Summary
Canonical transient receptor potential 1 (TRPC1) plasmalemmal cation channels mediate Ca2+ and Na+ fluxes and control respective cytoplasmic ion signals in rat cortical astrocytes. Mechanical stimulation of astrocytes results in increases in cytosolic Ca2+ and Na+ levels that are in part due to entry of extracellular cations through TRPC1 containing channels. Inhibition of the TRPC1 pore with an antibody against the selective filter of TRPC1 reduced cytosolic Ca2+ accumulation caused by mechanical stimulation. In contrast, this immunological treatment increased the cytosolic Na+ peak accumulation induced by mechanical stimulation. We propose that TRPC channels are amenable to changes in selective filtering as mutations in previous studies and antibody binding in our present study differentially affect the flux of Ca2+ and Na+. TRPC1 containing channels might represent focal points for co-ordination of Ca2+ and Na+ signalling in astroglia and this can have consequences on Ca2+- and Na+-dependent processes such as regulated exocytosis and lactate production, respectively, which in turn can modulate neuronal synaptic transmission.
Keywords: astrocyte, calcium, differential filtering, sodium, store operate calcium entry
1. Introduction
Astrocytes, the main homeostatic cells of the central nervous system (CNS) express multiple plasmalemmal ion channels that contribute to cytosolic ion signalling, which is central for astroglial homeostatic functions [1-3]. These channels are activated by multiple stimuli such as neurotransmitters or mechanical stimulation. This latter mechanism is physiologically relevant for astrocytes since they have remarkable morphological plasticity and may rapidly change their volume. The majority of plasmalemmal ion channels expressed in astrocytes belong (with obvious exception of selective K+ channels) to nonselective cationic channels permeable to Ca2+, Na+ and K+. Activation of these channels results in Ca2+ and Na+ signalling events that control various functional responses [4, 5].
Canonical transient receptor potential 1 (TRPC1) channel is one of seven mammalian TRPC subtypes widely expressed in various tissues [6]. TRPC1 is a nonselective cation channel with equal permeability for Ca2+ and Na+ and is different from other TRPC channels some of which are highly Ca2+ permeable with PCa /Pmonovalent between 2 and 9 [7]. Astroglial cells express several types of TRPC proteins, which, through heteromeric assembly with the TRPC1 subunit (that is known to be obligatory for channel formation), form functional channels [8-11]. TRPC channels in astrocytes are responsible for generation of store-operated Ca+ entry (SOCE) although the mechanism linking them to the endoplasmic reticulum (ER) Ca2+ store remains elusive. Targeting the TRPC1 subunit in astrocytes with a blocking antibody or by reducing TRPC1 expression with a silencing RNA treatment substantially reduced the SOCE in cultured astroglial cells [8, 10]. The TRPC 1-mediated SOCE contributes to astroglial Ca2+ signalling and it has been shown to modulate Ca2+-dependent vesicular glutamate release in cortical astrocytes in response to mechanical stimulation [12, 13]. The TRPC channel in astrocytes can also provide substantial Na+ influx that may occur in response to mechanical stimulation or develop alongside with SOCE following depletion of the ER Ca2+ store. This Na+ influx can be instrumental for local Na+ signals critical for glial homeostatic response [4, 14]. In this study we characterized TRPC1-mediated Ca2+ and Na+ signalling in mechanically-stimulated cultured astrocytes and demonstrate that Ca2+ and Na+ fluxes can be dissociated following the treatment with a TRPC1 blocking antibody.
2. Materials and methods
2.1. Astrocyte cultures
Solitary astrocytes from visual cortices of 1- to 2- day-old Sprague Dawley rats were maintained in vitro as previously described [15]. Briefly, visual cortices were dissected and enzymatically treated with papain (20 i.u./ml, 1 hr at 37 °C) in the presence of L-cysteine (0.2 mg/ml); digestion was arrested by trypsin inhibitor (10 mg/ml; type II-O; 5 min at room temperature). Tissue was mechanically dissociated and neural cells were seeded into culture flasks containing culture medium composed of α-minimum essential medium (α-MEM, without phenol red; Life Technologies Corp. Invitrogen™, Carsbad, CA, USA) supplemented with foetal bovine serum (10% v/v; Thermo Scientific HyClone, Logan, UT, USA), glucose (20 mM), L-glutamine (2 mM), sodium pyruvate (1 mM), sodium bicarbonate (14 mM), penicillin (100 i.u./ml), and streptomycin (100 μg/ml), pH 7.35. After allowing cells to adhere to the bottom of the flasks for 1 hour, they were washed and provided with new media. Cells were then maintained at 37°C in a 95% air/ 5% CO2 environment for 5 to 7 days to reach ∼60% confluency. At that juncture, the cell cultures were purified for astrocytes using previously described procedure [16]. Purified astrocytes were detached from the flasks using trypsin (10,000 Nα-benzoyl-arginine ethyl ester hydrochloride units/ml; Sigma-Aldrich, St. Louis, MO, USA). After inhibition of trypsin activity by addition of complete culture medium, cells were pelleted using centrifugation (100 × g for 10 minutes), resuspended and plated onto round (12 mm in diameter) glass coverslips (Thermo Fisher Scientific) pre-coated with polyethyleneimine (1mg/ml; Sigma). Purified astrocytes were kept in culture medium at 37°C in a 95% air/ 5% CO2 atmosphere incubator for 5 - 8 days when used in experiments. The purity of astrocytic culture (>99%) was confirmed: (i) by indirect immunocytochemistry using anti-glial fibrillary acidic protein antibody and (ii) by visualization of accumulation of a dipeptide, β-Ala-Lys, conjugated to 7-amino-4-methylcoumarin-3-acetic acid as previously described [16]. Astrocytes in our culture system are flat polygonal cells and thus have a simplified morphology compared to astrocytes in situ [16, 17].
2.2. Anti-TRPC1 antibody treatment
Astrocytes grown on coverslips were incubated in external solution (pH 7.35) consisting of sodium chloride (140 mM), potassium chloride (5 mM), calcium chloride (2 mM), magnesium chloride (2 mM), HEPES (10 mM), and glucose (5 mM), with or without 30 μg/ml of anti-TRPC1 antibody (cat. No. ACC-010, Alomone labs, Jerusalem, Israel) for 30 min at room temperature (22-25 °C) as described previously [10]. Antibody incubation was performed after loading cells with either the Ca2+ indicator fluo-3 acetoxymethyl (AM) ester or the Na+ indicator CoroNa™Green AM and de-esterification of the indicators. Antibody was kept in solution during the entire imaging procedure lasting ∼200 seconds for Ca2+ and Na+ measurements.
2.3. Intracellular Ca2+ imaging
Cytosolic Ca2+ concentration ([Ca2+]i) in somata of cultured solitary astrocytes were assessed using the Ca2+ indicator fluo-3 as described earlier [17]. Briefly, astrocytes were loaded with fluo-3 AM (10 μg/ml; Life Technologies Corp. Invitrogen™) in external solution containing pluronic acid (0.025% w/v) for 30 min at room temperature. To allow de-esterification of fluo-3 AM, cells were subsequently kept in external solution for 30 min at room temperature. Coverslips were transferred into a recording chamber mounted on the inverted microscope, and astrocytes were visualized with a standard fluorescein isothiocyanate (FITC) filter set (Chroma Technology, Rockingham, VT, USA). Fluorescence intensities obtained from somata of indicator-loaded astrocytes were corrected (digital subtraction) for the background fluorescence measured from regions of coverslips containing no cells. Fluorescence data were expressed as ΔF/F0 (%) with the cell baseline fluorescence (F0) representing the average of the first 5 images before mechanical stimulation while ΔF represents the change in fluorescence emission. The [Ca2+]i was determined using calibration of fluo-3 as described elsewhere [18].
2.4. Intracellular Na+ imaging
Cytosolic Na+ concentration ([Na+]i) in somata of cultured solitary astrocytes was monitored using the Na+ indicator CoroNa™Green AM (10 μM; Life Technologies Corp. Invitrogen™) [15, 19]. Astrocytes were loaded with the indicator, imaged, and data were collected and processed as described above for Ca2+ imaging. Because CoroNa™Green tends to leak out of the cell, its intracellular fluorescence intensity substantially decays over time [19]. Consequently, using a linear regression and extrapolation of the baseline fluorescence of individual traces, we corrected them for the leak of the dye. The [Na+]i was determined using calibration of CoroNa™Green as described elsewhere [15].
2.5. Image acquisition and processing
An inverted microscope (TE 300; Nikon, Melville, NY, USA), equipped with differential interference contrast and wide-field fluorescence illumination, was used in all experiments. Experiments were performed using a 60X Plan Apo oil-immersion objective (1.4 numerical aperture; Nikon). Images were acquired using a CoolSNAP-HQ cooled charge-coupled device camera (Roper Scientific Inc., Tucson, AZ, USA) driven by V++ imaging software (Digital Optics Ltd., Auckland, New Zealand). All raw data/images had their pixel intensities within the camera's dynamic range (0-4095). The ΔF/F0 of the control and treatment groups were ranked and normalized to control to accommodate for variations in culture conditions, and to allow comparisons between experimental batches, as we previously described [15].
2.6. Mechanical stimulation
To stimulate a solitary astrocyte of interest, we employed mechanical contact using a glass pipette filled with external solution as we described elsewhere [15, 17]. This approach allows spatio-temporal control of the stimulus application without affecting plasma membrane integrity. The establishment of the patch pipette contact with the plasma membrane was determined by an increase in pipette resistance monitored using a patch-clamp amplifier (PC-ONE; Dagan, Minneapolis, MN, USA) that delivered -20 mV, 10 ms square pulses at 50 Hz. Once established, cell contact was maintained for ∼ 1 s. The strength of the stimulus, expressed as ΔR/R0 (%), where R0 represents the pipette resistance (2.6 - 4.8 MΩ) prior to establishing a pipette-astrocyte contact, and ΔR represents the increase in the resistance (0.05 – 0.28 MΩ) during the contact, had comparable intensities under all conditions tested (Mann-Whitney U-test, P = 0.253-0.351).
2.7. Statistical analysis
The comparison of the pipette resistance increases in different conditions and effects of an anti-TRPC1 antibody on mechanically-induced intracellular Ca2+ and Na+ loads were tested using Mann-Whitney U-test. Data are expresses as means ± SEMs.
3. Results
3.1. Cytosolic Ca2+ responses to mechanical stimulation in rat cortical astrocytes is reduced by an anti-TRPC1 antibody
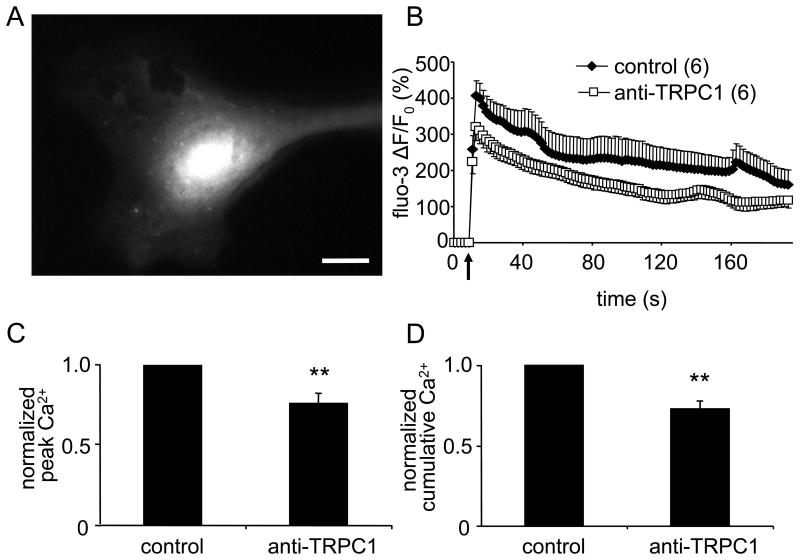
Mechanical stimulation of cortical astrocytes generates a rapid increase in cytosolic Ca2+ that slowly decays to the basal level [17, 20]. Previous studies conducted by us and others [8, 10] provided conclusive evidence that this increase in part results from Ca2+ entry through TRPC1 containing channels, and that application of an anti-TRPC1 antibody directed at amino acid residues 557-571 [21, 22] inhibited this Ca2+ entry [10, 12]. In this study mechanical stimulation of solitary astrocytes with glass pipettes induced transient increase in [Ca2+]i (Fig. 1). Incubation of astrocytes with an anti-TRPC1 antibody significantly reduced the peak of the [Ca2+]i transient; ΔF/F0 fell from 408 ± 42 % in control to 320 ± 38% (n = 6, Fig. 1). These changes in fluo-3 fluorescence correspond to cytosolic Ca2+ concentrations of ∼1.7 μM and ∼ 860 nM, respectively; resting [Ca2+]i levels were ∼ 70 nM. Both the peak of [Ca2+]i transients (Fig. 1C) and cumulative (area under the curve) [Ca2+]i responses (Fig. 1D) in anti-TRPC1 treated astrocytes were significantly lower than those of control cells (n = 6, Mann-Whitney U-test, P < 0.01).
Figure 1.
Cytosolic Ca2+ accumulation in astrocytes induced by mechanical stimulation is reduced by an antibody that binds to the pore forming region of TRPC1.
(A) A representative image of a solitary rat cortical astrocyte at rest and loaded with fluo-3. Scale bar = 20 μm. (B) Average kinetics of fluo-3 fluorescence from mechanically stimulated astrocytes. (C,D) Bar graphs showing normalized peak and cumulative cytosolic Ca2+ responses to mechanical stimulation. Both responses were significantly reduced when astrocytes were treated with an anti-TRPC1 antibody (30 μg/ml, 30 min). Points and bars represent means ± SEM of measurements. SEMs are shown in single directions for clarity. Arrow indicates the time when the mechanical stimulus occurred. Numbers in parentheses indicate the number of astrocytes studied in each group. Asterisks denote a significant change in measurement when compared to control group, i.e. untreated astrocytes (Mann-Whitney U-test; **p < 0.01).
3.2. The peak cytosolic Na+ response to mechanical stimulation in rat cortical astrocytes is increased by an anti-TRPC1 antibody
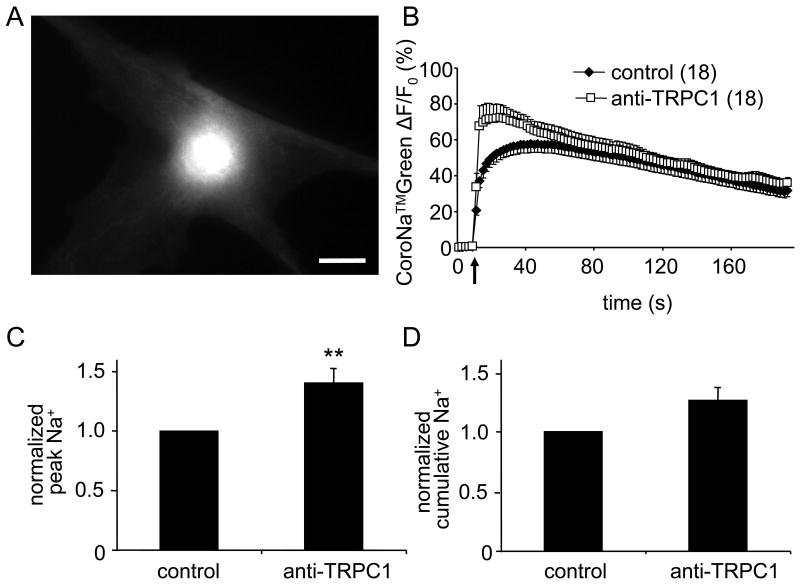
Mechanical stimulation of astrocytes triggered a large transient increase in [Na+]i (Fig. 2). Changes in peak fluorescence measured in control conditions averaged 58 ± 5 % (ΔF/F0; n = 18) that correspond to a [Na+]i increase to ∼35.5 mM from the resting level of ∼17 mM. Incubation of cultured astrocytes with an anti-TRPC1 antibody increased mechanically-induced [Na+]i transients. An increase in the ΔF/F0 peak averaged 72 ± 7% (Fig. 2; n = 18, Mann-Whitney U-test, P < 0.01) that corresponded to the peak [Na+]i transient of ∼42.6 mM. At the same time the cumulative [Na+]i response measured from anti-TRPC1 antibody treated cells was not significantly different when compared to control cells (Fig. 2D).
Figure 2.
Cytosolic Na+ accumulation in astrocytes induced by mechanical stimulation is augmented by an antibody that binds to the pore forming region of TRPC1.
(A) A representative image of a solitary rat cortical astrocyte at rest and loaded with CoroNa™Green. Scale bar = 20 μm. (B) Average kinetics of CoroNa™Green fluorescence from mechanically stimulated astrocytes. (C,D) Bar graphs showing normalized peak and cumulative cytosolic Na+ responses to mechanical stimulation. The peak value was significantly increased when astrocytes were treated with an anti-TRPC1 antibody (Mann-Whitney U-test; **p < 0.01). Points, bars, arrow and numbers in parentheses as in Fig 1.
4. Discussion
Mounting highly heterogeneous and precisely controlled homeostatic responses to a continuously changing interstitial environment of the CNS is the raison d'etre of astroglia. The range of these responses is remarkable as astrocytes regulate fluxes of physiologically relevant ions such as K+, Na+, Ca2+, H+, C1-, HCO3-, maintain glutamine-glutamate and glutamine-GABA shuttles, supply lactate to active neuronal compartments, control water movements, secrete scavengers of reactive oxygen species and many more [23, 24]. These responses are critically important for the functional connectivity in the CNS because they maintain neuronal excitability and synaptic transmission. For this purpose astrocytes continuously monitor neuronal activity by multiple neurotransmitter receptors and ion channels [25, 26]. Activation of these channels and receptors triggers often highly localized fluctuations of Ca2+ and Na+ in astroglial cytosol that constitute a substrate for glial excitability [4, 27].
Astrocytes possess a high degree of morphological plasticity, as indeed neuronal activity results in highly dynamic redistribution of water and transient changes in the volume of perisynaptic astroglial processes [28, 29]; similarly astroglia exhibits a volume response to changes in extracellular osmotic pressure [30]. These dynamic changes in cell volume activate signaling cascades through mechano-sensitive channels that mediate ion fluxes in response to mechano-stimulation of the plasmalemma resulting in cytosolic Ca2+ and Na+ signals [15]. Array of mechano-sensitive astroglial channels most likely consist of several channel types, of which TRPC1 is a sound candidate. Indeed stretching the membrane of frog oocytes or CHO-K1 cells transfected with TRPC1 activated ionic currents, whereas inhibition of TRPC1 synthesis by antisense RNA reduced these mechano-sensitive current responses [31]. Here we further corroborate this hypothesis by demonstrating that treatment of astrocytes with an antibody against TRPC1 channel inhibited [Ca2+]i transients triggered by mechanical stimulation. Of note, however, other mechano-sensitive channels may underlie cation fluxes. Recently, channels of Piezo1 and Piezo2 types have been identified as mechano-sensitive channels in dorsal root ganglion neurones [32]. The Piezo1 channel can be selectively inhibited by peptide toxin GsMT×4, isolated from tarantula venom, and GsMT×4 was reported to inhibit stretch-activated channels in astrocyte membranes [33].
We further confirmed that mechanical stimulation of astrocytes triggers large (up to 20 mM in amplitude) increases in [Na+]i which also were found to be sensitive to anti-TRPC1 antibody. In contrast with [Ca2+]i responses, however, inhibition of the TRPC1 channels by the anti-TRPC1 antibody resulted in a significant increase in the peak amplitude of [Na+]i response. Thus binding of the antibody to the TRPC1 channel decreases Ca2+ flux with a parallel increase in Na+ flux. Somewhat similar observations were gathered in site-directed mutagenesis studies of the TRPC1 and TRPC3 channels. Substitution of seven acidic residues to basic amino acids in the channel region of TRPC1 inhibited Ca2+ movement without affecting Na+ fluxes [34]. A single mutation (E630Q) of the selective filter of TRPC3 markedly inhibited Ca2+ current, whereas increasing Na+ currents through the channel especially at negative membrane potentials [35]. Incidentally, the Basic Local Alignment Search Tool (BLAST®) comparison of rat TRPC1 and TRPC3 proteins reveals that the TRPC1 antibody peptide target (amino acids 557-571) and the mutated site of TRPC3 are both located in putative selective filters that are not highly conserved [34, 35]. Taken together, these studies suggest that at least some TRPC channels are amenable to mutations that disrupt Ca2+ but preserve (or even increase) Na+ entry. This observation may be important to differentially modulate TRP channels in heath and disease.
It should be noted that we have not addressed the ability of anti-TRPC1 antibody to modulate ion dynamics in astrocytes at rest. It is likely that the antibody would require an opening of the channel to bind to the pore region. Such opening events in unstimulated astrocytes at rest are considered rare as TRPC1 channels are activated when the ER store is getting depleted. Nonetheless, binding of the antibodies to TRPC1 channels in astrocytes at rest may cause cell depolarization and reduce the driving force for Ca2+ and Na+ entry to the cytosol. In such a scenario, in mechanically-stimulated astrocytes treated with the anti-TRPC1 antibody there could be a more profound decrease in [Ca2+]i and a less prominent increase of [Na+]i than those we observed. Another intriguing, albeit unlikely, possibility is that the bound antibody, which blocks the Ca2+ selectivity filter, could dilate the TRPC1 channel during mechanical stimulation. Such conditions could allow an increase of Na+ flux which would be manifested as an increase in the amplitude of the [Na+]i transient as we recorded.
Another fundamental function of TRPC1 containing channels in astroglia lies in their role in SOCE. This mechanism of the Ca2+ entry is universally present in neuroglial cells and is ubiquitous in astrocytes. Stimulation of astroglia in vitro or in situ with neurotransmitters initiates complex [Ca2+]i responses comprising of the ER Ca2+ release-dependent initial peak and the SOCE dependent long-lasting plateau [8, 10, 36-38]; it should be noted that SOCE contributes even to the peak response [10]. Similarly receptor independent depletion of ER Ca2+ store results in prominent SOCE in astrocytes [39, 40]. Sites of astroglial SOCE are localized close to the ER thus increasing the efficacy of Ca2+ store replenishment [8, 41]. The SOCE pathway is also prominent in pathologically modified glial cells such as for example in glioblastoma [42].
TRPC1 containing channels have been identified as a main mechanism for SOCE in astroglia [5, 8, 10]. This endows them with another highly important role in coordination of ionic signalling in astrocytes. Being store-operated TRPC1 channels establish a functional link between metabotropically induced Ca2+ signalling with Na+ influx thus coordinating Ca2+ and Na+ signalling in astroglial sub-compartments. In this study we found that TRPC1 channels can potentially regulate their permeability to Ca2+ and Na+ thus being capable of dynamic control over local ionic signalling. Such changes of [Ca2+]i and [Na+]i could affect Ca2+- and/or Na+-dependent processes. Increase of [Ca2+]i can trigger regulated exocytosis of glutamate [43], while increase of [Na+]i can trigger glycolysis leading to lactate production [44]; both events are important for modulation of synaptic transmission and plasticity [45, 46]. Naturally the physiological mechanism of such regulation of TRPC1 permeability remains unknown (and it may include enzymatic or post-translational modification) and requires further investigation.
The TRPC1 channels were also reported to co-localize with astroglial plasmalemmal Na+/Ca2+ exchangers (NCX), which in turn are concentrated in perisynaptic processes being also closely associated with Na+/K+ pumps, glutamate ionotropic receptors, and glutamate/GABA Na+-dependent transporters [4]. This strategic localization allowing focal Na+ and Ca2+ fluxes could be of critical importance for fast neuronal-astroglial signalling at the single synaptic level. For example TRPC1-mediated changes in [Ca2+]i and [Na+]i determined in this study can substantially affect reversal potential of astroglial NCX (ENCX); this has been suggested as a key mechanism underpinning TRPC3 signalling [47]. Indeed, the resting ENCX in our experimental conditions is estimated at -98 mV, it shifts to -79 mV in astrocytes mechanically stimulated in control conditions and to -109 mV in cells stimulated in the presence of an anti-TRPC1 antibody (see Table 1 for details). These fluctuations in ENCX have important functional consequences. In normal conditions mechanical stimulation promotes reversal mode of NCX and Ca2+ entry [15], whereas after TRPC1 modification cell stimulation favours forward mode of NCX resulting in Ca2+ extrusion and additional Na+ entry. As outlined above, this can govern Ca2+- and/or Na+-dependent processes in astrocytes that can in turn modulate the operation of the tripartite synapse.
Table 1. Reversal potential of astroglial plasmalemmal NCX.
| Cytosolic ion concentrations | Condition | ENCX |
|---|---|---|
| [Na+]i= 16.6 mM | resting | -98 mV |
| [Ca2+]i = 73 nM | ||
| [Na+]i=35.5 mM | mechanical stimulation; | -76 mV |
| [Ca2+]i=1.7 μM | control | |
| [Na+]i=42.6 mM | mechanical stimulation; | -107 mV |
| [Ca2+]i= 861 nM | TRPC1 antibody treatment |
Reversal potential of NCX (ENCX) at 25°C was calculated using our recorded cytosolic Na+ and Ca2+ concentrations together with concentrations of these ions in the external solution ([Na+]o = 140 mM and [Ca2+]o = 2 mM) and presumed NCX 3:1 stoichiometry.
In conclusion the TRPC1 channels appear as a focal point for co-ordination of Ca2+ and Na+ signalling in astroglia. The capability of TRPC1 to dynamically modify Ca2+ and Na+ fluxes may be relevant for this coordination by increasing plastic potential of ionic signalling in astroglia.
Acknowledgments
This work was supported by the National Science Foundation (CBET 0943343). Reno C. Reyes was additionally funded by UCSF Neuroscience and Schizophrenia T32 (MH 089920).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Parpura V, Verkhratsky A. Homeostatic function of astrocytes: Ca2+ and Na+ signalling. Transl Neurosci. 2012;3:334–344. doi: 10.2478/s13380-012-0040-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parpura V, Verkhratsky A. Neuroglia at the crossroads of homoeostasis, metabolism and signalling: evolution of the concept. ASN Neuro. 2012;4:201–205. doi: 10.1042/AN20120019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verkhratsky A, Steinhauser C. Ion channels in glial cells. Brain Res Brain Res Rev. 2000;32:380–412. doi: 10.1016/s0165-0173(99)00093-4. [DOI] [PubMed] [Google Scholar]
- 4.Kirischuk S, Parpura V, Verkhratsky A. Sodium dynamics: another key to astroglial excitability? Trends Neurosci. 2012;35:497–506. doi: 10.1016/j.tins.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Verkhratsky A, Rodriguez JJ, Parpura V. Calcium signalling in astroglia. Mol Cell Endocrinol. 2012;353:45–56. doi: 10.1016/j.mce.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 6.Ambudkar IS, Ong HL. Organization and function of TRPC channelosomes. Pflugers Archiv : European journal of physiology. 2007;455:187–200. doi: 10.1007/s00424-007-0252-0. [DOI] [PubMed] [Google Scholar]
- 7.Owsianik G, Talavera K, Voets T, Nilius B. Permeation and selectivity of TRP channels. Annu Rev Physiol. 2006;68:685–717. doi: 10.1146/annurev.physiol.68.040204.101406. [DOI] [PubMed] [Google Scholar]
- 8.Golovina VA. Visualization of localized store-operated calcium entry in mouse astrocytes. Close proximity to the endoplasmic reticulum. J Physiol. 2005;564:737–749. doi: 10.1113/jphysiol.2005.085035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grimaldi M, Maratos M, Verma A. Transient receptor potential channel activation causes a novel form of [Ca2+]i oscillations and is not involved in capacitative Ca2+ entry in glial cells. J Neurosci. 2003;23:4737–4745. doi: 10.1523/JNEUROSCI.23-11-04737.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malarkey EB, Ni Y, Parpura V. Ca2+ entry through TRPC1 channels contributes to intracellular Ca2+ dynamics and consequent glutamate release from rat astrocytes. Glia. 2008;56:821–835. doi: 10.1002/glia.20656. [DOI] [PubMed] [Google Scholar]
- 11.Pizzo P, Burgo A, Pozzan T, Fasolato C. Role of capacitative calcium entry on glutamate-induced calcium influx in type-I rat cortical astrocytes. J Neurochem. 2001;79:98–109. doi: 10.1046/j.1471-4159.2001.00539.x. [DOI] [PubMed] [Google Scholar]
- 12.Parpura V, Grubisic V, Verkhratsky A. Ca2+ sources for the exocytotic release of glutamate from astrocytes. Biochim Biophys Acta. 2011;1813:984–991. doi: 10.1016/j.bbamcr.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Zorec R, Araque A, Carmignoto G, Haydon PG, Verkhratsky A, Parpura V. Astroglial excitability and gliotransmission: an appraisal of Ca2+ as a signalling route. ASN Neuro. 2012;4 doi: 10.1042/AN20110061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rose CR, Karus C. Two sides of the same coin: Sodium homeostasis and signaling in astrocytes under physiological and pathophysiological conditions. Glia. 2013 doi: 10.1002/glia.22492. [DOI] [PubMed] [Google Scholar]
- 15.Reyes RC, Verkhratsky A, Parpura V. Plasmalemmal Na+/Ca2+ exchanger modulates Ca2+-dependent exocytotic release of glutamate from rat cortical astrocytes. ASN Neuro. 2012;4 doi: 10.1042/AN20110059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montana V, Ni Y, Sunjara V, Hua X, Parpura V. Vesicular glutamate transporter-dependent glutamate release from astrocytes. J Neurosci. 2004;24:2633–2642. doi: 10.1523/JNEUROSCI.3770-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hua X, Malarkey EB, Sunjara V, Rosenwald SE, Li WH, Parpura V. Ca2+-dependent glutamate release involves two classes of endoplasmic reticulum Ca2+ stores in astrocytes. J Neurosci Res. 2004;76:86–97. doi: 10.1002/jnr.20061. [DOI] [PubMed] [Google Scholar]
- 18.Parpura V, Haydon PG. Physiological astrocytic calcium levels stimulate glutamate release to modulate adjacent neurons. Proc Natl Acad Sci U S A. 2000;97:8629–8634. doi: 10.1073/pnas.97.15.8629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meier SD, Kovalchuk Y, Rose CR. Properties of the new fluorescent Na+ indicator CoroNa Green: comparison with SBFI and confocal Na+ imaging. J Neurosci Methods. 2006;155:251–259. doi: 10.1016/j.jneumeth.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Reyes RC, Parpura V. Mitochondria modulate Ca2+-dependent glutamate release from rat cortical astrocytes. J Neurosci. 2008;28:9682–9691. doi: 10.1523/JNEUROSCI.3484-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu SZ, Beech DJ. TrpC1 is a membrane-spanning subunit of store-operated Ca2+ channels in native vascular smooth muscle cells. Circ Res. 2001;88:84–87. doi: 10.1161/01.res.88.1.84. [DOI] [PubMed] [Google Scholar]
- 22.Antoniotti S, Lovisolo D, Fiorio Pla A, Munaron L. Expression and functional role of bTRPC1 channels in native endothelial cells. FEBS letters. 2002;510:189–195. doi: 10.1016/s0014-5793(01)03256-2. [DOI] [PubMed] [Google Scholar]
- 23.Kettenmann H, Ransom BR. Neuroglia. Oxford University Press; Oxford: 2013. p. 864. [Google Scholar]
- 24.Verkhratsky A, Butt AM. Glial Physiology and Pathophysiology. Wiley-Blackwell; Chichester: 2013. [Google Scholar]
- 25.Lalo U, Pankratov Y, Parpura V, Verkhratsky A. Ionotropic receptors in neuronal-astroglial signalling: what is the role of “excitable” molecules in non-excitable cells. Biochim Biophys Acta. 2011;1813:992–1002. doi: 10.1016/j.bbamcr.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 26.Verkhratsky A, Parpura V. Recent advances in (patho)physiology of astroglia. Acta Pharmacol Sin. 2010;31:1044–1054. doi: 10.1038/aps.2010.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agulhon C, Petravicz J, McMullen AB, Sweger EJ, Minton SK, Taves SR, Casper KB, Fiacco TA, McCarthy KD. What is the role of astrocyte calcium in neurophysiology? Neuron. 2008;59:932–946. doi: 10.1016/j.neuron.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haj-Yasein NN, Jensen V, Ostby I, Omholt SW, Voipio J, Kaila K, Ottersen OP, Hvalby O, Nagelhus EA. Aquaporin-4 regulates extracellular space volume dynamics during high-frequency synaptic stimulation: A gene deletion study in mouse hippocampus. Glia. 2012;60:867–874. doi: 10.1002/glia.22319. [DOI] [PubMed] [Google Scholar]
- 29.Amiry-Moghaddam M, Ottersen OP. The molecular basis of water transport in the brain. Nat Rev Neurosci. 2003;4:991–1001. doi: 10.1038/nrn1252. [DOI] [PubMed] [Google Scholar]
- 30.Kimelberg HK, Sankar P, O'Connor ER, Jalonen T, Goderie SK. Functional consequences of astrocytic swelling. Prog Brain Res. 1992;94:57–68. doi: 10.1016/s0079-6123(08)61739-2. [DOI] [PubMed] [Google Scholar]
- 31.Maroto R, Raso A, Wood TG, Kurosky A, Martinac B, Hamill OP. TRPC1 forms the stretch-activated cation channel in vertebrate cells. Nat Cell Biol. 2005;7:179–185. doi: 10.1038/ncb1218. [DOI] [PubMed] [Google Scholar]
- 32.Bae C, Sachs F, Gottlieb PA. The mechanosensitive ion channel Piezo1 is inhibited by the peptide GsMT×4. Biochemistry. 2011;50:6295–6300. doi: 10.1021/bi200770q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suchyna TM, Johnson JH, Hamer K, Leykam JF, Gage DA, Clemo HF, Baumgarten CM, Sachs F. Identification of a peptide toxin from Grammostola spatulata spider venom that blocks cation-selective stretch-activated channels. J Gen Physiol. 2000;115:583–598. doi: 10.1085/jgp.115.5.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu X, Singh BB, Ambudkar IS. TRPC1 is required for functional store-operated Ca2+ channels. Role of acidic amino acid residues in the S5-S6 region. The Journal of biological chemistry. 2003;278:11337–11343. doi: 10.1074/jbc.M213271200. [DOI] [PubMed] [Google Scholar]
- 35.Poteser M, Schleifer H, Lichtenegger M, Schernthaner M, Stockner T, Kappe CO, Glasnov TN, Romanin C, Groschner K. PKC-dependent coupling of calcium permeation through transient receptor potential canonical 3 (TRPC3) to calcineurin signaling in HL-1 myocytes. Proc Natl Acad Sci U S A. 2011;108:10556–10561. doi: 10.1073/pnas.1106183108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kirischuk S, Tuschick S, Verkhratsky A, Kettenmann H. Calcium signalling in mouse Bergmann glial cells mediated by α1-adrenoreceptors and H1 histamine receptors. Eur J Neurosci. 1996;8:1198–1208. doi: 10.1111/j.1460-9568.1996.tb01288.x. [DOI] [PubMed] [Google Scholar]
- 37.Moller T, Nolte C, Burger R, Verkhratsky A, Kettenmann H. Mechanisms of C5a and C3a complement fragment-induced [Ca2+]i signaling in mouse microglia. J Neurosci. 1997;17:615–624. doi: 10.1523/JNEUROSCI.17-02-00615.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tuschick S, Kirischuk S, Kirchhoff F, Liefeldt L, Paul M, Verkhratsky A, Kettenmann H. Bergmann glial cells in situ express endothelinB receptors linked to cytoplasmic calcium signals. Cell Calcium. 1997;21:409–419. doi: 10.1016/s0143-4160(97)90052-x. [DOI] [PubMed] [Google Scholar]
- 39.Lo KJ, Luk HN, Chin TY, Chueh SH. Store depletion-induced calcium influx in rat cerebellar astrocytes. Br J Pharmacol. 2002;135:1383–1392. doi: 10.1038/sj.bjp.0704594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singaravelu K, Lohr C, Deitmer JW. Regulation of store-operated calcium entry by calcium-independent phospholipase A2 in rat cerebellar astrocytes. J Neurosci. 2006;26:9579–9592. doi: 10.1523/JNEUROSCI.2604-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pivneva T, Haas B, Reyes-Haro D, Laube G, Veh RW, Nolte C, Skibo G, Kettenmann H. Store-operated Ca2+ entry in astrocytes: different spatial arrangement of endoplasmic reticulum explains functional diversity in vitro and in situ. Cell Calcium. 2008;43:591–601. doi: 10.1016/j.ceca.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 42.Hartmann J, Verkhratsky A. Relations between intracellular Ca2+ stores and store-operated Ca2+ entry in primary cultured human glioblastoma cells. J Physiol. 1998;513:411–424. doi: 10.1111/j.1469-7793.1998.411bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parpura V, Zorec R. Gliotransmission: Exocytotic release from astrocytes. Brain Res Rev. 2010;63:83–92. doi: 10.1016/j.brainresrev.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Magistretti PJ. Neuron-glia metabolic coupling and plasticity. J Exp Biol. 2006;209:2304–2311. doi: 10.1242/jeb.02208. [DOI] [PubMed] [Google Scholar]
- 45.Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ, Alberini CM. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell. 2011;144:810–823. doi: 10.1016/j.cell.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perea G, Araque A. Astrocytes potentiate transmitter release at single hippocampal synapses. Science. 2007;317:1083–1086. doi: 10.1126/science.1144640. [DOI] [PubMed] [Google Scholar]
- 47.Eder P, Poteser M, Romanin C, Groschner K. Na+ entry and modulation of Na+/Ca2+ exchange as a key mechanism of TRPC signaling. Pflugers Archiv : European journal of physiology. 2005;451:99–104. doi: 10.1007/s00424-005-1434-2. [DOI] [PubMed] [Google Scholar]