Abstract
In this work, a polystyrene (PS)-polydimethylsiloxane (PDMS) hybrid device was developed to enable the integration of cell culture with analysis by microchip electrophoresis and electrochemical detection. It is shown that this approach combines the fundamental advantages of PDMS devices (the ability to integrate pumps and valves) and PS devices (the ability to permanently embed fluidic tubing and electrodes). The embedded fused-silica capillary enables high temporal resolution measurements from off-chip cell culture dishes and the embedded electrodes provide close to real-time analysis of small molecule neurotransmitters. A novel surface treatment for improved (reversible) adhesion between PS and PDMS is described using a chlorotrimethylsilane stamping method. It is demonstrated that a Pd decoupler is efficient at handling the high current (and cathodic hydrogen production) resulting from use of high ionic strength buffers needed for cellular analysis; thus allowing an electrophoretic separation and in-channel detection. The separation of norepinephrine (NE) and dopamine (DA) in highly conductive biological buffers was optimized using a mixed surfactant system. This PS-PDMS hybrid device integrates multiple processes including continuous sampling from a cell culture dish, on-chip pump and valving technologies, microchip electrophoresis, and electrochemical detection to monitor neurotransmitter release from PC 12 cells.
1 Introduction
Microfluidic technologies are an attractive approach for studying biological systems and have been used for a wide variety of cellular applications.1–3 Microfluidic devices have been developed to culture/immobilize cells because of their ability to closely mimic in vivo systems. In addition, microchip devices enable the integration of multiple processes and minimize dilution, making them ideal for cellular analysis. Many of the previously described devices have integrated cell culture/immobilization on microchip devices, but many of these devices do not incorporate an analysis component. The integration of an analysis component enables studies where cell-to-cell or drug-to-cell interactions can be studied.
There have been several demonstrations of integrating multiple processes on microchip devices, where analysis step is used to monitor biological events (both in vivo and in vitro). A recent study demonstrated the effectiveness of integrating flowing cells, a porous polycarbonate membrane, and a plate reader for measurement of nitric oxide released from red blood cells.4 On-chip red blood cell lysis and electrochemical analysis of intracellular glutathione have also been integrated in a microchip format.5 The incorporation of a microchip-based separation has also been demonstrated using both off- and on-chip sampling techniques. Microdialysis sampling has been coupled with microchips in a manner where electrophoretic separations and laser-induced fluorescence detection are used to monitor primary amino acid neurotransmitters in a rat brain.6 This approach was later extended to include segmented flow for improved temporal resolution.7 A recent study integrated single cell transport, lysis, injection, electrophoresis, and fluorescence detection to enable the analysis of intracellular nitric oxide in single cells in a high-throughput manner.8 A method has also been developed to perform on-chip synchronization of bacterial cells. This automated system integrated cell seeding, culture, incubation, and fluorescence detection for analysis.9 Previous work from our group included the development of microchip devices that integrated multiple processes such as cell immobilization, on-chip valving technologies, microchip electrophoresis, and either post-column derivatization for fluorescence detection or electrochemical detection.10,11 A key aspect of this work was the ability to incorporate a separation step (microchip-based electrophoresis) so that numerous neurotransmitters/products that are released from the cells could be separated. Electrochemical detection was an attractive detection mode because it required no derivatization steps and enabled close to real-time analysis.
The traditional substrate for microfluidic devices has been PDMS because it is inexpensive, transparent, flexible, amenable to rapid prototyping, can include integrated valves/pumps, and has the ability to reversibly seal to itself or other microchip substrates. In order to incorporate a microchip electrophoresis separation with electrochemical detection, a palladium decoupler can be utilized to provide an electrophoretic ground and absorb the hydrogen produced from the reduction of water at the cathode.12,13 A widely used approach for integrating electrodes in microfluidic devices is through traditional sputtering and lithographic processing of electrodes on a glass plate, followed by bonding of the electrode plate with a PDMS fluidic network.5,12,14,15 These thin-layer glass plate electrodes do enable integration of microchip electrophoresis with electrochemical detection, but they can be expensive to fabricate, require a specialized facility for fabrication, and fabricating multiple electrodes of differing composition can be difficult. A simple and reproducible fabrication approach has been described that incorporates multiple electrode materials and allow electrode polishing to generate a fresh electrode surface when desired.16,17 It has been shown that the resulting embedded Pd decouplers have improved performance and are capable of dissipating the hydrogen production from the electrolysis of water associated with highly conductive biological buffers. The use of higher field strengths is possible with the embedded Pd decouplers (as opposed to the thin-layer Pd decouplers) due to the increased surface area of the Pd, which allows for more H2 dissipation. Important for this work, the ability to embed components such as tubing for fluidic interconnects and electrodes for analysis enables integration of multiple processes.17,18
The device described here is a PS-PDMS hybrid device that bridges the fundamental advantages of utilizing PDMS devices (the ability to incorporate pumps and valves) and PS devices (the ability to permanently embed fluidic tubing to easily incorporate off-chip sampling and electrodes for analysis). The device is more modular for integrating cell analysis and enables high-throughput cellular studies that will also have high temporal resolution. As opposed to previous work where it was shown that PS is a more biocompatible microchip substrate,19–23 in this work we utilize PS due to its ability to easily embed tubing and multiple electrode materials. We describe a novel surface treatment using chlorotrimethylsilane to create a more robust reversible seal between the PS and PDMS substrates. It is shown that the Pd decoupler is of high performance, enabling the injection of high ionic strength cell buffers, with the decoupler handling the high current (and cathodic H2 production) that results. Electrophoretic separations are also made more difficult in high ionic strength buffers due to anti-stacking issues.24,25 The separation of NE and DA is optimized in this work using a mixed surfactant separation buffer. In addition, it is shown that an embedded capillary allows modular coupling of the valving microchip with immobilized cells and a low dead-volume interface. The development of the described PS-PDMS hybrid device incorporates multiple processes including continuous sampling from a cell culture dish, on-chip pump and valving technologies, microchip electrophoresis, and electrochemical detection to monitor neurotransmitter release from PC 12 cells.
2 Experimental
2.1 Chemicals and materials
The following chemicals and materials were used as received: Sylgard 184 (Ellsworth Adhesives, Germantown, WI, USA); Nano SU-8 developer, SU-8 50, SU-8 10 photoresist (Microchem, Newton, MA, USA); AZ 4620 positive resist and AZ 400 developer (AZ Resist, Somerville, NJ, USA); catechol, dopamine, norepinephrine, boric acid, sodium dodecyl sulfate, potassium chloride, sodium chloride, magnesium chloride, sodium phosphate, TES sodium salt, Tween® 20, HEPES, chlorotrimethylsilane, 3-aminopropyl trimethoxysilane and hexamethyldisilazane (Sigma Aldrich, St. Louis, MO, USA); 25 µm Pt wire and 1 mm palladium wire (Alfa Aesar, Ward Hill, MA, USA); soldering wire and heat shrink tubes (Radioshack); isopropanol and acetone (Fisher Scientific, Springfield, NJ, USA); colloidal silver (Ted Pella, Redding, CA, USA); BAS electrode polishing kit (Bioanalytical Systems, West Lafayette, IN, USA); 150 µm i.d. capillary (Polymicro Technologies, Phoenix, AZ, USA); 33 µm carbon fiber (Avco Specialty Materials, Textron, Lowell, MA, USA) and PS powder (250 µm particle size, Goodfellow, Oakdale, PA, USA).
2.2 Fabrication and assembly
Bilayer PDMS valving microchips were fabricated as previously described, using a negative resist for the valving layer and a positive resist for the fluidic layer.10,26 The PDMS structures were cured together after a partial cure step to form a uniform, bilayer microchip. The channel heights were measured using a profilometer (Dektak3 ST, Veeco Instruments, Woodbury, NY, USA). For the temporal resolution and injection characterization studies, the separation and pushback channels were 40 µm wide and 20 µm tall, with the flow channel being 200 µm in width. For cell studies, the flow channel was 300 µm wide, and the separation and pushback channels were 30 µm wide, 17 µm tall, and 2.5 cm long.
PS devices with embedded electrodes were fabricated as previously described.16 Briefly, electrodes (1 mm diameter Pd for the decoupler and either 25 µm Pt or 33 µm carbon fiber, all connected to a copper connecting wire with colloidal silver) were placed in a vertical orientation into electrode insertion holes and commercially available PS powder was melted at 250 °C in a uniform manner. For all studies, a 1 mm (in diameter) Pd decoupler was embedded 1 mm away from either a 25 µm Pt detection electrode or a 33 µm carbon fiber detection electrode. Fused-silica capillary was also embedded in the device18 with the fabrication procedure being similar to that of making PS-embedded electrodes. A capillary is threaded through a hole in the aluminum weigh dish and plugged with PDMS to prevent PS particles from clogging the capillary during heating. In this manner, PS devices are fabricated with embedded fused-silica capillary and electrodes. A film-based design for fabrication of the positive photoresist flow layer is used to ensure proper alignment of the capillary, the Pd decoupler, and the working electrode before PS is added for heating. The film is placed on the inside of the aluminum boat and flipped 180° vertically from the way which the PDMS chip would appear on the PS base. A 23 gauge syringe (Becton Dickinson and Company, Franklin Lakes, NJ, USA) is used to punch the holes in the aluminum boat for the capillary, decoupler, and electrode. The needle is pushed through completely to make an insertion hole for the capillary and decoupler, while only the syringe tip is pushed through for the electrode to leave less room for error in the alignment of the electrode. Each of the components of the device is then threaded through their respective insertion holes, followed by addition of the PS powder, the heating step, and cooling to room temperature. The PS was removed from the aluminum dish and shaped by wet polishing using a range (200–1200) of grits (Buehler, Lake Bluff, IL, USA) to achieve a fine polish. While devices with the Pt detection electrode can be polished with the finest grit for an extended period of time, it was found that the carbon fiber devices, if polished for an extended period of time with a fine grit, led to the carbon fiber protruding from the surface sometimes several microns in height (resulting in subsequent issues with flow through the channel). For these devices, the fine polishing step was abbreviated so that it was long enough to just remove any scratches, with the resulting fiber protruding 0.5 µm or less from the surface (measured with a profilometer).
For all studies, the PDMS microchip was reversibly sealed over the electrodes. Before sealing the microchip over the PS-embedded electrodes, the PDMS microchip was stamped with chlorotrimethylsilane (Sigma Aldrich, St. Louis, MO, USA). The procedure consisted of pipetting 500 µL of neat chlorotrimethysilane onto a separate PS, glass, or silicon surface, followed by evaporation of silane for several minutes, and sealing of the PDMS microchip on the treated surface for at least an hour. The PDMS was then removed and sealed on the untreated PS with embedded electrodes. Contact angle measurements were taken on the PDMS microchip before and after treatment with chlorotrimethylsilane. A 1 µL droplet of water was pipetted onto the PS surface and a QX3 microscope (Digital Blue, Atlanta, GA, USA) was used to take an image of the droplet. The dimensions of the droplet were then measured to calculate the contact angle using the sessile drop method.27
2.3 Injection characterization
The PDMS microchip contains a set of on-chip peristaltic pumps and two injection valves (shown in Figure 1). All valves and pumps were dead end filled with water before operation.11 The device operates by offsetting the actuation of each pump to create a peristaltic pump that continuously samples from the reservoir. The peristaltic pumps operate at 5 psi using an 8-channel valve controller (Fluidigm Corporation, San Francisco, CA, USA). A bi-directional syringe pump (Eldex MicroPro, Napa, CA, USA) was also used to pull the sample through the flow channels. In this manner, solution was constantly withdrawn from the reservoir, towards the injection interface.
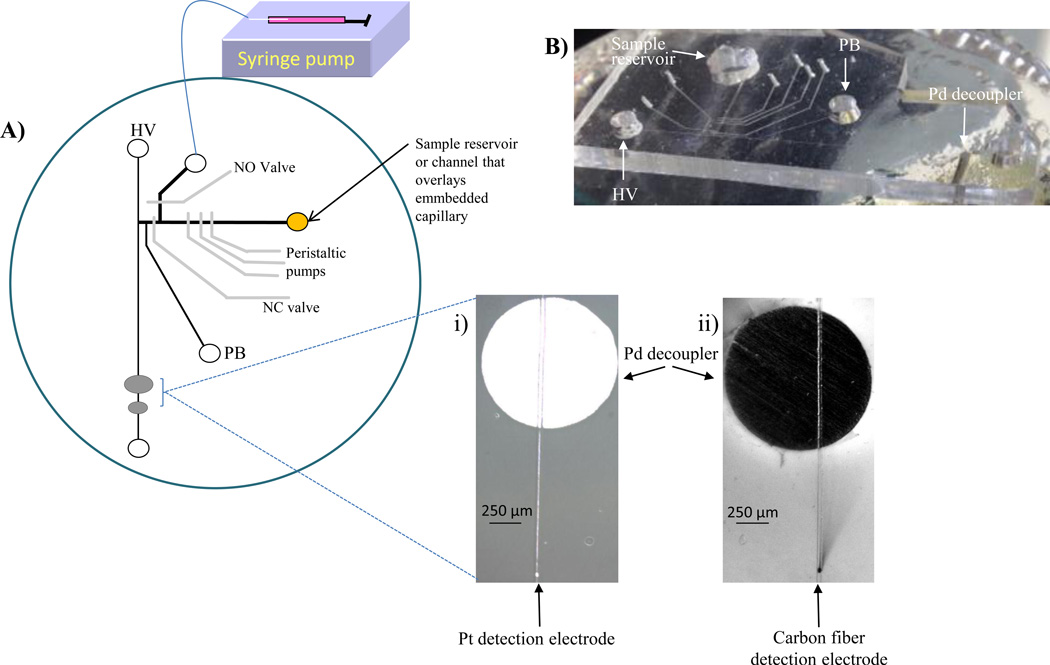
Figure 1.
Polystyrene device with PDMS valving chip for PC 12 cell analysis. A) Schematic of device. On-chip perisaltic pumps are used to continuously sample from the sample reservoir and injection valves (NO and NC valves) are utilized to discretely inject into an electrophoresis channel. A high voltage (HV) and pushback voltage (PB) are used to separate via microchip electrophoresis and amperometric detection is performed at either a i) 25 µm Pt or ii) 33 µm carbon fiber electrode. A syringe pump in withdraw mode allows an improvement in temporal resolution. B) Side-view of the PDMS microchip sealed over PS-embedded electrodes.
Injections were made by actuating the pneumatic valves using MAC valves (Mac Fluid Power Engineering, St. Louis, MO, USA) by means of a timer-based control unit. The normally closed (NC) valve was opened for a short period of time (3–5 seconds) while the normally open (NO) valve was closed to introduce a discrete plug into the separation channel. To characterize the device, injections were made into the separation channel as the frequency of the pumps (0–20 Hz) and the withdraw rate (0–12 µL/min) were changed.
2.4 Microchip electrophoresis with electrochemical detection
The analytes were electrophoretically separated and detected downstream at the detection electrode. Amperometric detection was performed at +0.9 V with a CH Instruments potentiostat (Austin, TX, USA). An embedded 25 µm Pt wire or 33 µm carbon fiber served as the detection electrode and a 500 µm Pt wire served as the auxiliary and reference (quasi-reference) electrode (placed in the waste reservoir). Various separation buffers were investigated, and the optimized buffer used was 25 mM TES, 25 mM SDS, 0.5 mM Tween® 20 (pH=7.4). Cells were stimulated with a buffer with elevated K+ made up of the following: 80 mM KCl, 50 mM NaCl, 0.7 mM MgCl2, 1 mM NaH2PO4, 2 mM CaCl2 and 10 mM HEPES and rinsed with physiological saline: 4.2 mM KCl, 150 mM NaCl, 0.7 mM MgCl2, 1 mM NaH2PO4, 2 mM CaCl2 and 10 mM HEPES.28 Dopamine and norepinephrine standard mixtures (100 µM each) were also prepared in the K+ stimulant buffer. The high voltage for the electrophoretic separation was +600 V and the pushback voltage was +75 V (Field strength = 160 V/cm). For temporal resolution studies, the catechol concentration (75 µM to 150 µM) was changed in the sample reservoir, injected into the separation channel and amperometrically detected at a Pt electrode. The withdraw rate was −3.0 µL/min and the peristaltic pumps were operated at 2.0 Hz. Conductivity measurements were taken for each buffer with a conductivity meter (Orion Instruments Model 162, Thermo Scientific, Springfield, NJ, USA).
2.5 PC 12 culture and immobilization
PC 12 cells (ATCC, Manassas, VA, USA) were cultured at 37 °C and 5% CO2 in PS T-25 flasks (Fisher Scientific, Springfield, NJ, USA). The T-25 flasks and surfaces for cell adhesion were pre-treated with 0.435 mg/mL collagen. The PC 12 cells were grown with media (F-12K supplemented with 1% penicillin-streptomycin solution, 2.5 % fetal bovine serum, and 15% horse serum, all from ATCC) being replaced every 1–2 days. Cells were grown to 90% confluency (as determined by optical microscopy) and either passaged into new flasks or used for experiments. Cells were cultured in 35 mm diameter petri dishes for analysis (Corning Incoporated, Corning, NY, USA). Once the cells were 90% confluent, the cells were pre-loaded29 with a solution of 1 mM NE and DA in physiological saline for one hour so that release of both NE and DA could be monitored. Cell counts were taken using a hemocytometer. After one hour, cells were rinsed three times with physiological saline and the embedded capillary was inserted into the dish. The volume over the cells remained constant throughout the experiment (400 µL). The cell buffer was used as the system blank before the K+ stimulant was added over the cells. Cell releasate was withdrawn (−1.0 µL/min) from the cell culture dish, through the embedded capillary, and onto the microchip towards the injection interface. Discrete injections were made into the separation channel and DA and NE were electrophoretically separated and amperometrically detected.
2.6 Imaging
Micrographs were captured with Streampix Digital Video Recording software (Norpix, Montereal, Canada) and Image Pro express software (Media Cybernetics, Silver Spring, MD, USA) was used to measure the fluorescent plug to calculate injection plug sizes. Non-fluorescent images were captured from a stereoscope (Olympus SZ61) operating in bright-field mode using a Sony 3CCD color camera (Leeds Precision Instruments, Minneapolis, MN, USA). Fluorescent images was taken using fluorescein (Sigma Aldrich, St. Louis, MO, USA) and an upright fluorescence microscope (Olympus EX 60, Center Valley, PA, USA) equipped with a 100 W Hg Arc lamp and a cooled 12-bit monochrome Qicam Fast digital CCD camera (Qimaging, Montreal, Canada). Micrographs were captured with Streampix Digital Video Recording software (Norpix, Montreal, Canada).
3 Results and Discussion
3.1 Fabrication and assembly
Many microchip substrates are fabricated from PDMS because it is optically transparent, inexpensive, flexible, gas permeable, and has the ability to reversibly or irreversibly seal to itself or other microchip substrates. However, there are some problems that are associated with studying biological systems on PDMS substrates. PC 12 cells exhibit poor cell adhesion on PDMS even with the aid of biological coatings such as collagen.23 It has also been shown that hydrophobic molecules can partition into the PDMS and un-crosslinked monomers from PDMS can leach into the cell environment.30 Recent work has shown that microchip devices can be fabricated from PS, a more biologically compatible substrate, for a variety of applications.19,21,22,31 The work described here utilizes PS for its ability to embed electrodes for analysis and fluidic tubing to provide a low dead-volume connection to direct flow from sample (or immobilized cells) to the analysis device.16,18 Importantly, the ability to embed the tubing allows us to integrate the chip with off-chip, well-established culture methods such as conventional petri dishes in a manner that does not significantly degrade the temporal resolution (discussed in more detail below). The PS embedded Pd decoupler has a large surface area and is capable of absorbing the increased hydrogen production at the decoupler due to increased ionic strength and current. The improved performance of the Pd decoupler allows separations in highly conductive buffers (also discussed below). The ability to embed multiple electrode materials is crucial in this work because it allows for a Pd decoupler to ground the electrophoretic voltage and enables in-channel detection with a different electrode material (platinum or carbon fiber). The design of the device shown in Figure 1 combines the advantages of PDMS substrates (including the ability to incorporate peristaltic pumps and injection valves) with the advantages of using PS-embedded electrodes for analysis and fluidic tubing to incorporate off-chip sampling. The integration of PDMS-based “Quake-type”32,33 valves is critical in isolating cell culture from the high voltages associated with the electrophoretic separation.
The use of PDMS substrates is advantageous because they are capable of being reversibly sealed to itself and other substrate materials, such as glass, which leads to easy integration with electrochemical detection. One disadvantage of a PDMS-thermoplastic hybrid device is the adhesion between the two substrates. The sealing between the two substrates worsens as smaller channels and more components (such as peristaltic pumps and injection valves) are introduced. Several groups have reported adhesion problems using PDMS-PS hybrid devices. Recently, the use of silane reagents to improve the adhesion by creating an irreversible bond between PDMS and thermoplastic materials has been reported.34–40 It has been shown that this adhesion is crucial when using PDMS-based valves. Gu et al. used 3-(trimethoxysilyl) propyl methacrylate (TMSPMA) to irreversibly bond PDMS to cyclic olefin copolymer (COC) and polymethylmethacrylate (PMMA) after oxidizing the substrates with corona discharge. This study showed that the device could withstand a pressure of 100 psi being applied to the PDMS-based valve before delamination occured.36 Other groups have utilized aminosilane and epoxysilane (after activating the substrates with oxygen plasma) to form an irreversible bond between a thermoplastic (COC or PMMA) and PDMS.35,38,40 Most recently, 3-aminopropyl trimethoxysilane (APTES) was successfully used to irreversibly bond PDMS-thermoplastic devices.34,37,39 The effect of these silanes on electrophoretic separations in PS-PDMS devices has also not been determined.
As previously mentioned, it would be advantageous to achieve a reversible bond between PDMS and PS substrates for easy integration with the embedded electrodes for electrochemical detection. Reversible sealing of the two substrates allows a fresh electrode surface to be generated via wet polishing as well as the ability to disassemble and reassemble the device as desired. A strong, reversible seal was achieved between PS and PDMS using a different stamping approach (discussed in next paragraph). Several different silanes, including hexamethyldisilazane (HMDS), APTES, and chlorotrimethylsilane, were investigated to reversibly improve the adhesion between the PS and PDMS. APTES did improve the adhesion between the two substrates, but it also affected the electrophoretic capabilities of the device, with the EOF being suppressed and even reversed. HMDS also improved the adhesion but the improved sealing between the substrates did not last as long as chlorotrimethylsilane treatment. Chlorotrimethylsilane was utilized to improve the adhesion between reversibly sealed PS and PDMS and enabled the separation of DA and NE in highly conductive, biological buffers.
The treatment consists of spreading a neat solution of chlorotrimethylsilane onto a surface of either PS, glass, or silicon and reversibly sealing the PDMS microchip on the coated substrate for at least 1 hour. During this time, the silane transfers into the PDMS, after which time the PDMS is removed and sealed on an untreated PS base containing embedded electrodes and tubing. The transfer of chlorotrimethylsilane into the PDMS improves the adhesion when the PDMS is reversibly sealed onto a different PS substrate. While there was a difference in the contact angles for the chlorotrimethylsilane treated (105.0 ± 0.5°) and untreated (111.1 ± 3.1°) PDMS devices, the chips were still relatively hydrophobic. The electroosmotic flow (EOF) of the microchip does change after it has been stamped with chlorotrimethylsilane. The EOF was measured using the current monitoring method41 and a boric acid buffer (pH 9.2). It was found that the EOF decreased significantly (p<<0.01) after chlortrimethylsilane stamping compared to an untreated microchip (6.3 ± 0.4 × 10−4 cm2 V−2 s−1 to 4.1 ± 0.2 ×10−4 cm2 V−2 s−1). The diminished EOF did not affect the separation efficiency of the device. Without chlorotrimethylsilane treatment, the normally closed injection valve does not fully close and cell buffer/stimulant solution was found to constantly leak into the separation portion of the chip. This led to increased currents and bubble formation at the Pd decoupler. The PDMS microchip can be reversibly sealed 2–3 times before treatment needs to be repeated. Figure S-1 demonstrates the improved adhesion (around the sample reservoirs) after chlorotrimethylsilane treatment.
3.2 Device characterization
The overall goal of this work is to create a microchip device that integrates cell culture with analysis via microchip electrophoresis and electrochemical detection. The specific application is to monitor the release of dopamine and norepinephrine from stimulated PC 12 cells (a commonly used neuronal mimic).42 If low dead-volume interconnections are made, microfluidic technology enables high temporal measurements to be made so there is minimal time between the biological event and analytical measurement.43 In our previously described device where on-chip valves were used to interface continuous flow in one portion of the device with microchip electrophoresis, peristaltic pumps were solely used to move sample from the reservoir to the injection interface. This work yielded an average flow rate of 49 ± 4 nL/min.10 An increased flow rate would be advantageous for detection of rapid concentration changes. The ability to operate a syringe pump in withdraw mode, as opposed to previous studies where a positive displacement was used, has been reported.23 The withdraw mode was less invasive on cells and alleviated any issues with air bubbles and cells becoming detached from the microchip substrate.
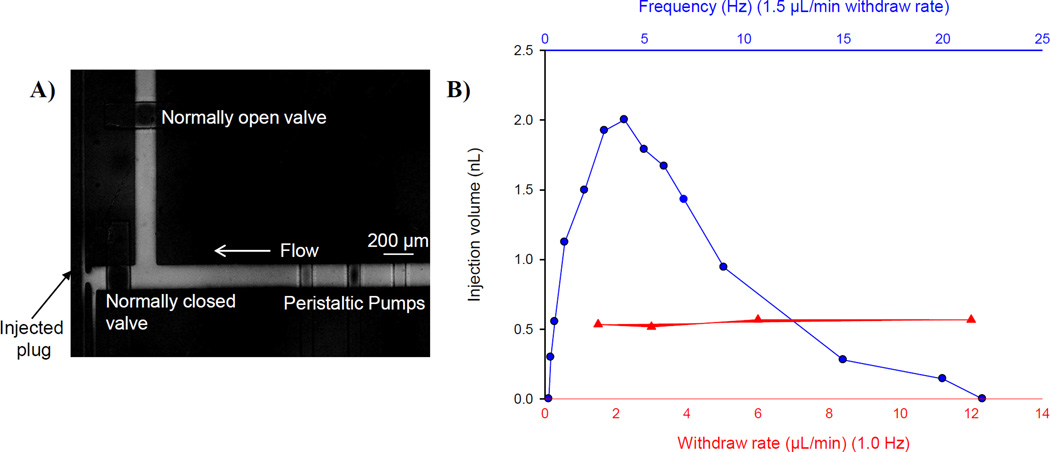
An increased flow rate is demonstrated with the use of both on-chip peristaltic pumps and a syringe pump in withdraw mode. Figure 2 depicts the injection characterization for this device. Figure 2A shows an on-chip injection made by opening the normally closed valve for ~3–5 seconds (and closing the normally open valve) to discretely inject a sample plug into the separation channel. When the normally open valve is closed, the peristaltic pumps are responsible for the hydrodynamic injection of sample into the electrophoresis channel and this closed valve isolates the withdraw pump from the rest of the fluidic network. As the withdraw rate varied from −1.5 to −12 µL/min, the injection volume remained constant (547 ± 25.2 pL, 4.6% RSD). As the frequency of the persistaltic pump changed from 0.2 to 25 Hz, the injection volume varied from 0–2 nL. These results can be seen in Figure 2B. This data demonstrates the ability of the normally open valve to fully close during an injection event and demonstrates that the injection volume is a result of the peristaltic pump frequency and the withdraw flow rate has no effect on the injection event. It is crucial to integrate both the peristaltic pumps (for on-chip injections) and the syringe pump in withdraw mode (to improve temporal resolution).
Figure 2.
Characterization of injection. A) Micrograph of injection event. Normally closed valve is opened for a pressure-based injection into the electrophoresis channel. B) Effect of frequency of peristaltic pumps and withdraw rate on injection volume. The injection volume changes as the frequency of the pumps changes and remains constant as the withdraw rate changes. This is evidence that the normally open valve completely closes during the injection event.
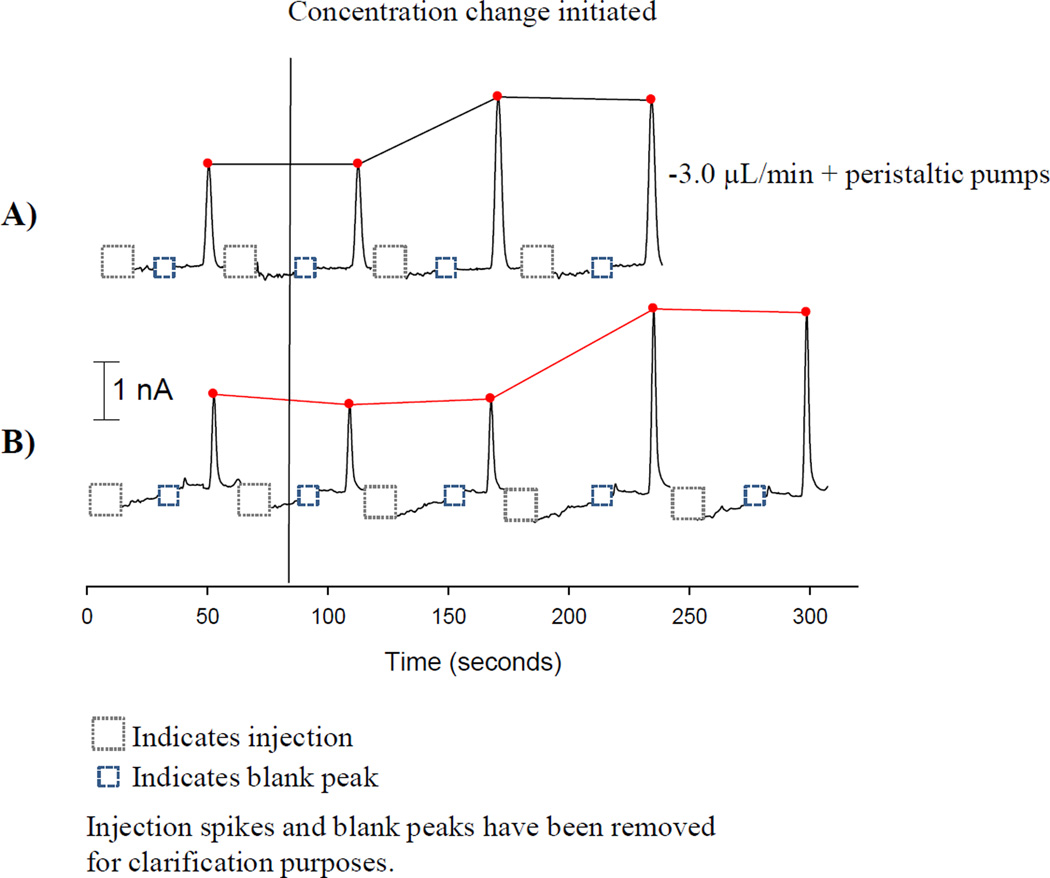
The ability to rapidly detect a concentration change is desired when studying complex biological systems. The temporal resolution of the device is improved with the incorporation of a syringe pump in withdraw mode as opposed to only using the peristaltic pumps. The concentration change was initiated within the on-chip reservoir (75 µM to 150 µM catechol). Injections were continuously made following amperometric detection of catechol. As can be seen in Figure 3A, the concentration change is evident one separation after the concentration change when both peristaltic pumps and a syringe pump are incorporated (−3.0 µL/min). The change in signal requires two injection events when only the peristaltic pumps are used (Figure 3B). The electrode responds 80 seconds after the concentration change for the withdraw and peristaltic pump combination and 160 seconds when only using the peristaltic pumps. When using the withdraw mode, the electrochemical response time is limited by the separation time. This data demonstrates how the use of a syringe pump in withdraw mode improves the temporal resolution of the device.
Figure 3.
Improvement in temporal resolution for cellular analysis. A) Incorporation of a syringe pump in withdraw mode provides an improvement in temporal resolution, with the concentration change monitored within one injection event. B) The utilization of peristaltic pumps alone requires two injection events to detect a concentration change.
3.3 Separation optimization
An efficient separation is crucial to this work because dopamine and norepinephrine are very similar in structure (only differ by a hydroxyl group and it is not possible to differentiate between the two with a direct electrochemical measurement).44 PC 12 cells can easily be stimulated to release dopamine and norepinephrine with the use of elevated levels of potassium, causing depolarization of the cell membrane and initiating an action potential and exocytosis.45 Physiological saline and K+ stimulant solutions have high ionic strengths, which can lead to anti-stacking issues and make a separation more difficult to achieve.24,25 For example, in this work the separation buffers have a conductivity value of 2 mS/cm, while the cell solutions such as the K+ stimulant solution have a conductivity of 16.7 mS/cm. Previous work has demonstrated the effectiveness of embedded Pd decouplers to dissipate the increased H2 production when using high ionic strength cell-compatible buffers.17 The improved performance of embedded Pd decouplers allows separations in highly conductive buffers without the need for dilution or less conductive receptor-based stimulants.17 The injection valves in this device enable isolation of cell-compatible buffers/stimulants from the separation buffer.
The use of mixed surfactant systems to improve microchip separations has been previously described, with a combination of ionic, zwitterionic, and nonionic surfactants being used to improved resolution in PDMS microchip devices.46,47 In this work with hybrid PS-PDMS devices, an anionic (SDS) and nonionic (Tween® 20) surfactant were investigated to optimize the separation. A recent study has shown that the when using these mixed surfactant systems in PDMS microchips, the SDS dominates the EOF behavior.47 In our studies, the EOF was measured using the current monitoring method41 and it was found that the SDS was the predominant factor in the EOF values, with the addition of 0.5 mM Tween® 20 to the TES/SDS system leading to a very slight change in the EOF (see Table S-1 for data).
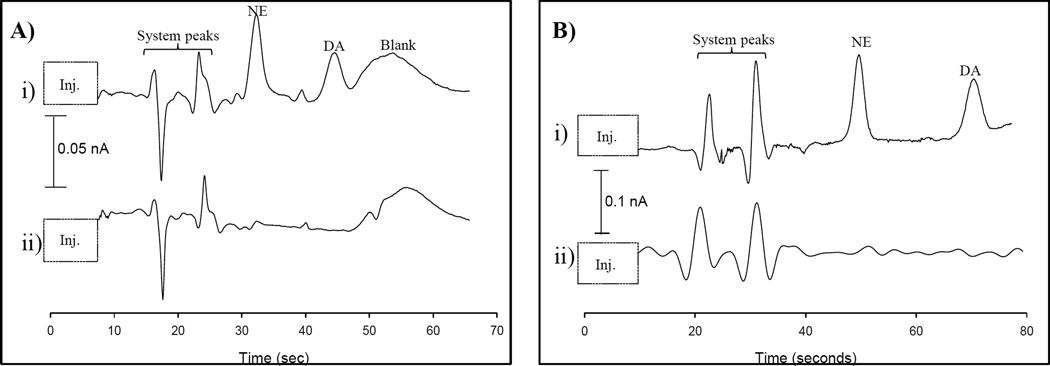
As shown in Figure S-2, the separation of a 100 µM NE/DA in a K+ stimulant solution changes with the addition of SDS and Tween® 20. In Figure S-2A, 25 mM TES was used as the separation buffer. Both NE and DA, as well as the blank peak, co-elute. It is evident that the use of TES does not provide a separation using this device. The separation resolution improves with the addition of 25 mM SDS (shown in Figure S-2B). The separation improves because NE and DA can partition differently into the SDS micelle, however, DA still co-elutes with a blank peak. The NE/DA separation is improved in Figure 4A using 25 mM TES, 25 mM SDS, and 0.5 mM Tween® 20. The mixed surfactant system is ideal because NE and DA can partition into the negatively charged SDS micelle and the non-ionic Tween® 20 can interact with the analytes through hydrophobic/hydrophilic interactions. The use of these two surfactants in concert allows for additional/competitive interactions that aid in the separation. DA is pulled away from the blank peak that is obtained with the K+ stimulant solution. However, as more injections are made, DA again begins to co-elute with the blank peak. The source of the blank peak was investigated by replacing the chloride salts in the stimulant solution with nitrate salts. The blank peak is eliminated when the chloride salts are removed from the stimulant solution. We postulate that there is some interaction between the chloride (~160 mM in sample solution) and the Pt working electrode but we have not performed exhaustive studies to confirm this. The separation is optimized in Figure 4B by replacing the 25 µm Pt detection electrode with a 33 µm carbon fiber detection electrode. The blank peak is eliminated using the same 25 mM TES, 25 mM SDS, and 0.5 mM Tween® 20 buffer. DA and NE are clearly resolved, with a resolution of 4.7. The number of theoretical plates for DA is 2600, which is typical for PDMS devices,48,49 and reasonable with the anti-stacking that occurs due to the high conductivities of the buffers. The limit of detection in the K+ stimulant solution is 4 µM for NE. The separation and electrode response is reproducible, with repetitive injections of 100 µM DA having an average peak height of 57 ± 1.4 pA (2.5% RSD, n=6). The use of a mixed surfactant system and a carbon fiber detection electrode makes this separation possible, with a short separation channel (2.5 cm) and reasonable analysis time for these high ionic strength systems (70 seconds).
Figure 4.
Optimized separation of DA and NE with a mixed surfactant system i) 100 µM DA/NE mixtures were prepared in K+ stimulant ii) Samples were prepared exactly as in i) with no addition of analytes. A) Buffer = 25 mM TES, 25 mM SDS, 0.5 mM Tween® (pH=7.4) using a Pt detection electrode B) Same parameters as A), but using a carbon fiber detection electrode
3.4 PC 12 cell analysis
Our group and others have described microchip devices that incorporate cell culture and analysis in a single, highly integrated device.11,20,50,51 With these totally integrated devices, if any part of the chip fails (such as delamination or cells dislodging), the entire experiment is compromised. Several devices have demonstrated the temporal resolution and minimal dilution to monitor near real-time release from cells, but many are low-throughput. Each chip may have a single layer of cells and this requires several chips for duplicate and control studies. The device described here is a modular device that can be used for more high-throughput studies. An important component of this approach is the ability to encapsulate both tubing and electrodes in PS substrates. The encapsulated tubing provides a low dead volume connection that is important to obtain high temporal resolution measurements, with the capillary being used to continuously sample from off-chip petri dishes. To investigate the ability of this approach in dynamic, biological systems the rise time of the device (time which signal increases from 10 to 90% of the maximum intensity) was characterized using the embedded fused-silica capillary to sample from an off-chip petri dish. Fluorescence detection was performed at the injection interface and the sample in the cell dish was changed from buffer to 500 µM fluorescein. The sample was withdrawn (−1.0 µL/min) through the 25 cm long, 150 µm inner diameter embedded capillary and onto the microchip. The calculated rise time was 31.8 ± 2.0 sec (6.3 % RSD), and even faster rise times are possible with higher withdraw flow rates.
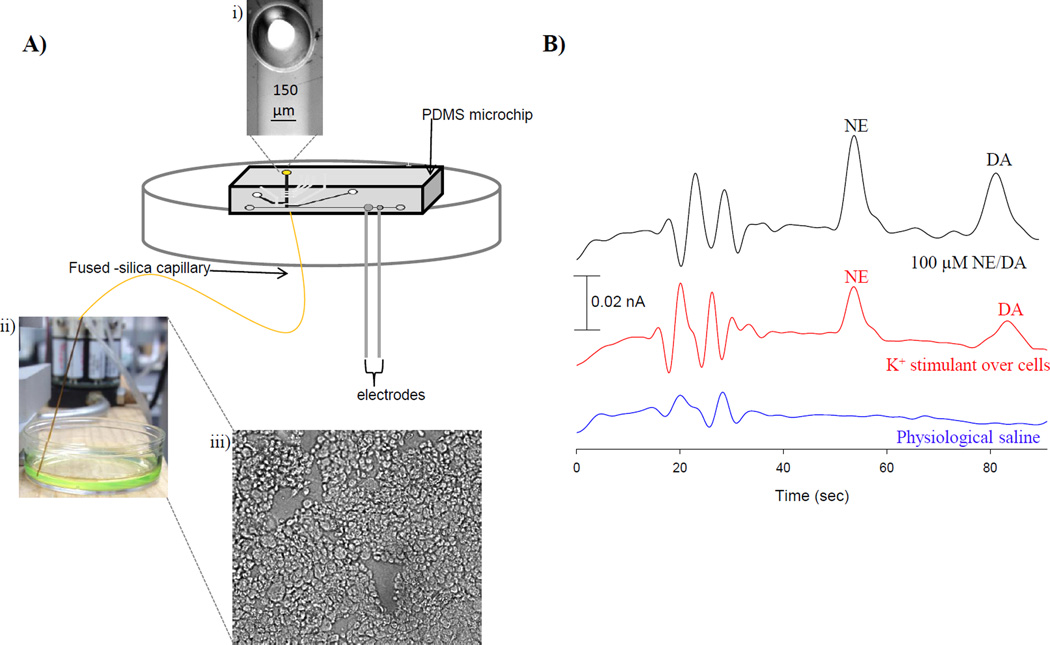
For cell studies the chlorotrimethylsilane stamped PDMS valving microchip was sealed over the PS base so that the channels overlaid the inner diameter of the embedded capillary (Figure 5A i) and the decoupler/detection electrode. Sample was withdrawn (−1.0 µL/min) from the cell culture dish (Figure 5 A ii) through the embedded capillary and towards the injection interface of the PDMS microchip. Initially, a mixture of 100 µM NE/DA in K+ stimulant was sampled, injected, separated, and detected. PC 12 cells were immobilized in 35 mm diameter cell culture dishes treated with 0.435 mg/mL collagen. Figure 5A iii shows a micrograph of the immobilized PC 12 cells, which were pre-loaded and incubated with 1 mM NE/DA (in physiological saline) for 1 hour prior to use. After one hour, the cells were rinsed three times with physiological saline prior to analysis.28 The volume over the cells remained constant throughout the experiment (400 µL). K+ stimulant was utilized to cause depolarization of the cell membrane and initiate an action potential and exocytosis. Physiological saline was used as used as a control to ensure the cells only released NE and DA when stimulated. The results can be seen in Figure 5B. When the cells were stimulated with K+, they released 31 ± 2 µM NE and 29 ± 2 µM DA, or an average of ~1 × 10−14 moles NE and DA per cell (n=5). This PS-PDMS hybrid device enables off-chip sampling of cells due to the low dead-volume interface that results from the embedded fused-silica capillary. This device is capable of measuring NE and DA in a more modular, high-throughput manner than previous studies.10
Figure 5.
PC 12 cell analysis. A) Schematic of assembly for PC 12 cell analysis. i) The PDMS microchip is sealed over the embedded fused-silica capillary. ii) The capillary is placed in solution and sample is withdrawn to the PDMS microchip. iii) Micrograph of immobilized PC 12 cells. B) Electropherograms of physiological saline, K+ stimulated release, and 100 µM NE/DA standards.
4. Conclusions
In this work, we developed a hybrid PS-PDMS device that integrates multiple processes for cellular analysis. The PDMS device incorporates pumping and valving technologies, while the PS device contains embedded electrodes for analysis and fluidic tubing to provide a low dead-volume interface for off-chip sampling. A new surface treatment utilizing chlorotrimethylsilane is described that improves the adhesion between PS and PDMS in reversibly sealed devices and allows easy integration with electrochemical detection. A detailed separation optimization study was completed for these relative high ionic strength stimulant solutions. Although this approach of using the chip to sample from a petri dish is a more modular approach, we are interested in expanding this approach in the future where the embedded capillary is utilized to couple a cell culture microchip to a valving/electrophoresis chip when analysis is desired. This approach would have improved temporal resolution and also be both modular and amenable to automated analysis of multiple components released from cells.
Supplementary Material
Acknowledgements
Support from the National Institute of General Medical Sciences (Award Number R15GM084470-03) is acknowledged.
References
- 1.El-Ali J, Sorger PK, Jensen KF. Nature. 2006;442:403–411. doi: 10.1038/nature05063. [DOI] [PubMed] [Google Scholar]
- 2.Price AK, Culbertson CT. Anal. Chem. 2007;79:2614–2621. doi: 10.1021/ac071891x. [DOI] [PubMed] [Google Scholar]
- 3.Johnson AS, Selimovic A, Martin RS. Anal Bioanal Chem. 2013 doi: 10.1007/s00216-012-6682-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halpin ST, Spence DM. Anal. Chem. 2010;82:7492–7497. doi: 10.1021/ac101130s. [DOI] [PubMed] [Google Scholar]
- 5.Batz NG, Martin RS. Analyst. 2009;34:372–379. doi: 10.1039/b813898b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sandlin ZD, Shou M, Shackman JG, Kennedy RT. Anal Chem. 2005;77:7702–7708. doi: 10.1021/ac051044z. [DOI] [PubMed] [Google Scholar]
- 7.Wang M, Roman GT, Perry ML, Kennedy RT. Anal. Chem. 2009;81:9072–9078. doi: 10.1021/ac901731v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Metto EC, Evans K, Barney P, Culbertson AH, Gunasekara DB, Caruso G, Hulvey MK, Fracassi da Silva F, Lunte SM, Culbertson CT. Anal Chem. 2013;85:10188–10195. doi: 10.1021/ac401665u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madren SM, Hoffman MD, Brown PJ, Kysela DT, Brun YV, Jacobson SC. Anal Chem. 2012;84:8571–8578. doi: 10.1021/ac301565g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bowen AL, Martin RS. Electrophoresis. 2010;31:2534–2540. doi: 10.1002/elps.201000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li MW, Martin RS. Analytst. 2008;133:1358–1366. doi: 10.1039/b807093h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lacher NA, Lunte SM, Martin RS. Anal. Chem. 2004;76:2482–2491. doi: 10.1021/ac030327t. [DOI] [PubMed] [Google Scholar]
- 13.Vickers JA, Henry CS. Electrophoresis. 2005;26:4641–4647. doi: 10.1002/elps.200500508. [DOI] [PubMed] [Google Scholar]
- 14.Amatore C, Da Mota N, Sella C, Thouin L. Anal. Chem. 2008;80:4976–4985. doi: 10.1021/ac800227t. [DOI] [PubMed] [Google Scholar]
- 15.Martin RS, Gawron AJ, Lunte SM, Henry CS. Anal. Chem. 2000;72:3196–3202. doi: 10.1021/ac000160t. [DOI] [PubMed] [Google Scholar]
- 16.Johnson AS, Anderson KB, Halpin ST, Kirkpatrick DC, Spence DM, Martin RS. Analyst. 2013;138:129–136. doi: 10.1039/c2an36168j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson AS, Selimovic A, Martin RS. Electrophoresis. 2011;32:3121–3128. doi: 10.1002/elps.201100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Becirovic V, Doonan SR, Martin RS. Anal. Methods. 2013;5:4220–4229. doi: 10.1039/C3AY40809D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Detwiler DA, Dobes NC, Sims CE, Kornegay JN, Allbritton NL. Anal Bioanal Chem. 2012;402:1083–1091. doi: 10.1007/s00216-011-5596-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ku C-J, D’Amico Oblak T, Spence DM. Anal. Chem. 2008;80:7543–7548. doi: 10.1021/ac801114j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Midwoud PM, Janse A, Merema MT, Groothuis GMM, Verpoorte E. Anal Chem. 2012;84:3938–3944. doi: 10.1021/ac300771z. [DOI] [PubMed] [Google Scholar]
- 22.Young EW, Berthier E, Guckenberger DJ, Sackmann E, Lamers C, eyvantsson I, Huttenlocher A, Beebe DJ. Anal Chem. 2011;83:1408–1417. doi: 10.1021/ac102897h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson KB, Halpin ST, Johnson AS, Martin RS, Spence DM. Analyst. 2013;138:137–143. doi: 10.1039/c2an36171j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gillogly JA, Lunte CE. Electrophoresis. 2005;26:633–639. doi: 10.1002/elps.200410061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weinberger R, editor. Practical Capillary Electrophoresis. San Diego, CA: Academic Press; 2000. [Google Scholar]
- 26.Li MW, Spence DM, Martin RS. Electroanalysis. 2005;17:1171–1180. [Google Scholar]
- 27.Erbil HY. Surface Chemistry of Solid and Liquid Interfaces. Oxford, UK: Blackwell Publishing; 2006. [Google Scholar]
- 28.Kozminski KD, Gutman DA, Davila V, Sulzer D, Ewing A. Anal. Chem. 1998;70:3123–3130. doi: 10.1021/ac980129f. [DOI] [PubMed] [Google Scholar]
- 29.Moore JM, Papke JB, Cahill AL, Harkins AB. Am. J. Physiol. Cell Physiol. 2006;291:C270–C281. doi: 10.1152/ajpcell.00539.2005. [DOI] [PubMed] [Google Scholar]
- 30.Regehr KJ, Domenech M, Koepsel JT, Carver KC, Ellison-Zelski SE, Murphy WL, Schuler LA, Alarid ET, Beebe DJ. Lab Chip. 2009;9:2132–2139. doi: 10.1039/b903043c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen CS, Breslauer DN, Luna JI, Grimes A, Chin WC, Lee LP, Khine M. Lab Chip. 2008;8:622–624. doi: 10.1039/b719029h. [DOI] [PubMed] [Google Scholar]
- 32.Liu J, Hansen C, Quake SR. Anal Chem. 2003;75:4718–4723. doi: 10.1021/ac0346407. [DOI] [PubMed] [Google Scholar]
- 33.Unger MA, Chou HP, Thorsen T, Scherer A, Quake SR. Science. 2000;7:113–116. doi: 10.1126/science.288.5463.113. [DOI] [PubMed] [Google Scholar]
- 34.Aran K, Sasso LA, Kamdar N, Zahn JD. Lab Chip. 2010;10:548–552. doi: 10.1039/b924816a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cortese B, Mowlem MC, Morgan H. Sens. Actuators B. 2011;160:1473–1480. [Google Scholar]
- 36.Gu P, Liu K, Chen H, Nishida T, Fan ZH. Anal Chem. 2010;83:446–452. doi: 10.1021/ac101999w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee KS, Ram RJ. Lab Chip. 2009;9:618–1624. doi: 10.1039/b820924c. [DOI] [PubMed] [Google Scholar]
- 38.Ogilvie I, Sieben VJ, Cortese B, Mowlem MC, Morgan H. Lab Chip. 2011;11 doi: 10.1039/c1lc20069k. [DOI] [PubMed] [Google Scholar]
- 39.Sunkara V, Cho YK. ACS Appl. Mater. Interfaces. 2012;4:6537–6544. doi: 10.1021/am3015923. [DOI] [PubMed] [Google Scholar]
- 40.Tang L, Lee NY. Lab Chip. 2010;10:1274–1280. doi: 10.1039/b924753j. [DOI] [PubMed] [Google Scholar]
- 41.Huang X, Gordon MJ, Zare RN. Anal Chem. 1988;60:1837–1838. [Google Scholar]
- 42.Sombers LA, Ewing A. In: Electroanalytical Methods for Biological Materials. Brajter-Toth A, Chambers JQ, editors. New York: Marcel Dekker; 2002. pp. 279–327. Editon edn. [Google Scholar]
- 43.Wang M, Roman GT, Schultz K, Jennings C, Kennedy RT. Anal. Chem. 2008;80:5607–5615. doi: 10.1021/ac800622s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heien ML, Philips PEM, Stuber GD, Seipel AT, Wightman RM. Analyst. 2003;128:1413–1419. doi: 10.1039/b307024g. [DOI] [PubMed] [Google Scholar]
- 45.Sombers LA, Ewing AE. In: Electroanalytical Methods for Biological Methods. Brajter-Toth A, Chambers JQ, editors. New York: Brajter-Toth; 2002. pp. 279–327. Editon edn. [Google Scholar]
- 46.Badal MY, Wong M, Chiem N, Salimi-Moosavi H, Harrison DJ. J Chromatogr A. 2002;947:277–286. doi: 10.1016/s0021-9673(01)01601-6. [DOI] [PubMed] [Google Scholar]
- 47.Guan Q, Noblitt SD, Henry CS. Electrophoresis. 2012;33:2875–2883. doi: 10.1002/elps.201200255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vickers JA, Caulum MM, Henry CS. Anal. Chem. 2006;78:7446–7452. doi: 10.1021/ac0609632. [DOI] [PubMed] [Google Scholar]
- 49.Lacher NA, de Rooij NF, Verpoorte E, Lunte SM. J Chromatogr A. 2003;1004:225–235. doi: 10.1016/s0021-9673(03)00722-2. [DOI] [PubMed] [Google Scholar]
- 50.Clark AM, Sousa KM, Chisolm CN, MacDougald OA, Kennedy RT. Anal Bioanal Chem. 2010;397:2939–2947. doi: 10.1007/s00216-010-3897-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Genes LI, Tolan NV, Hulvey MK, Martin RS, Spence DM. Lab Chip. 2007;7:1256–1259. doi: 10.1039/b712619k. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.