Abstract
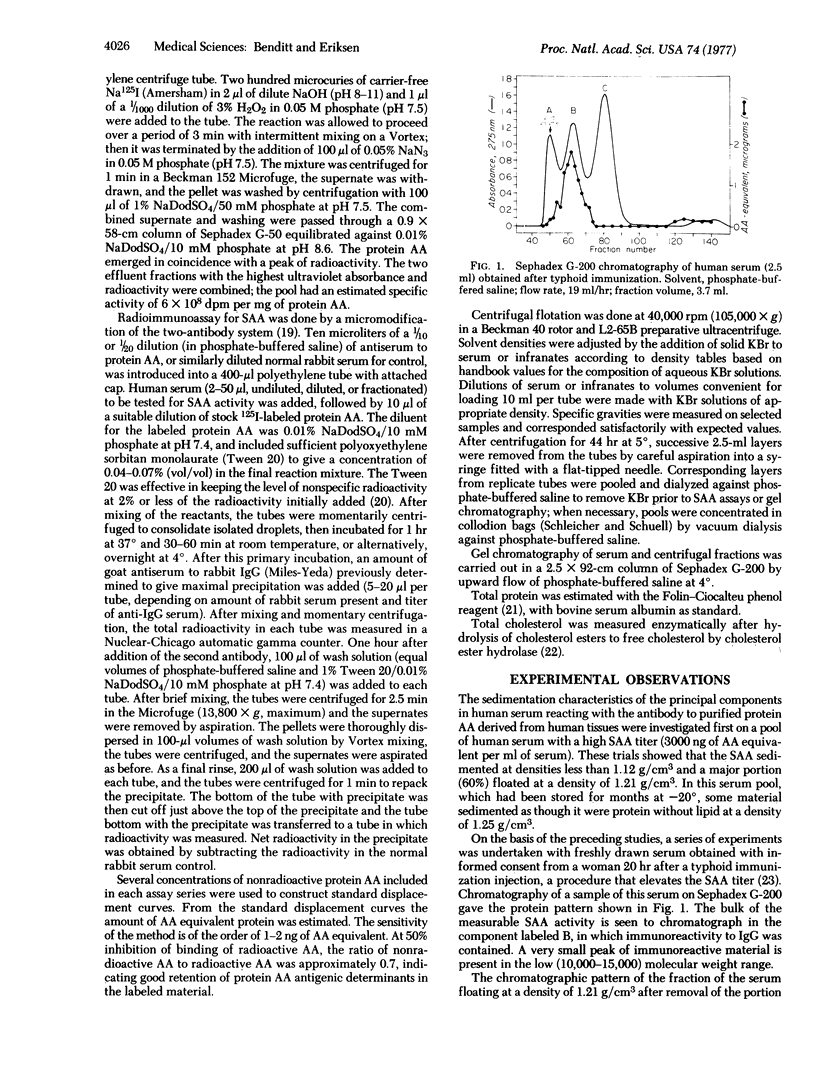
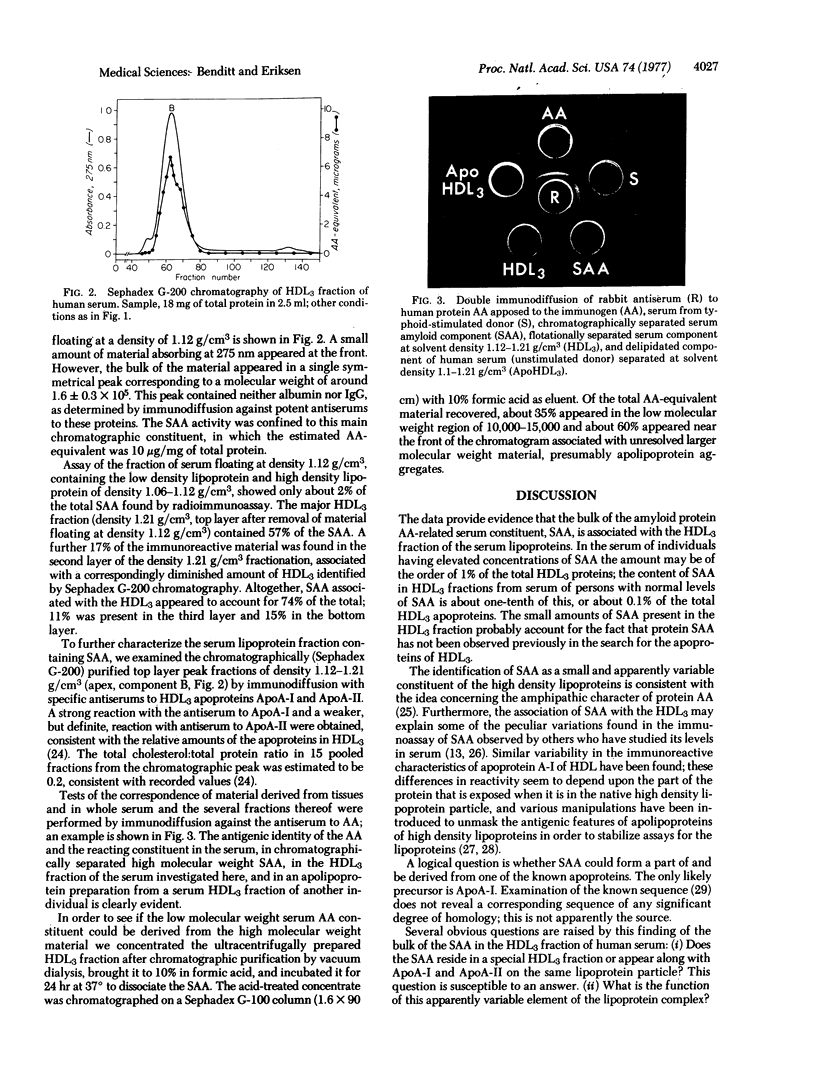
Human serums contain a protein antigenically related to protein AA, the principal protein of a major class of amyloid substance. The serum antigen, SAA, occurs mainly in a high molecular weight form, 1 to 2 X 10(5). This work shows that the bulk of the SAA sediments at density 1.12 g/cm3 and floats at density 1.21 g/cm3, as does the high density lipoprotein HDL3. SAA is associated with the apolipoproteins ApoA-I and ApoA-II. The total cholesterol:total protein ratio of the fraction with density 1.12-1.21 g/cm3 is 0.2:1, consistent with that of SAA constituent of the HDL3 fraction into low molecular weight species of the order of 13,000. The quantity of SAA may vary from 0.1% or more of the total protein of HDL3.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allain C. C., Poon L. S., Chan C. S., Richmond W., Fu P. C. Enzymatic determination of total serum cholesterol. Clin Chem. 1974 Apr;20(4):470–475. [PubMed] [Google Scholar]
- Anders R. F., Natvig J. B., Michaelsen T. E., Husby G. Isolation and characterization of amyloid-related serum protein SAA as a low molecular weight protein. Scand J Immunol. 1975;4(4):397–401. doi: 10.1111/j.1365-3083.1975.tb02642.x. [DOI] [PubMed] [Google Scholar]
- Baker H. N., Delahunty T., Gotto A. M., Jr, Jackson R. L. The primary structure of high density apolipoprotein-glutamine-I. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3631–3634. doi: 10.1073/pnas.71.9.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benditt E. P., Eriksen N. Chemical classes of amyloid substance. Am J Pathol. 1971 Oct;65(1):231–252. [PMC free article] [PubMed] [Google Scholar]
- Benson M. D., Skinner M., Lian J., Cohen A. S. "A" protein of amyloidosis. Isolation of a cross-reacting component from serum by affinity chromatography. Arthritis Rheum. 1975 Jul-Aug;18(4):315–322. doi: 10.1002/art.1780180404. [DOI] [PubMed] [Google Scholar]
- Eriksen N., Ericsson L. H., Pearsall N., Lagunoff D., Benditt E. P. Mouse amyloid protein AA: Homology with nonimmunoglobulin protein of human and monkey amyloid substance. Proc Natl Acad Sci U S A. 1976 Mar;73(3):964–967. doi: 10.1073/pnas.73.3.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etzrodt H., Schleyer M., Schröder K. E., Pfeiffer F. The use of Tween 20 in the double antibody radioimmunoassay. Hoppe Seylers Z Physiol Chem. 1976 Sep;357(9):1205–1208. doi: 10.1515/bchm2.1976.357.2.1205. [DOI] [PubMed] [Google Scholar]
- Fielding C. J., Havel R. J. Lipoprotein lipase. Arch Pathol Lab Med. 1977 May;101(5):225–229. [PubMed] [Google Scholar]
- Franklin E. C. Some properties of antisera to serum amyloid A protein (SAA): inhibition of precipitation by complexing of SAA to albumin. J Exp Med. 1976 Dec 1;144(6):1679–1682. doi: 10.1084/jem.144.6.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenner G. G., Terry W. D., Isersky C. Amyloidosis: its nature and pathogenesis. Semin Hematol. 1973 Jan;10(1):65–86. [PubMed] [Google Scholar]
- Hermodson M. A., Kuhn R. W., Walsh K. A., Neurath H., Eriksen N., Benditt E. P. Amino acid sequence of monkey amyloid protein A. Biochemistry. 1972 Aug 1;11(16):2934–2938. doi: 10.1021/bi00766a002. [DOI] [PubMed] [Google Scholar]
- Husby G., Natvig J. B. A serum component related to nonimmunoglobulin amyloid protein AS, a possible precursor of the fibrils. J Clin Invest. 1974 Apr;53(4):1054–1061. doi: 10.1172/JCI107642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husby G., Natvig J. B., Sletten K., Nordstoga K., Anders R. F. An experimental model in mink for studying the relation between amyloid fibril protein AA and the related serum protein SAA. Scand J Immunol. 1975;4(8):811–816. doi: 10.1111/j.1365-3083.1975.tb03721.x. [DOI] [PubMed] [Google Scholar]
- Ignaczak T. F., Sipe J. D., Linke F. P., Glenner G. G. Immunochemical studies on the nature of the serum component (SAA) related to secondary amyloidosis. J Lab Clin Med. 1977 May;89(5):1092–1104. [PubMed] [Google Scholar]
- Levin M., Franklin E. C., Frangione B., Pras M. The amino acid sequence of a major nonimmunoglobulin component of some amyloid fibrils. J Clin Invest. 1972 Oct;51(10):2773–2776. doi: 10.1172/JCI107098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M., Pras M., Franklin E. C. Immunologic studies of the major nonimmunoglobulin protein of amyloid. I. Identification and partial characterization of a related serum component. J Exp Med. 1973 Aug 1;138(2):373–380. doi: 10.1084/jem.138.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder E., Anders R. F., Natvig J. B. Connective tissue origin of the amyloid-related protein SAA. J Exp Med. 1976 Nov 2;144(5):1336–1346. doi: 10.1084/jem.144.5.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchalonis J. J. An enzymic method for the trace iodination of immunoglobulins and other proteins. Biochem J. 1969 Jun;113(2):299–305. doi: 10.1042/bj1130299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal C. J., Franklin E. C., Frangione B., Greenspan J. Isolation and partial characterization of SAA-an amyloid-related protein from human serum. J Immunol. 1976 May;116(5):1415–1418. [PubMed] [Google Scholar]
- Schacterle G. R., Pollack R. L. A simplified method for the quantitative assay of small amounts of protein in biologic material. Anal Biochem. 1973 Feb;51(2):654–655. doi: 10.1016/0003-2697(73)90523-x. [DOI] [PubMed] [Google Scholar]
- Schonfeld G., Bradshaw R. A., Chen J. Structure of high density lipoprotein. The immunologic reactivities of the COOH- and NH2-terminal regions of apolipoprotein A-I. J Biol Chem. 1976 Jul 10;251(13):3921–3926. [PubMed] [Google Scholar]
- Segrest J. P., Jackson R. L., Morrisett J. D., Gotto A. M., Jr A molecular theory of lipid-protein interactions in the plasma lipoproteins. FEBS Lett. 1974 Jan 15;38(3):247–258. doi: 10.1016/0014-5793(74)80064-5. [DOI] [PubMed] [Google Scholar]
- Segrest J. P. Molecular packing of high density lipoproteins: a postulated functional role. FEBS Lett. 1976 Oct 15;69(1):111–115. doi: 10.1016/0014-5793(76)80665-5. [DOI] [PubMed] [Google Scholar]
- Segrest J. P., Pownall H. J., Jackson R. L., Glenner G. G., Pollock P. S. Amyloid A: amphipathic helixes and lipid binding. Biochemistry. 1976 Jul 27;15(15):3187–3191. doi: 10.1021/bi00660a005. [DOI] [PubMed] [Google Scholar]
- Shen B. W., Scanu A. M., Kézdy F. J. Structure of human serum lipoproteins inferred from compositional analysis. Proc Natl Acad Sci U S A. 1977 Mar;74(3):837–841. doi: 10.1073/pnas.74.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner M., Cathcart E. S., Cohen A. S., Benson M. D. Isolation and identification by sequence analysis of experimentally induced guinea pig amyloid fibrils. J Exp Med. 1974 Sep 1;140(3):871–876. doi: 10.1084/jem.140.3.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein O., Stein Y. High density lipoproteins reduce the uptake of low density lipoproteins by human endothelial cells in culture. Biochim Biophys Acta. 1976 May 27;431(2):363–368. doi: 10.1016/0005-2760(76)90157-0. [DOI] [PubMed] [Google Scholar]
- Weinstein D. B., Carew T. E., Steinberg D. Uptake and degradation of low density lipoprotein by swine arterial smoot muscle cells with inhibition of cholesterol biosynthesis. Biochim Biophys Acta. 1976 Mar 26;424(3):404–421. doi: 10.1016/0005-2760(76)90030-8. [DOI] [PubMed] [Google Scholar]




