of HPV DNA in clinical samples.
| HPV detection assay |
||
|---|---|---|
| PCR-based | DNA ISH | |
| Tissue substrate | More efficient on frozen tissue | Optimized for formalin-fixed and paraffin-embedded tissues |
| Sensitivity | Very high (<1 viral copy per host genome) | High (up to 1 viral copy per host genome) |
| Specificity | Suboptimal - susceptible to viral contaminant or “passenger virus” | High |
| Tissue context | Not perceptible | Visualization of viral distribution in tissues |
| Transferability | Restricted to the molecular laboratory | Available to the surgical pathology laboratory |
| Confirmation of clinical relevance | Poor | Superb |
RNA in situ hybridization
The ultimate goal of any developing technology for HPV detection in clinical samples is to approach the gold standard for sensitivity and specificity while maximizing efficiency, simplicity, reproducibility and transferability to the diagnostic laboratory. Although the most direct and compelling evidence of HPV-related tumorigenesis is the documentation of transcriptionally active HPV in tumor cells, the detection of E6/E7 transcripts is technically challenging. The recent development of RNA in situ hybridization probes complementary to E6/E7 mRNA now permits direct visualization of viral transcripts in routinely processed tissues (Figure 4). In formalin-fixed and paraffin-embedded samples of oropharyngeal carcinomas, the sensitivity of this method has been shown to match the sensitivity of p16 immunohistochemical staining and exceed that of HPV DNA in situ hybridization [48–51]. Testing for HPV E6/E7 transcripts by RNA ISH is an ideal platform for HPV detection in clinical samples. First, it confirms the presence of integrated and transcriptionally active virus by permitting the visualization of viral transcripts directly in tissue sections. Second, it is technically feasible and easily transferrable into the diagnostic pathology laboratory. Indeed, the imminent availability of the HPV RNA in situ hybridization method to a widely available automated staining platform promises to enhance standardization across diagnostic laboratories, decrease turnaround time for large case volumes, and improve reproducibility among clinical trials. Third, the transcription of viral mRNA provides a natural target amplification step that may dramatically improve viral detection in clinical samples and clarify the status of those perplexing tumors that are p16 positive by immunohistochemistry but HPV negative by DNA ISH. Fourth, it is prognostically useful: The presence of E6/E7 mRNA transcripts is tightly coupled to the expression of other powerful prognostic markers (e.g. p16 expression), and strongly correlates with patient outcomes [48].
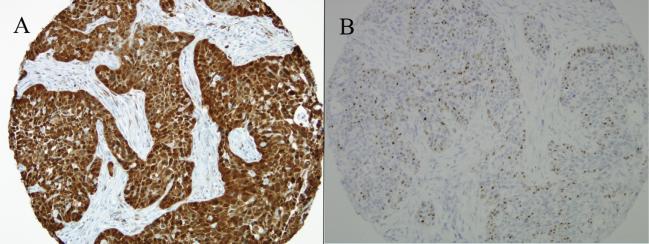
Figure 4.
This HPV-related oropharyngeal carcinoma is strongly p16 positive by immunohistochemistry (A), and it demonstrates transcriptional activity as visualized by RNA in situ hybridization for HPV E6/E7 mRNA transcripts (B).
P16 immunohistochemical staining as a surrogate marker of HPV
Immunostaining for p16 protein has recently been regarded as a practical alternative or complimentary procedure for HPV testing of oropharyngeal cancers based on a high correlation between the HPV detection and p16 overexpression in recent studies [44,52–54]. In head to head comparisons using HR-HPV E6/E7 mRNA expression as the gold standard for HPV status, Jordan et al. [44] recently reported that both p16 immunohistochemistry (sensitivity, 96.8%; specificity, 83.8%) and HPV16 in situ hybridization (sensitivity, 88.0%; specificity, 94.7%) showed excellent performance in HPV detection. The simplicity, low cost and high sensitivity of p16 immunohistochemistry have prompted consideration of replacing more intensive ISH and PCR-based methods as a standalone HPV test [53]. At the same time, the absence of a direct and exclusive mechanistic link between HPV DNA integration and p16 expression warns against a casual application of p16 testing alone, particularly in ways that do not take into account tumor site. In sites like the oral cavity and larynx that are not preferentially targeted by HPV, the likelihood that p16 overexpression truly reflects the presence of transcriptionally active HPV (i.e. positive predictive value) is very low [51,55,56]. For these non-oropharyngeal cancers, and even for a subset of oropharyngeal HNSCCs, the possibility of encountering elevated p16 expression by non-viral related mechanisms must be considered.
To be truly useful as a surrogate marker of HPV infection, the interpretation of p16 immunohistochemistry must be informed by various histological, anatomical and clinical considerations [57]. First, p16 IHC may substitute for HPV testing when strong staining is present in the nucleus and cytoplasm of the tumor cells throughout all or most (>70%) of the tumor (Fig. 4). Focal or weak staining should be supported by other forms of HPV testing. Second, while the sensitivity and specificity of p16 staining as a marker of HPV infection is sufficiently high to serve as a reliable test for squamous cell carcinomas of oropharyngeal origin, these values are either unknown or unacceptably low for HNSCCs arising in non-oropharyngeal sites. Third, interpretation of p16 staining must be informed by the morphologic features of the tumor as outlined above. P16 IHC staining may substitute for HPV detection in those oropharyngeal carcinomas that demonstrate the typical morphology of HPV-related HNSCC. Additional HPV testing should be performed in p16 negative oropharyngeal carcinomas that exhibit classic HPV-related histomorphology, and in p16 positive oropharyngeal carcinomas that do not exhibit classic HPV morphology. Fourth, p16 IHC is currently used primarily as a prognostic indicator for patients with oropharyngeal carcinoma, and any expanded clinical role for HPV detection may necessitate more stringent detection methods. Many of these guidelines have been adopted by the Cancer Care Ontario's evidence based guidelines for p16 testing of HNSCCs (https://www.cancercare.on.ca/common/pages/UserFile.aspx?fileId=279836).
HPV detection strategies: single versus multi-modality HPV analysis
The power of p16 immunohistochemical staining lays in its high sensitivity for detecting all high risk types of HPV, but it suffers from suboptimal specificity. Use of p16 staining as standalone test for HPV detection is associated with a small false positive rate where p16 expression is driven by some non-viral mechanism. These p16 positive/HPV-negative oropharyngeal carcinomas have been associated with less favorable survival than p16-positive/HPV-positive cancers, suggesting that selection of patients for de-escalation clinical trials may benefit from supplementary detection assays rather than p16 staining alone [58]. DNA in situ hybridization, on the other hand, offers a high degree of specificity at the expense of suboptimal sensitivity. The failure to detect all cases of HPV-related oropharyngeal cancer will deny some patients the opportunity to benefit from novel therapeutic strategies and potentially expose them to unnecessarily high levels of treatment-related toxicity.
Multimodality detection strategies look to utilize the strengths of individual assays in combination to optimize the overall reliability of HPV detection (Fig. 5). Current multimodality strategies utilize a stepwise approach that begins with p16 immunohistochemical staining. Those oropharyngeal squamous cell carcinomas that are p16 positive are then analyzed with more rigorous HPV-specific detection assays such as HPV DNA in situ hybridization [54] and/or a PCR-based assay [43]. Although the multimodality approach may provide the most accurate analysis of HPV status, it does represent a deviation from a growing trend in HPV testing that highly values rapid turnaround, simplicity, and cost restraint. More painstaking HPV detection algorithms may be most appropriate when there is no allowance for error in determining true HPV status, such as selection of patients for “de-escalation” therapy or therapeutic HPV vaccine trials.
Figure 5.
Algorithms using multimodality methods of HPV detection generally begin with p16 immunohistochemical staining. In situ hybridization assays and/or PCR-based assays are used in p16 positive carcinomas to confirm the presence of HPV.
HPV testing of cytologic specimens
Even as the need and expectation for HPV testing of head and neck cancers is growing, opportunities for HPV testing of tissue samples are diminishing: The sensitivity of HPV-related HNSCC to chemotherapy and ionizing radiation has limited the role of surgical resection, and diagnostic tissue biopsies/resections may not be available in a substantial portion of patients with small or occult primaries. Into this quandary steps the cytopathologist. Fine needle aspirates of metastatic HNSCCs and brushes of oropharyngeal cancers provide a valuable substrate for HPV analysis ([40,59]. The feasibility HPV detection in cervical lymph node fine needle aspirates (FNAs) has been confirmed in a limited number of studies using both p16 immunostaining and in situ hybridization platforms. Using an in situ hybridization approach on aspirated cells processed as cell blocks, Begum et al. [30] detected HPV16 in 53% of metastatic oropharyngeal carcinomas but in none of those meta-static carcinomas arising from non-oropharyngeal sites. Others have applied these same detection strategies directly to ethanolfixed smears of fine needle aspirates, circumventing the need for the construction of cell blocks [60–62].
In cytologic specimens, p16 staining is impacted by various technical and biological factors that limit its role as a method of HPV analysis. First, established cutoff values for p16 expression based on the percentage and intensity of staining in tissue samples are more difficult to apply in cytologic specimens that are vulnerable to sampling error and lack of cellular integrity. In fine needle aspirates of cystic lymph node metastases where interpretation is often plagued by low tumor cellularity and cell degradation, p16 staining can be weak or absent in HPV-positive tumors. Second, p16 staining is sometimes noted in neck lesions unrelated to the presence of HPV. For example, p16 positivity is also noted in almost half of all branchial cleft cysts evaluated by fine needle aspiration, but this staining reflects neither the presence of HPV nor malignant potential [63]. In effect, the use of p16 staining as a standalone test can be treacherous and would benefit from other methods that provide more direct evidence for the presence or absence of virus.
Direct transfer of cytologic samples into the liquid media minimizes specimen preparation and eliminates the need for specimen processing as cell blocks. Various liquid phase assays already in widespread use for HPV analysis of cervical cancer risk may be directly applicable to the head and neck context. The Hybrid Capture 2 (HC2) HPV DNA Test is an in vitro nucleic acid hybridization assay with signal-amplification using microplate chemiluminescence for the detection of 13 high risk HPV types (including 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68) in cervical specimens. In a limited study of 24 patients with HNSCC, the HC2 assay was found to be highly reliable in discerning HPV status [64]. In this study population, there was 100% correlation between HC2 analysis of the cytologic specimens (brushings and FNAs) and DNA in situ hybridization analysis of the paired surgical resection specimens (primary tumor resections and lymph node metastases. Although the HC2 method appears to be reliable in determining the HPV status of directly sampled HNSCCs, it may not be sufficiently sensitivity to be used as part of a screening strategy for early cancer detection. Using oral rinses as their test substrate, Jarboe et al. [65] were unable to detect HPV using the HC2 assay in oral rinses from patients with known HPV-related oropharyngeal cancers.
Like the HC2 method, the Cervista® HPV HR test is a liquid phase assay that is clinically validated for HPV detection in cervical cytologic specimens. Its analytical sensitivity is comparable to that of HC2, but the addition of a housekeeping gene as an internal control to ensure sufficient cellularity diminishes the likelihood of false negative results. In one feasibility study, this method was found to be effective in detecting the presence of HPV in FNAs from patients with metastatic HNSCC, again demonstrating that HPV detection and genotyping can be achieved without the need for tissue acquisition or complex specimen processing [66]. Similarly, the Roche cobas® HPV test is a PCR-based assay that permits distinction between HPV16, HPV18 and other HR-HPV types. Again, the addition of a housekeeping gene helps to eliminate false negative results. In one recent applied to HNSCCs, the sensitivity relative to HPV DNA in situ hybridization was 100%, but the specificity was only 86% [67].
Summary
HPV-related head and neck cancers represent a biologically and clinically distinct disease. Accordingly, determination of HPV status is important as it impacts all aspects of patient care including prognosis, tumor staging (i.e. identifying site of tumor origin) and selection of patients most likely to benefit from certain therapeutic options. In the ongoing effort to establish a consensus approach for HPV testing, the challenge for the oncologic community is to implement standardized HPV testing using a method that is highly accurate, technically feasible, cost effective, and readily transferrable to the diagnostic pathology laboratory. Each currently used test is associated with own unique strengths and weaknesses. Development of detection assays optimized for cytologic samples may open the door to more widespread implementation of HPV testing, and may obviate the need for tissue acquisition.
Footnotes
Conflict of interest statement
None declared.
References
- 1.Adelstein DJ, Ridge JA, Gillison ML, Chaturvedi AK, D'Souza G, Gravitt PE, et al. Head and neck squamous cell cancer and the human papillomavirus: summary of a National Cancer Institute state of the science meeting, November 9–10, 2008, Washington, D.C. Head Neck. 2009;31:1393–422. doi: 10.1002/hed.21269. [DOI] [PubMed] [Google Scholar]
- 2.Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–20. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 3.Ramqvist T, Dalianis T. An epidemic of oropharyngeal squamous cell carcinoma (OSCC) due to human papillomavirus (HPV) infection and aspects of treatment and prevention. Anticancer Res. 2011;31:1515–9. [PubMed] [Google Scholar]
- 4.Marur S, D'Souza G, Westra WH, Forastiere AA. Hpv-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol. 2010;11:781–9. doi: 10.1016/S1470-2045(10)70017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Junor EJ, Kerr GR, Brewster DH. Oropharyngeal cancer. Fastest increasing cancer in scotland, especially in men. BMJ. 2010;340:c2512. doi: 10.1136/bmj.c2512. [DOI] [PubMed] [Google Scholar]
- 6.Chaturvedi AK, Anderson WF, Lortet-Tieulent J, Curado MP, Ferlay J, Franceschi S, et al. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol. 2013;31:4550–9. doi: 10.1200/JCO.2013.50.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robinson M, Sloan P, Shaw R. Refining the diagnosis of oropharyngeal squamous cell carcinoma using human papillomavirus testing. Oral Oncol. 2010;46:492–6. doi: 10.1016/j.oraloncology.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Chiesa F, Mauri S, Tradati N, Calabrese L, Giugliano G, Ansarin M, et al. Surfing prognostic factors in head and neck cancer at the millennium. Oral Oncol. 1999;35:590–6. doi: 10.1016/s1368-8375(99)00043-3. [DOI] [PubMed] [Google Scholar]
- 9.Helliwell TR. Molecular markers of metastasis in squamous carcinomas. J Pathol. 2001;194:289–93. doi: 10.1002/1096-9896(200107)194:3<289::AID-PATH912>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 10.Lopes MA, Nikitakis NG, Reynolds MA, Ord RA, Sauk Jr J. Biomarkers predictive of lymph node metastases in oral squamous cell carcinoma. J Oral Maxillofac Surg. 2002;60:142–7. doi: 10.1053/joms.2002.29804. [DOI] [PubMed] [Google Scholar]
- 11.Pai SI, Westra WH. Molecular pathology of head and neck cancer: Implications for diagnosis, prognosis, and treatment. Ann Rev Pathol. 2009;4:49–70. doi: 10.1146/annurev.pathol.4.110807.092158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–9. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 14.Weinberger PM, Yu Z, Haffty BG, Kowalski D, Harigopal M, Brandsma J, et al. Molecular classification identifies a subset of human papillomavirus-associated oropharyngeal cancers with favorable prognosis. J Clin Oncol. 2006;24:736–47. doi: 10.1200/JCO.2004.00.3335. [DOI] [PubMed] [Google Scholar]
- 15.Ragin CC, Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysis. Int J Cancer. 2007;121:1813–20. doi: 10.1002/ijc.22851. [DOI] [PubMed] [Google Scholar]
- 16.Bonilla-Velez J, Mroz EA, Hammon RJ, Rocco JW. Impact of human papillomavirus on oropharyngeal cancer biology and response to therapy: implications for treatment. Otolaryngol Clin North Am. 2013;46:521–43. doi: 10.1016/j.otc.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao M, Rosenbaum E, Carvalho AL, Koch W, Jiang W, Sidransky D, et al. Feasibility of quantitative PCR-based saliva rinse screening of hpv for head and neck cancer. Int J Cancer. 2005;117:605–10. doi: 10.1002/ijc.21216. [DOI] [PubMed] [Google Scholar]
- 18.Agrawal Y, Koch WM, Xiao W, Westra WH, Trivett AL, Symer DE, et al. Oral human papillomavirus infection before and after treatment for human papillomavirus 16-positive and human papillomavirus 16-negative head and neck squamous cell carcinoma. Clin Cancer Res. 2008;14:7143–50. doi: 10.1158/1078-0432.CCR-08-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chuang AY, Chuang TC, Chang S, Zhou S, Begum S, Westra WH, et al. Presence of hpv DNA in convalescent salivary rinses is an adverse prognostic marker in head and neck squamous cell carcinoma. Oral Oncol. 2008;44:915–9. doi: 10.1016/j.oraloncology.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Begum S, Cao D, Gillison M, Zahurak M, Westra WH. Tissue distribution of human papillomavirus 16 DNA integration in patients with tonsillar carcinoma. Clin Cancer Res. 2005;11:5694–9. doi: 10.1158/1078-0432.CCR-05-0587. [DOI] [PubMed] [Google Scholar]
- 21.Lyford-Pike S, Peng S, Young GD, Taube JM, Westra WH, Akpeng B, et al. Evidence for a role of the pd-1:Pd-l1 pathway in immune resistance of hpv-associated head and neck squamous cell carcinoma. Cancer Res. 2013;73:1733–41. doi: 10.1158/0008-5472.CAN-12-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Mofty SK, Lu DW. Prevalence of high-risk human papillomavirus DNA in nonkeratinizing (cylindrical cell) carcinoma of the sinonasal tract: a distinct clinicopathologic and molecular disease entity. Am J Surg Pathol. 2005;29:1367–72. doi: 10.1097/01.pas.0000173240.63073.fe. [DOI] [PubMed] [Google Scholar]
- 23.Bishop JA, Guo TW, Smith DF, Wang H, Ogawa T, Pai SI, et al. Human papillomavirus-related carcinomas of the sinonasal tract. Am J Surg Pathol. 2013;37:185–92. doi: 10.1097/PAS.0b013e3182698673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis Jr JS, Westra WH, Thompson LD, Barnes L, Cardesa A, Hunt JL, et al. The sinonasal tract: another potential “hot spot” for carcinomas with transcriptionally-active human papillomavirus. Head Neck Pathol. 2013 doi: 10.1007/s12105-013-0514-4. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singhi AD, Califano J, Westra WH. High-risk human papillomavirus in nasopharyngeal carcinoma. Head Neck. 2012;34:213–8. doi: 10.1002/hed.21714. [DOI] [PubMed] [Google Scholar]
- 26.Maxwell JH, Kumar B, Feng FY, McHugh JB, Cordell KG, Eisbruch A, et al. HPV-positive/p16-positive/ebv-negative nasopharyngeal carcinoma in white North Americans. Head Neck. 2010;32:562–7. doi: 10.1002/hed.21216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maniakas A, Ayad T, Guertin L, Nguyen-Tan PF, Gologan OE, Christopoulos A. North American survery on hpv and p16 testing in head and neck cancer. Otolaryngol-Head Neck Surg. 2014;149:181. doi: 10.1016/j.oraloncology.2014.07.004. [abstract] [DOI] [PubMed] [Google Scholar]
- 28.Mehrad M, Zhao H, Gao G, Wang X, Lewis Jr JS. Transcriptionally-active human papillomavirus is consistently retained in the distant metastases of primary oropharyngeal carcinomas. Head Neck Pathol. 2013 doi: 10.1007/s12105-013-0509-1. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Begum S, Gillison ML, Ansari-Lari MA, Shah K, Westra WH. Detection of human papillomavirus in cervical lymph nodes: a highly effective strategy for localizing site of tumor origin. Clin Cancer Res. 2003;9:6469–75. [PubMed] [Google Scholar]
- 30.Begum S, Gillison ML, Nicol TL, Westra WH. Detection of human papillomavirus-16 in fine-needle aspirates to determine tumor origin in patients with metastatic squamous cell carcinoma of the head and neck. Clin Cancer Res. 2007;13:1186–91. doi: 10.1158/1078-0432.CCR-06-1690. [DOI] [PubMed] [Google Scholar]
- 31.Weichert W, Schewe C, Denkert C, Morawietz L, Dietel M, Petersen I. Molecular hpv typing as a diagnostic tool to discriminate primary from metastatic squamous cell carcinoma of the lung. Am J Surg Pathol. 2009;33:513–20. doi: 10.1097/PAS.0b013e3181938319. [DOI] [PubMed] [Google Scholar]
- 32.Ruzevick J, Olivi A, Westra WH. Metastatic squamous cell carcinoma to the brain: an unrecognized pattern of distant spread in patients with hpv-related head and neck cancer. J Neuro-oncol. 2013;112:449–54. doi: 10.1007/s11060-013-1075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bishop JA, Ogawa T, Chang X, Illei PB, Gabrielson E, Pai SI, et al. HPV analysis in distinguishing second primary tumors from lung metastases in patients with head and neck squamous cell carcinoma. Am J Surg Pathol. 2012;36:142–8. doi: 10.1097/PAS.0b013e3182395c7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Westra WH. Detection of human papillomavirus in clinical samples. Otolaryngol Clin North Am. 2012;45:765–77. doi: 10.1016/j.otc.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 35.Mirghani H, Amen F, Moreau F, Guigay J, Ferchiou M, Melkane AE, et al. Human papilloma virus testing in oropharyngeal squamous cell carcinoma: what the clinician should know. Oral Oncol. 2014;50:1–9. doi: 10.1016/j.oraloncology.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 36.Chung CH, Gillison ML. Human papillomavirus in head and neck cancer: its role in pathogenesis and clinical implications. Clin Cancer Res. 2009;15:6758–62. doi: 10.1158/1078-0432.CCR-09-0784. [DOI] [PubMed] [Google Scholar]
- 37.Rautava J, Syrjanen S. Biology of human papillomavirus infections in head and neck carcinogenesis. Head Neck Pathol. 2012;6(Suppl 1):S3–15. doi: 10.1007/s12105-012-0367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Westra WH. The morphologic profile of hpv-related head and neck squamous carcinoma: implications for diagnosis, prognosis, and clinical management. Head Neck Pathol. 2012;6(Suppl 1):S48–54. doi: 10.1007/s12105-012-0371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singhi AD, Stelow EB, Mills SE, Westra WH. Lymphoepithelial-like carcinoma of the oropharynx: a morphologic variant of hpv-related head and neck carcinoma. Am J Surg Pathol. 2010;34:800–5. doi: 10.1097/PAS.0b013e3181d9ba21. [DOI] [PubMed] [Google Scholar]
- 40.Holmes BJ, Westra WH. The expanding role of cytopathology in the diagnosis of hpv-related squamous cell carcinoma of the head and neck. Diagn Cytopathol. 2014;42:85–93. doi: 10.1002/dc.23014. [DOI] [PubMed] [Google Scholar]
- 41.Begum S, Westra WH. Basaloid squamous cell carcinoma of the head and neck is a mixed variant that can be further resolved by hpv status. Am J Surg Pathol. 2008;32:1044–50. doi: 10.1097/PAS.0b013e31816380ec. [DOI] [PubMed] [Google Scholar]
- 42.Goldenberg D, Begum S, Westra WH, Khan Z, Sciubba J, Pai SI, et al. Cystic lymph node metastasis in patients with head and neck cancer: an hpv-associated phenomenon. Head Neck. 2008;30:898–903. doi: 10.1002/hed.20796. [DOI] [PubMed] [Google Scholar]
- 43.Smeets SJ, Hesselink AT, Speel EJ, Haesevoets A, Snijders PJ, Pawlita M, et al. A novel algorithm for reliable detection of human papillomavirus in paraffin embedded head and neck cancer specimen. Int J Cancer. 2007;121:2465–72. doi: 10.1002/ijc.22980. [DOI] [PubMed] [Google Scholar]
- 44.Jordan RC, Lingen MW, Perez-Ordonez B, He X, Pickard R, Koluder M, et al. Validation of methods for oropharyngeal cancer hpv status determination in us cooperative group trials. Am J Surg Pathol. 2012;36:945–54. doi: 10.1097/PAS.0b013e318253a2d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cohen MA, Basha SR, Reichenbach DK, Robertson E, Sewell DA. Increased viral load correlates with improved survival in hpv-16-associated tonsil carcinoma patients. Acta Otolaryngol. 2008;128:583–9. doi: 10.1080/00016480701558880. [DOI] [PubMed] [Google Scholar]
- 46.Mellin H, Dahlgren L, Munck-Wikland E, Lindholm J, Rabbani H, Kalantari M, et al. Human papillomavirus type 16 is episomal and a high viral load may be correlated to better prognosis in tonsillar cancer. Int J Cancer. 2002;102:152–8. doi: 10.1002/ijc.10669. [DOI] [PubMed] [Google Scholar]
- 47.Huang CC, Qiu JT, Kashima ML, Kurman RJ, Wu TC. Generation of type-specific probes for the detection of single-copy human papillomavirus by a novel in situ hybridization method. Mod Pathol. 1998;11:971–7. [PubMed] [Google Scholar]
- 48.Ukpo OC, Flanagan JJ, Ma XJ, Luo Y, Thorstad WL, Lewis Jr JS. High-risk human papillomavirus e6/e7 mrna detection by a novel in situ hybridization assay strongly correlates with p16 expression and patient outcomes in oropharyngeal squamous cell carcinoma. Am J Surg Pathol. 2011;35:1343–50. doi: 10.1097/PAS.0b013e318220e59d. [DOI] [PubMed] [Google Scholar]
- 49.Wang F, Flanagan J, Su N, Wang LC, Bui S, Nielson A, et al. RNAscope: a novel in situ rna analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn. 2012;14:22–9. doi: 10.1016/j.jmoldx.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schache AG, Liloglou T, Risk JM, Jones TM, Ma XJ, Wang H, et al. Validation of a novel diagnostic standard in hpv-positive oropharyngeal squamous cell carcinoma. Br J Cancer. 2013;108:1332–9. doi: 10.1038/bjc.2013.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bishop JA, Ma XJ, Wang H, Luo Y, Illei PB, Begum S, et al. Detection of transcriptionally active high-risk hpv in patients with head and neck squamous cell carcinoma as visualized by a novel e6/e7 mrna in situ hybridization method. Am J Surg Pathol. 2012;36:1874–82. doi: 10.1097/PAS.0b013e318265fb2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoffmann M, Ihloff AS, Gorogh T, Weise JB, Fazel A, Krams M, et al. P16(ink4a) overexpression predicts translational active human papillomavirus infection in tonsillar cancer. Int J Cancer. 2010;127:1595–602. doi: 10.1002/ijc.25174. [DOI] [PubMed] [Google Scholar]
- 53.Lewis Jr JS. P16 immunohistochemistry as a standalone test for risk stratification in oropharyngeal squamous cell carcinoma. Head Neck Pathol. 2012;6(Suppl. 1):S75–82. doi: 10.1007/s12105-012-0369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Singhi AD, Westra WH. Comparison of human papillomavirus in situ hybridization and p16 immunohistochemistry in the detection of human papillomavirus-associated head and neck cancer based on a prospective clinical experience. Cancer. 2010;116:2166–73. doi: 10.1002/cncr.25033. [DOI] [PubMed] [Google Scholar]
- 55.Lingen MW, Xiao W, Schmitt A, Jiang B, Pickard R, Kreinbrink P, et al. Low etiologic fraction for high-risk human papillomavirus in oral cavity squamous cell carcinomas. Oral Oncol. 2013;49:1–8. doi: 10.1016/j.oraloncology.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 56.Chernock RD, Wang X, Gao G, Lewis Jr JS, Zhang Q, Thorstad WL, et al. Detection and significance of human papillomavirus, cdkn2a(p16) and cdkn1a(p21) expression in squamous cell carcinoma of the larynx. Mod Pathol. 2013;26:223–31. doi: 10.1038/modpathol.2012.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.El-Naggar AK, Westra WH. P16 expression as a surrogate marker for hpv-related oropharyngeal carcinoma: a guide for interpretative relevance and consistency. Head Neck. 2012;34:459–61. doi: 10.1002/hed.21974. [DOI] [PubMed] [Google Scholar]
- 58.Rietbergen MM, Brakenhoff RH, Bloemena E, Witte BI, Snijders PJ, Heideman DA, et al. Human papillomavirus detection and comorbidity: critical issues in selection of patients with oropharyngeal cancer for treatment de-escalation trials. Ann Oncol. 2013;24:2740–5. doi: 10.1093/annonc/mdt319. [DOI] [PubMed] [Google Scholar]
- 59.Faquin WC. Human papillomavirus (hpv) assays for testing fine-needle aspiration specimens in patients with head and neck squamous cell carcinoma. Cancer Cytopathol. 2013 doi: 10.1002/cncy.21374. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 60.Grimes R, Garcia-Buitrago MT, Jorda M, Ganjei-Azar P, Ferrell A, Gomez-Fernandez C. P16inka immunocytochemistry in fine-needle aspiration cytology smears of metastatic head and neck squamous cell carcinoma. Acta Cytol. 2012;57:33–7. doi: 10.1159/000342501. [DOI] [PubMed] [Google Scholar]
- 61.Umudum H, Rezanko T, Dag F, Dogruluk T. Human papillomavirus genome detection by in situ hybridization in fine-needle aspirates of metastatic lesions from head and neck squamous cell carcinomas. Cancer. 2005;105:171–7. doi: 10.1002/cncr.21027. [DOI] [PubMed] [Google Scholar]
- 62.Zhang MQ, El-Mofty SK, Davila RM. Detection of human papillomavirus-related squamous cell carcinoma cytologically and by in situ hybridization in fine-needle aspiration biopsies of cervical metastasis: a tool for identifying the site of an occult head and neck primary. Cancer. 2008;114:118–23. doi: 10.1002/cncr.23348. [DOI] [PubMed] [Google Scholar]
- 63.Cao D, Begum S, Ali SZ, Westra WH. Expression of p16 in benign and malignant cystic squamous lesions of the neck. Hum Pathol. 2010;41:535–9. doi: 10.1016/j.humpath.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 64.Bishop JA, Maleki Z, Valsamakis A, Ogawa T, Chang X, Pai SI, et al. Application of the hybrid capture 2 assay to squamous cell carcinomas of the head and neck: a convenient liquid-phase approach for the reliable determination of human papillomavirus status. Cancer Cytopathol. 2012;120:18–25. doi: 10.1002/cncy.20175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jarboe EA, Willis M, Bentz B, Buchmann L, Hunt J, Ellis G, et al. Detection of human papillomavirus using hybrid capture 2 in oral brushings from patients with oropharyngeal squamous cell carcinoma. Am J Clin Pathol. 2011;135:766–9. doi: 10.1309/AJCPEI84KRPYWUPK. [DOI] [PubMed] [Google Scholar]
- 66.Solomides CC, Bibbo M, Wang ZX. Assessment of fine needle aspiration specimen adequacy for high-risk hpv detection and genotyping in oropharyngeal squamous cell carcinoma. Acta Cytol. 2012;56:196–8. doi: 10.1159/000335730. [DOI] [PubMed] [Google Scholar]
- 67.Kerr DA, Pitman MB, Sweeney B, Arpin RN, 3rd, Wilbur DC, Faquin WC. Performance of the roche cobas 4800 high-risk human papillomavirus test in cytologic preparations of squamous cell carcinoma of the head and neck. Cancer Cytopathol. 2013 doi: 10.1002/cncy.21372. [DOI] [PubMed] [Google Scholar]