Abstract
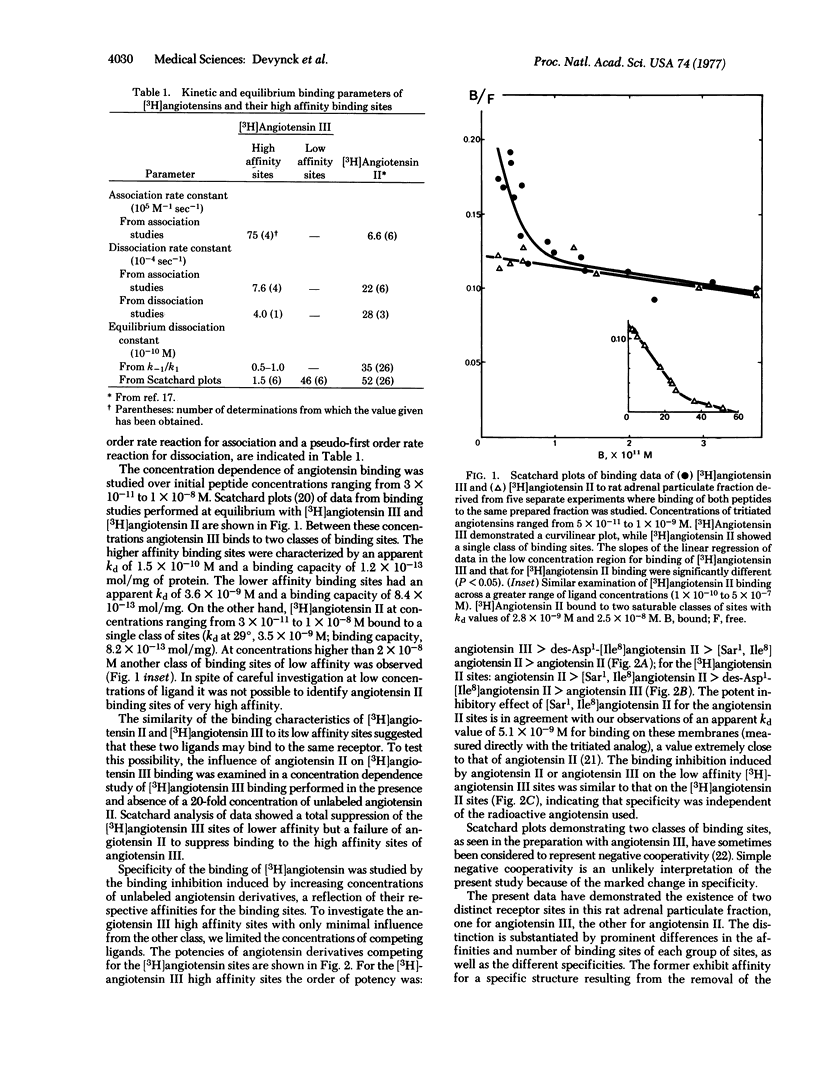
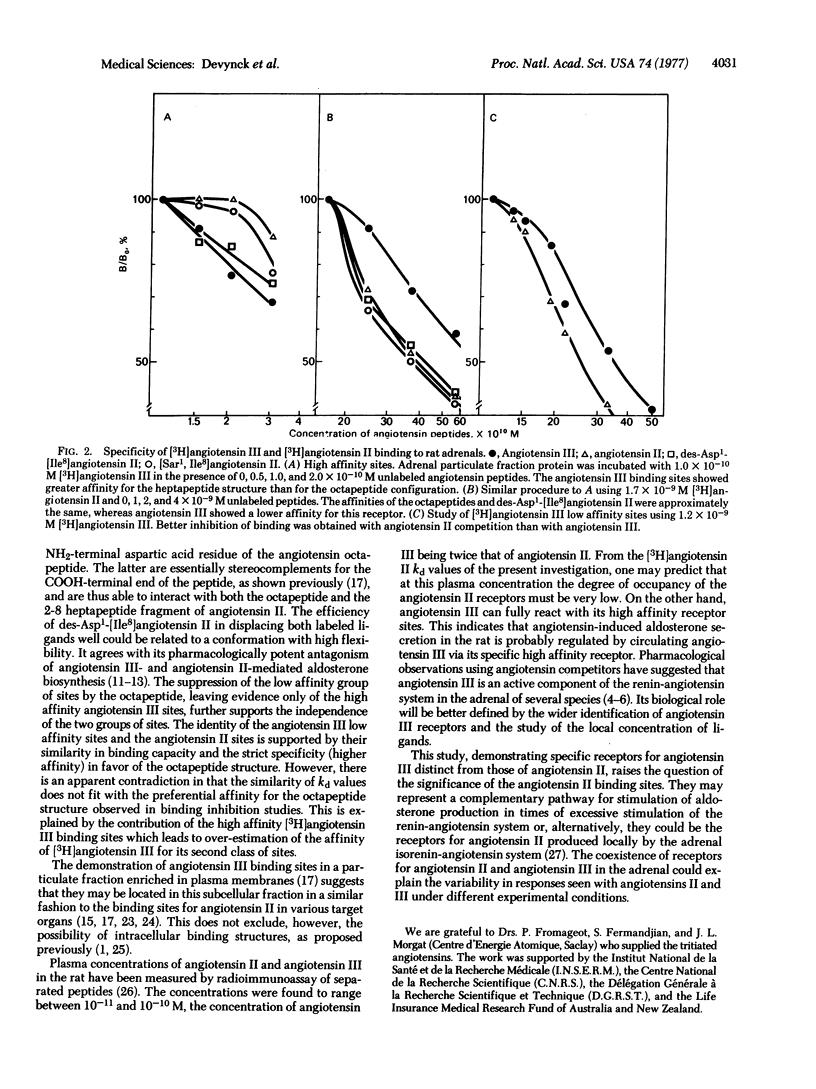
The specific binding of angiotensin II and des-Asp1-angiotensin II ("angiotensin III") III") to rat adrenals was studied with the use of the tritiated peptides. The binding sites having maximal affinity for angiotensin II were characterized by an equilibrium dissociation constant of 3.3 to 5.2 X 10(-9) M. Angiotensin III was able to interact with these sites, and also with a class of sites with very high affinity, characterized by an equilibrium dissociation constant of 1 to 2 X 10(-10) M. These sites exhibited a greater affinity for the heptapeptide angiotensin III than for the octapeptide angiotensin II. These findings, together with the known potent aldosterone stimulating effect of angiotensin III and its presence in rat plasma, suggest that this heptapeptide could be the physiologically important steroidogenic angiotensin in this species.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blair-West J. R., Coghlan J. P., Denton D. A., Funder J. W., Scoggins B. A., Wright R. D. The effect of the heptapeptide (2-8) and hexapeptide (3-8) fragments of angiotensin II on aldosterone secretion. J Clin Endocrinol Metab. 1971 Apr;32(4):575–578. doi: 10.1210/jcem-32-4-575. [DOI] [PubMed] [Google Scholar]
- Bravo E. L., Khosla M. C., Bumpus F. M. Vascular and adrenocortical responses to a specific antagonist of angiotensin II. Am J Physiol. 1975 Jan;228(1):110–114. doi: 10.1152/ajplegacy.1975.228.1.110. [DOI] [PubMed] [Google Scholar]
- Bumpus F. M., Khosla M. C. Inhibition of the pressor and aldosterone-releasing effects of angiotensin II. Clin Sci Mol Med Suppl. 1975 Jun;2:15s–18s. doi: 10.1042/cs048015s. [DOI] [PubMed] [Google Scholar]
- Campbell W. B., Pettinger W. A. Organ specificity of angiotensin II and Des-aspartyl angiotensin II in the conscious rat. J Pharmacol Exp Ther. 1976 Aug;198(2):450–456. [PubMed] [Google Scholar]
- Campbell W. B., Schmitz J. M., Itskovitz H. D. (Des-Asp1) angiotensin I: a study of its pressor and steroidogenic activities in conscious rats. Endocrinology. 1977 Jan;100(1):46–51. doi: 10.1210/endo-100-1-46. [DOI] [PubMed] [Google Scholar]
- Chang K. J., Jacobs S., Cuatrecasas P. Quantitative aspects of hormone-receptor interactions of high affinity. Effect of receptor concentration and measurement of dissociation constants of labeled and unlabeled hormones. Biochim Biophys Acta. 1975 Oct 6;406(2):294–303. doi: 10.1016/0005-2736(75)90011-5. [DOI] [PubMed] [Google Scholar]
- Devynck M. A., Meyer P. Angiotensin receptors in vascular tissue. Am J Med. 1976 Nov;61(5):758–767. doi: 10.1016/0002-9343(76)90157-1. [DOI] [PubMed] [Google Scholar]
- Devynck M. A., Rouzaire-Dubois B., Chevillotte E., Meyer P. Variations in the number of uterine angiotensin receptors following changes in plasma angiotensin levels. Eur J Pharmacol. 1976 Nov;40(1):27–37. doi: 10.1016/0014-2999(76)90350-2. [DOI] [PubMed] [Google Scholar]
- Forget G., Heisler S. Preparation and characterization of adrenocortical plasma membrane angiotensin II receptors. Can J Physiol Pharmacol. 1976 Oct;54(5):698–707. doi: 10.1139/y76-097. [DOI] [PubMed] [Google Scholar]
- Fredlund P., Saltman S., Catt K. J. Stimulation of aldosterone production by angiotensin II peptides in vitro: enhanced activity of the (1-sarcosine) analogue. J Clin Endocrinol Metab. 1975 Apr;40(4):746–749. doi: 10.1210/jcem-40-4-746. [DOI] [PubMed] [Google Scholar]
- Ganten D., Schelling P., Vecsei P., Ganten U. Iso-renin of extrarenal origin. "The tissue angiotensinogenase systems". Am J Med. 1976 May 31;60(6):760–772. doi: 10.1016/0002-9343(76)90890-1. [DOI] [PubMed] [Google Scholar]
- Glossmann H., Baukal A. J., Catt K. J. Properties of angiotensin II receptors in the bovine and rat adrenal cortex. J Biol Chem. 1974 Feb 10;249(3):825–834. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Larner A., Vaughan E. D., Tsai B. S., Peach M. J. Role of converting enzyme in the cardiovascular and adrenal cortical responses to (des-Asp1)-angiotensin I. Proc Soc Exp Biol Med. 1976 Sep;152(4):631–634. doi: 10.3181/00379727-152-39456. [DOI] [PubMed] [Google Scholar]
- Lohmeier T. E., Davis J. O., Freeman R. H. DES-ASP1--angiotensin II: possible role in mediating responses of the renin-angiotensin system. Proc Soc Exp Biol Med. 1975 Jun;149(2):515–518. doi: 10.3181/00379727-149-38840. [DOI] [PubMed] [Google Scholar]
- Morgat J. L., Hung L. T., Fromageot P. Preparation of highly labelled (3H)angiotensin II. Biochim Biophys Acta. 1970 May 26;207(2):374–376. doi: 10.1016/0005-2795(70)90032-2. [DOI] [PubMed] [Google Scholar]
- Peach M. J., Sarstedt C. A., Vaughan E. D., Jr Changes in cardiovascular and adrenal cortical responses to angiotensin III induced by sodium deprivation in the rat. Circ Res. 1976 Jun;38(6 Suppl 2):117–121. doi: 10.1161/01.res.38.6.117. [DOI] [PubMed] [Google Scholar]
- Pernollet M. G., Devynck M. A., Matthews P. G., Meyer P. Post-nephrectomy changes in adrenal angiotensin II receptors in the rat; influence of exogenous angiotensin and a competitive inhibitor. Eur J Pharmacol. 1977 Jun 15;43(4):361–372. doi: 10.1016/0014-2999(77)90042-5. [DOI] [PubMed] [Google Scholar]
- Robertson A. L., Jr, Khairallah P. A. Angiotensin II: rapid localization in nuclei of smooth and cardiac muscle. Science. 1971 Jun 11;172(3988):1138–1139. doi: 10.1126/science.172.3988.1138. [DOI] [PubMed] [Google Scholar]
- Sarstedt C. A., Vaughan E. D., Jr, Peach M. J. Selective inhibition by des-1-Asp-8-lle-angiotensin ii of the steroidogenic response to restricted sodium intake in the rat. Circ Res. 1975 Sep;37(3):350–358. doi: 10.1161/01.res.37.3.350. [DOI] [PubMed] [Google Scholar]
- Semple P. F., Morton J. J. Angiotensin II and angiotensin III in rat blood. Circ Res. 1976 Jun;38(6 Suppl 2):122–126. doi: 10.1161/01.res.38.6.122. [DOI] [PubMed] [Google Scholar]
- Spielman W. S., Davis J. O. The renin-angiotensin system and aldosterone secretion during sodium depletion in the rat. Circ Res. 1974 Oct;35(4):615–624. doi: 10.1161/01.res.35.4.615. [DOI] [PubMed] [Google Scholar]
- de Meyts P., Roth J., Neville D. M., Jr, Gavin J. R., 3rd, Lesniak M. A. Insulin interactions with its receptors: experimental evidence for negative cooperativity. Biochem Biophys Res Commun. 1973 Nov 1;55(1):154–161. doi: 10.1016/s0006-291x(73)80072-5. [DOI] [PubMed] [Google Scholar]


