Abstract
Objectives. We sought to assess 6-month outcomes for HIV-infected people released from New York City jails with a transitional care plan.
Methods. Jail detainees in New York City living with HIV who accepted a transitional care plan during incarceration were asked to participate in a multi-site evaluation aimed at improving linkages to community-based care. The evaluation included a 6-month follow-up; HIV surveillance data were used to assess outcomes for those considered lost to follow-up.
Results. Participants (n = 434) completed baseline surveys during incarceration in a jail in New York City. Of those seen at 6 months (n = 243), a greater number were taking antiretroviral medications (92.6% vs 55.6%), had improved antiretroviral therapy adherence (93.2% vs 80.7%), and reported significant reductions in emergency department visits (0.20 vs 0.60 visits), unstable housing (4.15% vs 22.4%), and food insecurity (1.67% vs 20.7%) compared with baseline.
Conclusions. Transitional care coordination services facilitate continuity of care and improved health outcomes for HIV-positive people released from jail.
New York City remains the epicenter of the HIV epidemic in the United States, with more people living with HIV/AIDS than in any other region in the country.1–3 AIDS case rates in New York City are nearly 3 times the national average, and HIV remains a leading cause of death for residents.1 The New York City jail system, the second largest in the country with an average daily census of about 12 500, is comprised of 12 jails with 9 active jails on Rikers Island and 3 local borough detention centers (Bronx, Brooklyn, and Manhattan). Demographically, the jail population mirrors that of New York City communities hit hardest by the HIV epidemic, with much of the jail population coming from the same communities most affected by HIV and other health and socioeconomic disparities.4 A 2006 blinded serosurvey by the New York City Department of Health and Mental Hygiene (DOHMH) found that approximately 5% of the New York City jail population (4.7% of men and 9.8% of women) was infected with HIV.5 In 2011, 3.5% of those admitted to a jail in New York City self-disclosed as being HIV positive at their medical intake or at subsequent encounters with DOHMH staff, and another 1.1% were identified through an opt-in HIV testing program.
All people detained for at least 24 hours in New York City jails receive a medical intake, which includes a medical and mental health screening. Medical and mental health care and transitional care planning services in New York City jails are overseen by DOHMH’s Correctional Health Services (CHS). In accordance with the National HIV/AIDS Strategy, the HIV Continuum of Care Model instituted by CHS includes discharge planning services for people living with HIV/AIDS.6 In New York City jails, this service is provided by Transitional Health Care Coordination (THCC), part of CHS. THCC staff provides discharge and care coordination plans which include referrals for community-based primary care, case management, and other social services for HIV-positive detainees. For a more detailed description of the THCC program, see Jordan et al.7
Given that most jail detainees return to their communities, jail settings can present an opportunity for public health interventions and provide clinicians the opportunity to reengage with patients who had fallen out of care in the community.8–10 For some people living with HIV/AIDs, the correctional setting may be the first time they receive care for their HIV infection.11 Yet continuity of care following release remains a challenge. A study of people living with HIV/AIDs released from prison in Texas found that only a fraction filled their antiretroviral therapy (ART) prescriptions within the first 10 days of release and less than one third did so within the first 60 days.12 However, having a transitional care plan prepared prior to release, coupled with case management, has been found to facilitate continuity of care.13,14 Spaulding et al. found that meeting with an HIV provider within 30 days of release from jail was associated with having an undetectable viral load 6 months after release from jail.15
In 2007, the DOHMH received a grant from the Health Resources and Services Administration’s Special Projects of National Significance (SPNS) to participate in the “Enhancing Linkages to HIV Primary Care in Jail Settings Initiative” (EnhanceLink), a multi-site evaluation project designed to identify HIV-positive people in jail and link them to community-based care following release.8,16 This article describes some of the outcomes for the DOHMH participants enrolled in EnhanceLink and draws on New York City HIV surveillance data to provide limited information on those participants who were not seen at follow-up, but who had HIV-related lab values reported within the follow-up timeframe, allowing us to compare HIV clinical markers with those who were seen at follow-up.
METHODS
From April 2008 through May 2011, incarcerated persons held in New York City jails who self-identified as living with HIV and who accepted offers of a discharge plan by a THCC patient care coordinator were invited to participate. People were eligible if they were: 18 years or older, likely to be released to the community within 6 months, and willing to receive medical care for their HIV infection. Participants also consented to receive case management services from 1 of 3 subgrantee community-based organizations that conducted 6-month follow-up interviews. Persons newly diagnosed with HIV or who were receiving mental health discharge planning during their jail stay were excluded because they were eligible for discharge planning services from other DOHMH programs. Once eligibility was determined, the patient care coordinator obtained consent and administered a paper-based baseline survey in either English or Spanish. Participants received a $20 deposit to their jail commissary account for their time and needed to be released to the community prior to a cut-off date to be included in the multisite evaluation. Participants included in the multisite evaluation who were not seen for a follow-up interview were considered lost to follow-up.
Demographic information (name, birth date, gender, and race), along with their New York State ID number (used by the criminal justice system), of participants defined as lost to follow-up were provided to DOHMH’s Bureau of HIV/AIDS Prevention and Control (BHIV) for matching with New York City HIV surveillance data to determine what percentage of those lost to follow-up had follow-up HIV labs after their release from jail, to inform the local program evaluation. Participants were considered lost to follow-up (n = 191) if they were eligible for but did not complete a follow-up interview. To be eligible for follow-up, participants needed to have been released from jail or prison and in the community long enough to have received community-based care and services, and reside in the New York City area.
The information shared is already captured by BHIV because all HIV-related laboratory results in New York City are reported to BHIV, which maintains the New York City HIV/AIDS Surveillance registry. Because of variations in lab reporting and the time frame of interest, the limit for undetectable HIV viral load was set to less than 400 copies. The registry is a repository of all HIV-related lab data in New York City reported to DOHMH for surveillance purposes. Access is strictly limited to BHIV staff that work with the surveillance registry data. After matching identifying information, BHIV staff reported the number of participants lost to follow-up who had HIV-related laboratory test results, post-release in aggregate form, maintaining the confidentiality of client-level data. The multisite evaluation follow-up had a 60-day window centered on the 6-month target date. Because people might have had their blood drawn for HIV labs prior to meeting with a clinician, the time frame for follow-up lab data were broadened to 4 to 7 months post-release.
Measures
Baseline survey data included client demographic characteristics, housing stability and food insecurity, medical care and emergency department utilization, HIV care and treatment history. Housing and food insecurity were determined by asking, “In the 30 days before your most recent incarceration: . . . did you consider yourself to be homeless?” and “. . . was there any time for two or more days when you didn’t get anything, or barely anything, to eat?” Current engagement in HIV care was assessed by the question, “During the 30 days before your most recent incarceration, did you have a usual health care provider or place where you got HIV care?” Current HIV treatment was defined as “During the 7 days before your most recent incarceration, were you taking any HIV medications?” ART adherence was self-reported using a visual analog scale, ranging from 0% to 100%, labeled: “Taking medications as prescribed what percentage of the time?” Self-reported physical and mental health status were assessed using the 12-item Short-Form survey (SF-12).17,18 Current general health assessment comes from the SF-12, question 1, which asks respondents to rate their current health using a 5-point scale, with 1 equaling excellent and 5 equaling poor health. HIV-related clinical data (CD4 and viral load) and ART treatment status were collected after release from jail and at the 6-month interview. Blood was drawn for the first jail-based CD4 and viral load tests at the time of the medical intake, soon after admission to jail; subsequent labs were drawn every 3 months, or more often if necessary. We also asked participants about the number of times they were seen for care in an emergency department in the 6 months prior to incarceration.
Analysis
Descriptive statistical analyses were performed using the t-test, χ2 test, or the Fisher exact test, depending on the variable and expected distributions. Registry staff were supplied with de-identified data on the 6-month follow-up group to allow comparisons in clinical outcomes between those lost to follow-up and those in the 6-month follow-up group. Mean values, as well as categorical breakdowns for CD4 and viral load, were provided, allowing us to determine how many of those lost to follow-up had CD4 counts of less than 200, as well as the number with undetectable viral loads. Data were analyzed using Stata 11.2 (StataCorp, College Station, TX).
RESULTS
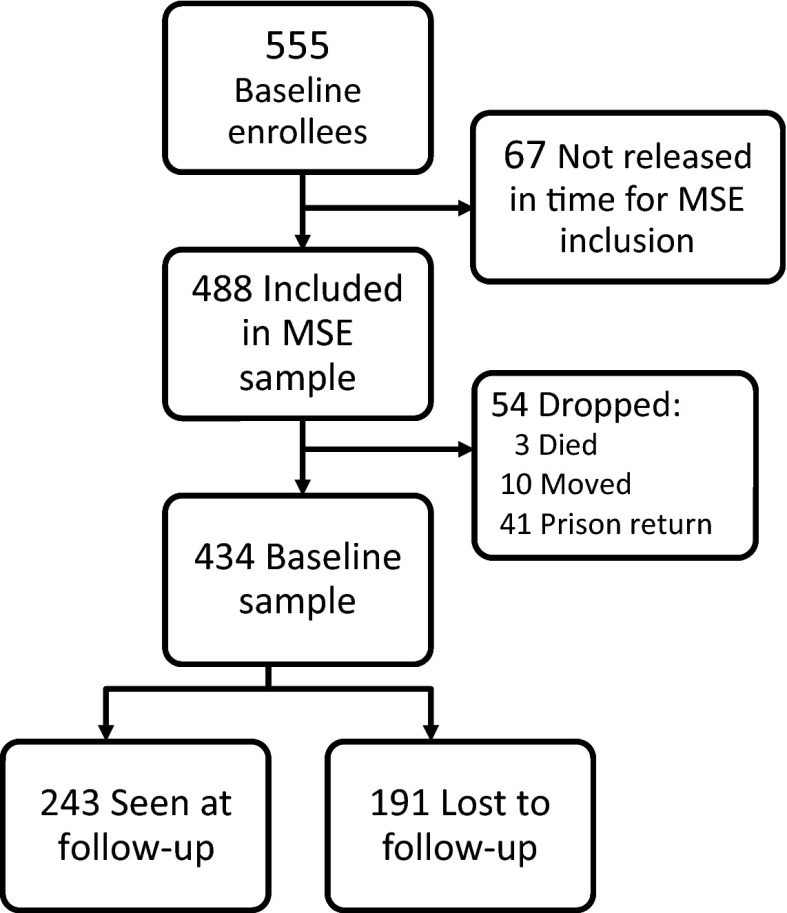
A total of 555 participants completed baseline surveys during their incarceration in a New York City jail. Of those, 488 (88%) were released in time to be included in the multisite evaluation (Figure 1). Of those 488, 54 were later determined to be ineligible for follow-up as a result of death, relocation, or subsequent incarcerations with prison sentences that extended beyond the follow-up time frame. This resulted in 434 participants who were eligible for follow-up, of whom 243 (56%) completed a 6-month survey the remaining 44% (n = 191) were in the group lost to follow-up. The demographic characteristics and baseline values of the 434 enrollees, with comparisons between 6-month follow-up group and those lost to follow-up, are shown in Table 1.
FIGURE 1—
Disposition of EnhanceLink participants: New York, NY, 2008–2011.
Note. MSE=multisite evaluation. Prison return indicates people who were sent to prison from an incarceration subsequent to release from the index incarceration.
TABLE 1—
Demographic Characteristics and Baseline Values for All Enrollees, With Comparisons Between Participants Seen at Follow-Up and Lost To Follow-Up: New York, NY, 2008–2011
| Characteristic | All Enrollees, No. (%) or Mean ±SD | Seen at Follow-Up, No. (%) or Mean ±SD | Lost To Follow-Up, No. (%) or Mean ±SD | P |
| Gender | < .001 | |||
| Male | 338 (78) | 208 (86) | 130 (68) | |
| Female | 87 (20) | 33 (13) | 54 (28) | |
| Transgender | 9 (2) | 2 (1) | 7 (4) | |
| Race/ethnicity | .078 | |||
| White | 31 (7.1) | 17 (7) | 14 (7.3) | |
| Black | 246 (56.7) | 127 (52.3) | 119 (62.3) | |
| Latino | 154 (35.5) | 98 (40.3) | 56 (29.3) | |
| Other | 3 (0.7) | 1 (0.4) | 2 (1) | |
| Age | 44.8 ±8.0 | 45.5 ±7.6 | 43.8 ±8.7 | .034 |
| < 30 y | 25 (3.5) | 8 (3.3) | 17 (9) | |
| 30–39 y | 74 (13.1) | 41 (17) | 33 (17) | |
| 40–49 y | 227 (49.3) | 128 (52.7) | 99 (52) | |
| 50–59 y | 100 (30) | 63 (26) | 37 (19) | |
| ≥ 60 y | 8 (4.1) | 3 (1) | 5 (3) | |
| Education | .17 | |||
| < high school or GED | 204 (47) | 105 (43) | 99 (52) | |
| High school diploma or GED | 165 (38) | 97 (40) | 68 (35.5) | |
| ≥ some college | 65 (15) | 41 (17) | 24 (12.5) | |
| Housing instability | 114 (26.4) | 54 (22.2) | 60 (31.4) | .035 |
| Food insecurity | 109 (25.3) | 50 (20.7) | 59 (31.9) | .012 |
| Had usual provider | 363 (83.6) | 211 (86.8) | 152 (79.6) | .043 |
| Currently on ART | 237 (54.6) | 135 (55.6) | 102 (53.4) | .656 |
| ART taken as directed, % of time | 80.3 ±28.4 | 80.7 ±29.7 | 81.7 ±25.3 | .774 |
| CD4 count | 383 ±262 | 374 ±263 | 395 ±261 | .422 |
| Viral load | 47 515 ±159 276 | 54 031 ±183 404 | 44 933 ±122 638 | .566 |
| ED visits, prior 6 moa | 0.67 ±1.4 | 0.60 ±1.2 | 0.77 ±1.6 | .202 |
| Current healthb | 3.29 ±1.03 | 3.22 ±1.05 | 3.29 ±1.01 | .475 |
| SF-12 physical composite score | 47.6 ±10.3 | 47.9 ±10.6 | 47.4 ±10.5 | .296 |
| SF-12 Mental Composite score | 44.3 ±10.2 | 44.8 ±9.5 | 44.1 ±11.4 | .392 |
Note. ART = antiretroviral therapy; ED = emergency department; GED = general equivalency diploma; SF-12 = 12-item Short-Form survey.
Self-reported number of times seen at ED 6 mo prior to incarceration or at 6-month follow-up interview.
Self-reported current health rating, with 1 = excellent, 5 = poor.
Seventy-eight percent of participants were male, 20% female, and 2% were transgender women. Over half (56.7%) of participants were non-Hispanic Black, 35.5% were Latino, with the remainder comprised of non-Hispanic White persons (7.1%) and persons of other races (0.7%). These numbers reflect the demographics of the general population of persons held in New York City jails, with the exception of women, who comprise approximately 10% of the jail population. The mean age at enrollment was 45 years; nearly half (47%) had not completed high school or the general educational development (GED). Approximately one quarter of all participants reported being unstably housed or going without food for 2 or more days in the 30 days prior to their index incarceration.
Participants considered lost to follow-up were more likely to be younger, non-Hispanic Black, female or transgender women, and to have reported housing instability and food insecurity at baseline compared with those in the 6-month follow-up group. Those lost to follow-up were also less likely to have had a usual medical provider in the community prior to incarceration compared with those seen at follow-up. Although not statistically significant, 52% of those lost to follow-up had less than a high school level education compared with 43% of those in the 6-month follow-up group. Over half (54.6%) of the participants reported taking ART in the week preceding their incarceration, with no significant differences between the 6-month follow-up group and those lost to follow-up. No differences in ART adherence, HIV clinical markers, ED visits, or SF-12 scores were observed between the 2 groups.
Among the 6-month follow-up group, the percentage of persons on ART increased from 55.5% to 92.6% (Table 2). Self-reported adherence to HIV medications for those in the 6-month follow-up group increased from nearly 81% at baseline to 93% at 6 months post-release. A drop in mean viral load and an increase in the percentage with undetectable viral load, and an increase in mean CD4 count among those seen at follow-up support this. The 6-month follow-up group reported significantly fewer visits to an emergency department since their release, from 0.60 per person in the 6 months prior to baseline, to 0.20 visits at follow-up. Housing instability decreased among those in the 6-month follow-up group, going from 22% who reported being unstably housed prior to incarceration to 4% at follow-up. Food insecurity among this group also decreased from 20% at baseline to less than 2% at follow-up. The 6-month follow-up group also self-reported feeling in better general health at follow-up compared with baseline when asked to rate their current health.
TABLE 2—
Comparison of Baseline Versus Follow-Up Values for Participants Seen at Follow-Up: New York, NY, 2008–2011
| Seen at Follow-Up (n = 243) |
||
| Variable | Baseline % or Mean ±SD | Follow-up % or Mean ±SD |
| CD4 count | 374 ±263 | 412 ±271* |
| Viral load | 54 031 ±183 404 | 13 738 ±23 310* |
| Currently on ART | 55.6 | 92.6* |
| ART taken as directed, % of time | 80.7 | 93.2* |
| Had usual provider | 86.8 | 92.9 |
| ED visits, prior 6 moa | 0.60 ±1.19 | 0.20 ±0.61* |
| Housing instability | 22.4 | 4.15* |
| Food insecurity | 20.7 | 1.7* |
| Current healthb | 3.22 ±1.05 | 2.81 ±0.79* |
| SF-12 physical composite score | 47.9 ±10.6 | 50.4 ±8.1* |
| SF-12 mental composite score | 44.8 ±9.5 | 47.5 ±6.9* |
Note. ED = emergency department.
Self-reported number of times seen at ED 6 mo prior to incarceration or at 6 month follow-up interview.
Self-reported current health rating, with 1 = excellent, 5 = poor.
*Significantly different from baseline numbers, with P < .05.
Of the 191 clients considered lost to follow-up whose identifying data were submitted to the HIV surveillance staff, 164 (86%) had lab data in the follow-up time frame of at least 4 months post-release. Fifty-three (32.3%) had lab values reported by a correctional facility. We anticipated that some enrollees would be reincarcerated, therefore having follow-up labs reported by a jail or prison was not a reason for exclusion and those clients remain in the analyses. Not all clients had both a CD4 and viral load test results: of the 111 in the lost to follow-up group who had values reported from community-based labs, 20 had only a CD4 count, 14 only a viral load result, and 77 (69%) had both. For those with labs reported by a correctional facility, 48 had only a viral load, 5 only a CD4, and none had both.
The 6-month follow-up and lost to follow-up groups had statistically significant differences in CD4 cell count by category at baseline, with 54.3% of the 6-month follow-up group having a CD4 count of fewer than 350, compared with 40.8% of those lost to follow-up (Table 3). Post-release, fewer people in the lost to follow-up group had an undetectable viral load (17.9%) compared with those in the 6-month follow-up group (35.2%). Those lost to follow-up also had a significantly greater percentage of people with viral load categorized as high (≥ 100 000 copies; 17.4%), versus 0.5% among the 6-month follow-up group. Similarly, the 6-month follow-up group had more than 1.5 times as many people with high CD4 counts (≥ 500), yet no statistically significantly differences were observed for post-release CD4 counts between the 2 groups.
TABLE 3—
Comparison of Clinical Data of Participants Seen at Follow-Up Versus Those Lost to Follow-Up: New York, NY, 2008-2011
| Follow-up Values |
|||
| Clinical Data | Seen at Follow-Up, No. (%) | Lost To Follow-Up (n = 164), No. (%) | P |
| CD4 count | .14 | ||
| < 200 | 52 (22.2) | 30 (29.4) | |
| 200–349 | 58 (24.7) | 28 (27.5) | |
| 350–499 | 54 (22.9) | 25 (24.5) | |
| ≥ 500 | 71 (30.2) | 19 (18.6) | |
| Viral load level | <.001 | ||
| Undetectable (< 400) | 80 (35.2) | 25 (17.9) | |
| Low (400–9999) | 77 (33.9) | 25 (17.9) | |
| Medium (10 000–99 999) | 69 (30.4) | 65 (46.8) | |
| High (≥ 100 000) | 1 (0.5) | 24 (17.4) | |
Note. Follow-up CD4 categories, 6-month follow-up group vs lost to follow-up group: χ2(3, n = 337) = 5.411; P = .144. Follow-up viral load categories, 6-month follow-up group vs lost to follow-up group: χ2(3, n = 366) = 58.84; P ≤ .001.
DISCUSSION
In New York City jails, HIV-positive detainees are offered comprehensive transitional care services, which include referrals to community-based care. This initiative enabled us to collect detailed data on participants and their prior engagement in care and barriers to care, such as unstable housing, which provided us with a better picture of our participants compared with what we typically obtain at intake. Though verification of linkage to care and 90-day retention in care is conducted, no further post-release assessment is typically conducted. Participation in this SPNS project allowed us to conduct post-release interviews and chart reviews with clients who were released to the community, which provided us with the opportunity to assess post-release health outcomes. That allowed us to better understand our clients and identify some factors that may be associated with improved outcomes.
Providing services to people held in jail presents a host of challenges. Compared with prisons, jails are relatively dynamic environments, with people going to and from court and unpredictable release dates, which can present challenges in providing medical care. But jail stays also present correctional health care workers with an opportunity to intervene and re-engage with those individuals who have fallen out of care.10,19–21 Reengagement in care is particularly important for people living with HIV who were not stably engaged in care or fell out of care in the community. Although current HIV treatments have resulted in reduced morbidity and mortality for people living with HIV, these outcomes require continued access to treatment and engagement in care.22,23 Many of the participants enrolled at our site had fallen out of care prior to their incarceration. Of those who reported taking ART prior to incarceration, many reported suboptimal treatment adherence levels. Poor adherence and discontinuity in care pose risks to health outcomes, as they can result in viral drug resistance leading to increased viral load and potentially limited treatment options in the future.
We found that many of the people who were lost to follow-up were more likely to have reported some degree of housing and food insecurity. However, among those lost to follow-up, the percentage of people with jail-based follow-up lab values is consistent with the systemwide average recidivism rate of approximately 1.4 incarcerations per person per year.24 Although HIV-positive people held in New York City jails are offered medical care and treatment, continuation of care upon release from jail remains a challenge, especially for those who were unstably housed or not fully engaged in care prior to their incarceration. Housing instability is a fundamental barrier to successful retention in care for most people since basic needs such as food and housing are typically prioritized over health care needs.25,26 An assessment of homelessness among the EnhanceLink multisite data found that homeless individuals were less likely to be actively engaged in care, prescribed ART, or attain viral suppression.27 Others have documented the difficulties HIV-positive persons face in maintaining continuity of care when leaving jail or prison.12,28 For those leaving jail, continuity of care may not be as important as getting their basic needs met and addressing more immediate and critical concerns. Having these needs assessed as part of the care coordination planning process would help to identify those persons in need of transitional housing upon release from jail.
All of the participants in this project received transitional care coordination services while incarcerated. Addressing all of a client’s most pressing needs, such as housing, substance abuse treatment, and mental health care needs as well as referrals to primary medical care, are core components of this approach. Althoff et al. found that prerelease discharge planning services delivered as part of the EnhanceLink initiative were associated with greater retention in community-based HIV care, and Spaulding et al. discovered the enhancement of linkage to care services in the initiative to be cost effective.15,29 Others who looked at factors associated with emergency department use among HIV-positive persons released from jail found that frequent users were less likely to have received prerelease discharge planning services.30 A more rigorous, randomized study, with controls, is likely needed to better understand the impact of discharge planning on health outcomes and service utilization. However, our program strives to provide services to all eligible persons living with HIV who are held in New York City jails. Therefore we would leave such a study to some other jurisdiction, perhaps one where discharge planning is being considered, but not yet currently available.
Whenever possible, prescreening for health insurance and enrollment into Medicaid is conducted to facilitate continuity of care. THCC refers people to providers and programs that offer transitional housing, as well as residential drug treatment programs or nursing homes for those in need. Accordingly, the participants who were seen at follow-up reported lower rates of unstable housing and food insecurity at their 6-month interview compared with their baseline interview. Those lost to follow-up were more likely at the time of their baseline interview to have reported unstable housing, food insecurity, and lack of a usual medical care provider. Combined, those indicators suggest a greater instability in their lives prior to landing in jail. This instability and lack of connection to care could also explain why those lost to follow-up were not seen for the 6-month survey. This is consistent with other studies which have found housing to be a critical issue in getting people linked to and retained in care.25,26,31–33
Limitations
Our project had several limitations, the first being that this was an observational study with no control group, because all participants received transitional care services during incarceration, making it difficult to assess the impact of those services on health outcomes. Second, survey data were self-reported and may be inaccurate for questions asking about events that occurred during varying times prior to incarceration. Also, data were collected during the participant’s incarceration, and it is possible that certain responses, such as current general health or questions regarding mental health status may have been influenced by the individual’s current state of incarceration. Finally, we relied on participants linking to and remaining in contact with one of our community-based partners long enough for our participants to be seen for a 6-month follow-up interview.
Although we were not able to conduct a follow-up survey with those lost to follow-up, by collaborating with the New York City HIV surveillance data staff, we were able to determine if the participants lost to follow-up had any HIV-related lab values during the follow-up time period, which we considered a proxy for engagement in care. The HIV surveillance data staff found that the majority (85%) of those lost to follow-up had HIV-related monitoring (CD4, viral load, or both) following their release from jail. However, nearly a third of those lab data were reported by a correctional facility, indicating that many had returned to jail following release from their index incarceration.
If we did not have access to the surveillance data and chose to consider all participants with missing data, from both the 6-month follow-up group and those lost to follow-up, as virally unsuppressed, we would have found that 18% of the 434 participants had attained viral suppression. Adding the number found in the surveillance data to be virally suppressed resulted in 24% of the 434 participants being virally suppressed. Among those we did see at follow-up, just over 35% had attained viral suppression, a percentage comparable with that found among Black persons using national HIV surveillance data.34
CONCLUSIONS
Based on these findings, we believe that providing HIV-infected persons appropriate jail-based health care and transitional care coordination services, which include a comprehensive discharge plan with appropriate referrals for community-based care, has a positive impact on post-release outcomes. A comprehensive discharge plan provides a road map for those returning to the community, particularly for those who had fallen out of care prior to incarceration. Linkages to care benefit not only the individual but also the community to which the person returns when released from jail. If the individual is successfully engaged and retained in care, this can lead to improved health outcomes not only for the individual, but for the community as well via reduced community viral load. Improved individual health may also lead to fewer emergency department and hospital-based care visits.
References
- 1. HIV Surveillance Report 2009. Centers for Disease Control and Prevention: Atlanta, GA; 2011.
- 2.Chiasson MA. New York City AIDS case mortality rates in the era of potent antiretroviral therapy. J Urban Health. 2000;77(2):255–257. doi: 10.1007/BF02390536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiewel EW, Hanna DB, Begier EM, Torian LV. High HIV prevalence and diagnosis rates in New York City black men. J Community Health. 2011;36(1):141–149. doi: 10.1007/s10900-010-9291-0. [DOI] [PubMed] [Google Scholar]
- 4.New York City Department of Health and Mental Hygiene. HIV Epidemiology & Field Services Semiannual Report. 2011. Available at: http://www.nyc.gov/html/doh/downloads/pdf/dires/2012-2nd-semi-rpt.pdf. Accessed November 27, 2014.
- 5.Begier EM, Bennani Y, Forgione L et al. Undiagnosed HIV infection among New York City jail entrants, 2006: results of a blinded serosurvey. J Acquir Immune Defic Syndr. 2010;54(1):93–101. doi: 10.1097/QAI.0b013e3181c98fa8. [DOI] [PubMed] [Google Scholar]
- 6.Obama B.National HIV/AIDS Strategy for the United States. 2010. Available at: http://aids.gov/federal-resources/national-hiv-aids-strategy/nhas.pdf. Accessed December 10, 2014.
- 7.Jordan AO, Cohen LR, Harriman G, Teixeira PA, Cruzado-Quinones J, Venters H. Transitional care coordination in New York City jails: facilitating linkages to care for people with HIV returning home from Rikers Island. AIDS Behav. 2013;17(Suppl 2):S212–219. doi: 10.1007/s10461-012-0352-5. [DOI] [PubMed] [Google Scholar]
- 8.Draine J, Ahuja D, Altice FL et al. Strategies to enhance linkages between care for HIV/AIDS in jail and community settings. AIDS Care. 2011;23(3):366–377. doi: 10.1080/09540121.2010.507738. [DOI] [PubMed] [Google Scholar]
- 9.Altice FL, Mostashari F, Friedland GH. Trust and the acceptance of and adherence to antiretroviral therapy. J Acquir Immune Defic Syndr. 2001;28(1):47–58. doi: 10.1097/00042560-200109010-00008. [DOI] [PubMed] [Google Scholar]
- 10.Avery AK, Ciomcia RW, Lincoln T et al. Jails as an opportunity to increase engagement in HIV care: findings from an observational cross-sectional study. AIDS Behav. 2013;17(Suppl 2):S137–S144. doi: 10.1007/s10461-012-0320-0. [DOI] [PubMed] [Google Scholar]
- 11.Braithwaite RL, Arriola KR. Male prisoners and HIV prevention: a call for action ignored. Am J Public Health. 2008;98(9 Suppl):S145–S149. doi: 10.2105/ajph.98.supplement_1.s145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baillargeon J, Giordano TP, Harzke AJ et al. Predictors of reincarceration and disease progression among released HIV-infected inmates. AIDS Patient Care STDS. 2010;24(6):389–394. doi: 10.1089/apc.2009.0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Needels K, James-Burdumy S, Burghardt J. Community case management for former jail inmates: its impacts on rearrest, drug use, and HIV risk. J Urban Health. 2005;82(3):420–433. doi: 10.1093/jurban/jti092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wakeman SE, Rich JD. HIV treatment in US prisons. HIV Ther. 2010;4(4):505–510. doi: 10.2217/hiv.10.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spaulding AC, Messina LC, Kim BI et al. Planning for success predicts virus suppressed: results of a non-controlled, observational study of factors associated with viral suppression among HIV-positive persons following jail release. AIDS Behav. 2013;17(Suppl 2):S203–S211. doi: 10.1007/s10461-012-0341-8. [DOI] [PubMed] [Google Scholar]
- 16.Spaulding AC, Booker CA, Freeman SH et al. Jails, HIV testing, and linkage to care services: an overview of the EnhanceLink initiative. AIDS Behav. 2013;17(Suppl 2):S100–S107. doi: 10.1007/s10461-012-0339-2. [DOI] [PubMed] [Google Scholar]
- 17.McLellan AT, Kushner H, Metzger D et al. The fifth edition of the addiction severity index. J Subst Abus Treat. 1992;9(3):199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- 18.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Rapp RC, Ciomcia R, Zaller N, Draine J, Ferguson A, Cagey R. The role of jails in engaging PLWHA in care: from jail to community. AIDS Behav. 2013;17(Suppl 2):S89–99. doi: 10.1007/s10461-012-0298-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frank L. Prisons and public health: emerging issues in HIV treatment adherence. J Assoc Nurses AIDS Care. 1999;10(6):24–32. doi: 10.1016/S1055-3290(06)60320-8. [DOI] [PubMed] [Google Scholar]
- 21.Spaulding AC, Seals RM, Page MJ, Brzozowski AK, Rhodes W, Hammett TM. HIV/AIDS among inmates of and releasees from US correctional facilities, 2006: declining share of epidemic but persistent public health opportunity. PLoS ONE. 2009;4(11):e7558. doi: 10.1371/journal.pone.0007558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakagawa F, Lodwick RK, Smith CJ et al. Projected life expectancy of people with HIV according to timing of diagnosis. AIDS. 2012;26(3):335–343. doi: 10.1097/QAD.0b013e32834dcec9. [DOI] [PubMed] [Google Scholar]
- 23.Palella FJ, Jr, Delaney KM, Moorman AC et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338(13):853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 24. Office of the Mayor, City of New York, Criminal Justice Coordinator, 2009. New York City Data Analytics Recidivism Tool (DART). Available at: http://recidivism.cityofnewyork.us. Accessed December 10, 2014.
- 25.Krieger J, Higgins DL. Housing and health: time again for public health action. Am J Public Health. 2002;92(5):758–768. doi: 10.2105/ajph.92.5.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolitski RJ, Kidder DP, Pals SL et al. Randomized trial of the effects of housing assistance on the health and risk behaviors of homeless and unstably housed people living with HIV. AIDS Behav. 2010;14(3):493–503. doi: 10.1007/s10461-009-9643-x. [DOI] [PubMed] [Google Scholar]
- 27.Zelenev A, Marcus R, Kopelev A et al. Patterns of homelessness and implications for HIV health after release from jail. AIDS Behav. 2013;17(Suppl 2):S181–S194. doi: 10.1007/s10461-013-0472-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sowell RL, Phillips KD, Seals BF, Julious CH, Rush C, Spruill LK. Social service and case management needs of HIV-infected persons upon release from prison/jail. Lippincott Case Manag. 2001;6(4):157–68. doi: 10.1097/00129234-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Althoff AL, Zelenev A, Meyer JP et al. Correlates of retention in HIV care after release from jail: results from a multi-site study. AIDS Behav. 2013;17(Suppl 2):S156–S170. doi: 10.1007/s10461-012-0372-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meyer JP, Qiu J, Chen NE, Larkin GL, Altice FL. Frequent emergency department use among released prisoners with human immunodeficiency virus: characterization including a novel multimorbidity index. Acad Emerg Med. 2013;20(1):79–88. doi: 10.1111/acem.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cunningham CO, Sohler NL, McCoy K, Heller D, Selwyn PA. Health care access and utilization patterns in unstably housed HIV-infected individuals in New York City. AIDS Patient Care STDS. 2005;19(10):690–695. doi: 10.1089/apc.2005.19.690. [DOI] [PubMed] [Google Scholar]
- 32.Cunningham WE, Andersen RM, Katz MH et al. The impact of competing subsistence needs and barriers on access to medical care for persons with human immunodeficiency virus receiving care in the United States. Med Care. 1999;37(12):1270–1281. doi: 10.1097/00005650-199912000-00010. [DOI] [PubMed] [Google Scholar]
- 33.Fontana L, Beckerman A. Recently released with HIV/AIDS: primary care treatment needs and experiences. J Health Care Poor Underserved. 2007;18(3):699–714. doi: 10.1353/hpu.2007.0058. [DOI] [PubMed] [Google Scholar]
- 34.Whiteside YO, Cohen SM, Bradley H, Skarbinski J, Hall HI, Lansky A. Progress along the continuum of HIV care among blacks with diagnosed HIV- United States, 2010. MMWR Morb Mortal Wkly Rep. 2014;63(5):85–89. [PMC free article] [PubMed] [Google Scholar]