Abstract
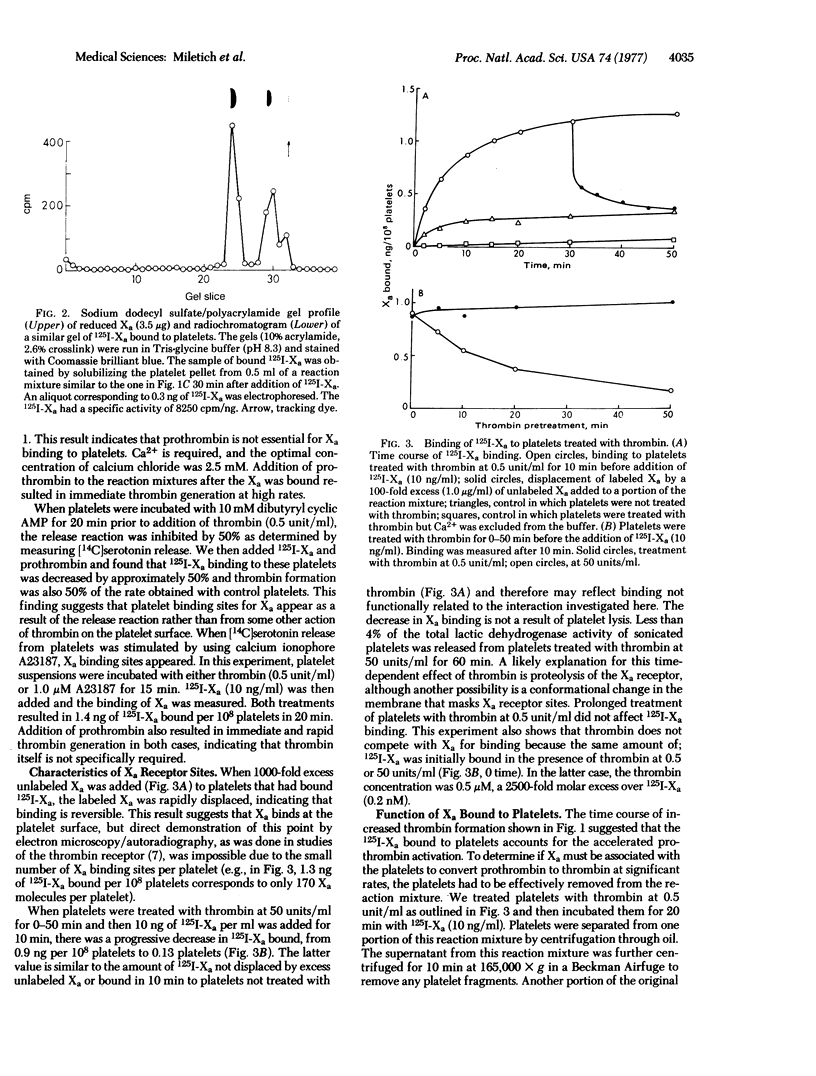
When human 125I-labeled Factor Xa is incubated with washed platelets, prothrombin, and Ca2+, a small amount of thrombin is formed which causes the platelet release reaction after a period of time that decreases as the Xa concentration is increased from 0.9 to 19 ng/ml. After a further lag period, the Xa binds reversibly to receptors on the platelet surface and rapid thrombin formation follows (3 units or 1 mug of thrombin formed per min per ng of Xa bound to 10(8) platelets). When platelets are treated with either htrombin (0.5 units/ml) or calcium ionophore A23187 prior to addition of Xa, binding begins immediately. Thrombin formation occurs at the platelet surface at rates that correlate with the amount of Xa bound. Dibutyryl cyclic AMP inhibits the release reaction, Xa binding, and rate of thrombin generation in parallel. The platelet Xa receptor is distinct from the previously described thrombin receptor and appears to be a protein because treatment of platelets with thrombin at 50 units/ml destroys Xa binding sites. The results suggest that specific receptors for Xa appear on the platelet surface after the release reaction occurs. The bound Xa catalyzes thrombin formation 1000-fold faster than does Xa added to reactions in which phospholipids are substituted for platelets.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BACHMANN F., DUCKERT F., KOLLER F. The Stuart-Prower factor assay and its clinical significance. Thromb Diath Haemorrh. 1958 May 1;2(1-2):24–38. [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Detwiler T. C., Feinman R. D. Kinetics of the thrombin-induced release of calcium (II) by platelets. Biochemistry. 1973 Jan 16;12(2):282–289. doi: 10.1021/bi00726a017. [DOI] [PubMed] [Google Scholar]
- Fenton J. W., 2nd, Campbell W. P., Harrington J. C., Miller K. D. Large-scale preparation and preliminary characterization of human thrombin. Biochim Biophys Acta. 1971 Jan 19;229(1):26–32. doi: 10.1016/0005-2795(71)90313-8. [DOI] [PubMed] [Google Scholar]
- Fenton J. W., 2nd, Fasco M. J. Polyethylene glycol 6,000 enhancement of the clotting of fibrinogen solutions in visual and mechanical assays. Thromb Res. 1974 Jun;4(6):809–817. doi: 10.1016/0049-3848(74)90024-3. [DOI] [PubMed] [Google Scholar]
- Ganguly P. Binding of thrombin to human platelets. Nature. 1974 Feb 1;247(5439):306–307. doi: 10.1038/247306a0. [DOI] [PubMed] [Google Scholar]
- HJORT P., RAPAPORT S. I., OWREN P. A. Evidence that platelet accelerator (platelet factor 1) is adsorbed plasma proaccelerin. Blood. 1955 Nov;10(11):1139–1150. [PubMed] [Google Scholar]
- Jevons S., Barton P. G. Biochemistry of blood platelets. Interaction of activated factor X with platelets. Biochemistry. 1971 Feb 2;10(3):428–434. doi: 10.1021/bi00779a012. [DOI] [PubMed] [Google Scholar]
- LEWIS J. H., FERGUSON J. H. Hypoproaccelerinemia. Blood. 1955 Apr;10(4):351–356. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MILSTONE J. H. THROMBOKINASE AS PRIME ACTIVATOR OF PROTHROMBIN: HISTORICAL PERSPECTIVES AND PRESENT STATUS. Fed Proc. 1964 Jul-Aug;23:742–748. [PubMed] [Google Scholar]
- Marcus A. J. Recent advances in platelet lipid metabolism research. Ann N Y Acad Sci. 1972 Oct 27;201:102–108. doi: 10.1111/j.1749-6632.1972.tb16291.x. [DOI] [PubMed] [Google Scholar]
- Marcus A. J., Zucker-Franklin D., Safier L. B., Ullman H. L. Studies on human platelet granules and membranes. J Clin Invest. 1966 Jan;45(1):14–28. doi: 10.1172/JCI105318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B. M., Wasiewski W. W., Fenton J. W., 2nd, Detwiler T. C. Equilibrium binding of thrombin to platelets. Biochemistry. 1976 Nov 2;15(22):4886–4893. doi: 10.1021/bi00667a021. [DOI] [PubMed] [Google Scholar]
- Osterud B., Rapaport S. I., Lavine K. K. Factor V activity of platelets: evidence for an activated factor V molecule and for a platelet activator. Blood. 1977 May;49(5):819–834. [PubMed] [Google Scholar]
- PAPAHADJOPOULOS D., HANAHAN D. J. OBSERVATIONS ON THE INTERACTION OF PHOSPHOLIPIDS AND CERTAIN CLOTTING FACTORS IN PROTHROMBIN ACTIVATOR FORMATION. Biochim Biophys Acta. 1964 Aug 19;90:436–439. doi: 10.1016/0304-4165(64)90220-x. [DOI] [PubMed] [Google Scholar]
- Shuman M. A., Majerus P. W. The perturbation of thrombin binding to human platelets by anions. J Clin Invest. 1975 Oct;56(4):945–950. doi: 10.1172/JCI108174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C. C., Cramer S. F., Steward P. G. The response of human peripheral blood lymphocytes to phytohemagglutinin: determination of cell numbers. Cell Immunol. 1975 Apr;16(2):237–250. doi: 10.1016/0008-8749(75)90115-x. [DOI] [PubMed] [Google Scholar]
- Suttie J. W., Jackson C. M. Prothrombin structure, activation, and biosynthesis. Physiol Rev. 1977 Jan;57(1):1–70. doi: 10.1152/physrev.1977.57.1.1. [DOI] [PubMed] [Google Scholar]
- Tollefsen D. M., Feagler J. R., Majerus P. W. Induction of the platelet release reaction by phytohemagglutinin. J Clin Invest. 1974 Jan;53(1):211–218. doi: 10.1172/JCI107540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollefsen D. M., Feagler J. R., Majerus P. W. The binding of thrombin to the surface of human platelets. J Biol Chem. 1974 Apr 25;249(8):2646–2651. [PubMed] [Google Scholar]
- WROBLEWSKI F., LADUE J. S. Lactic dehydrogenase activity in blood. Proc Soc Exp Biol Med. 1955 Oct;90(1):210–213. doi: 10.3181/00379727-90-21985. [DOI] [PubMed] [Google Scholar]