Abstract
Muscular dystrophies are a group of heterogeneous genetic disorders that cause progressive muscle weakness and wasting, dilated cardiomyopathy and early mortality. There are different types of muscular dystrophies with varying etiologies but they all have a common hallmark of myofiber degeneration, atrophy and decreased mobility. Mutation in sarcoglycan-delta (Sgcd), a subunit of dystrophin glycoprotein complex, causes Limb Girdle Muscular Dystrophy 2F (LGMD2F). Previously we have reported that Sgcd deficient (Sgcd−/−) mice exhibit angiotensin II (Ang II) induced autonomic and skeletal muscle dysfunction at a young age which contributes to onset of dilated cardiomyopathy and mortality at older ages. Two counter-regulatory renin angiotensin system (RAS) pathways have been identified – deleterious actions of angiotensin II (Ang II) acting on type 1 receptor (AT1R) versus protective actions of Ang-(1-7) acting on Mas receptors. We propose that the balance between the Ang II/AT1R and Ang-(1-7)/Mas axes is disturbed in Sgcd−/− mice. Control C57BL/6 and Sgcd−/− mice were treated with Ang-(1-7) included in hydroxypropyl β-cyclodextrin (drinking water) for 8–9 weeks beginning at 3 weeks of age. Ang-(1-7) treatment restored Ang II/AT1R vs. Ang-(1-7)/Mas balance, decreased oxidative stress and fibrosis in skeletal muscle, increased locomotor activity, and prevented autonomic dysfunction without lowering blood pressure in Sgcd−/− mice. Our results suggest that correcting the early autonomic dysregulation by administering Ang(1-7) or enhancing its endogenous production may provide a novel therapeutic approach in muscular dystrophy.
Keywords: sarcoglycans, renin angiotensin system, dystrophic glycoprotein complex, autonomic regulation, neuromuscular disorders, ACE/AngII/AT1R axis, ACE2/Ang1-7/Mas axis
INTRODUCTION
Sarcoglycans are members of the dystrophin glycoprotein complex (DGC) found in the sarcolemma that are important in maintaining integrity of the muscle against forces generated during contractions [1, 2]. Mutation of sarcoglycan-delta (Sgcd) causes limb girdle muscular dystrophy 2F (LGMD2F), characterized by progressive wasting of muscle and weakness, and dilated cardiomyopathy (DCM) in both humans and animals [3].
There is evidence of aberrant neurohumoral activation in muscular dystrophy [4, 5]. Amongst the numerous neurohumoral systems involved in cardiovascular homeostasis, the reninangiotensin system (RAS) is of paramount importance. Angiotensin converting enzyme (ACE) converts angiotensin I to angiotensin II (Ang II) that acts via angiotensin type 1 (AT1R) and type 2 (AT2R) receptors. Pathogenesis of a disease state due to the actions of RAS is primarily mediated by the Ang II/AT1R axis [6]. A powerful counter-regulatory RAS axis has been described recently [7, 8]. Ang II is cleaved by angiotensin converting enzyme 2 (ACE2) to form the heptapeptide angiotensin-(1-7) (Ang-(1-7)) that acts via the Mas receptors (Mas). Ang-(1-7) is an endogenous ligand for Mas [9]. Ang-(1-7) has been demonstrated to possess anti-fibrotic, anti-remodeling, antioxidant, sympathoinhibitory and vasodilatory properties [10, 11].
Recently, we demonstrated that Sgcd−/− mice exhibit severe autonomic and skeletal muscle dysfunction at a young age that worsens with aging, and contributes to DCM and early mortality (12, 13). This early-age dysregulation in Sgcd−/− mice is mediated by deleterious actions of Ang II binding to AT1R; the dysregulation was essentially abolished by chronic treatment with the AT1R antagonist losartan (13). Expression of AT1R and ACE activity are augmented in muscular dystrophy [13–15]. Activation of the ACE/Ang II/AT1R axis induces skeletal muscle fibrosis in muscular dystrophy by increasing transforming growth factor type β 1 (TGFβ1) and connective tissue growth factor [15, 16].
Most current therapies and translational approaches have focused on reversing skeletal muscle fibrosis and pathology, with relatively little attention to correcting the impaired autonomic regulation in muscular dystrophy. In this study, we hypothesized that chronic administration of Ang-(1-7) will counterbalance the deleterious actions of Ang II to prevent autonomic and skeletal muscle dysfunction in young Sgcd−/− mice.
MATERIALS AND METHODS
Animals
Experiments were performed on age-matched young (10-13 wks) control C57BL/6 and Sgcd−/− mice. Generation of homozygous Sgcd−/− mice has been described previously [2]. The mice were maintained in a 12:12 hr light-dark cycle (6:00 AM to 6:00 PM), fed normal mouse chow, and had access to water ad libitum. All procedures were performed in accordance with American Physiological Society and institutional guidelines.
Experimental Groups
At 3 weeks of age, control and Sgcd−/− mice pups were randomly assigned into four groups - untreated control, Ang-(1-7)-treated control, untreated Sgcd−/− and Ang-(1-7)-treated Sgcd−/− mice. The number of male and female mice in each group was balanced. Ang-(1-7) was included in hydroxypropyl β-cyclodextrin (CD-Ang-(1-7)) to ensure stability of the drug [17]. The formulation was administered in the drinking water (10 µg/ml) to the treated groups beginning at 3 weeks of age for 8–9 weeks.
Assessment of Blood Pressure, Heart Rate, Autonomic Regulation and Locomotor Activity
At 8–9 weeks of age, a radiotelemetry probe (PC10, DSI) was chronically implanted into the thoracic aorta via the left common carotid artery in mice anesthetized with ketamine and xylazine (91 µg/g and 9.1 µg/g, respectively, IP) as described previously [18, 19]. After one week of recovery from the implantation surgery, arterial blood pressure (BP), heart rate (HR), and locomotor activity were measured over three days using Dataquest ART Acquisition software [18, 19]. HR was derived from measurements of the arterial pulse intervals. In addition, BP was recorded continuously (2000 Hz) for one hour to collect beat-to-beat data for assessment of spontaneous baroreflex sensitivity (BRS), cardiac vagal and sympathetic tone, and vasomotor sympathetic tone [18, 19]. Resting cardiac vagal and sympathetic tones were measured as changes in HR in response to the muscarinic cholinergic receptor blocker methylatropine (1 mg/kg, IP; Sigma) and the β-adrenergic receptor blocker propranolol (1 mg/ kg, IP; Sigma), respectively, while resting vasomotor sympathetic tone was measured as the change in mean BP in response to the ganglionic blocker chlorisondamine (12 µg/g, IP; Tocris) [18, 19]. Responses to pharmacological drugs were measured when mice were inactive, as determined by the locomotor activity trace. BRS was calculated from spontaneous fluctuations in systolic BP and HR measured when the mice were active using the sequence technique [18, 19]. Measurements of spontaneous locomotor activity were derived from the changes in transmitter signal strength associated with movement of the mouse. All these measurements were made in untreated and Ang-(1-7)-treated control and Sgcd−/− mice at a young (10–13 weeks) age.
Measurement of Gene Expression, Fibrosis and Oxidative Stress in Skeletal Muscle
At the end of the study mice were anesthetized with sodium pentobarbital (1.5 mg/10 g of body weight, IP) to prepare skeletal muscle samples (quadriceps). 50% of the mice were perfused with 15 ml of PBS and zinc formalin fixative solution for embedding tissues in paraffin, while frozen muscle samples were prepared from the remaining mice.
(i) Fibrosis
Paraffin blocks of quadriceps were cut into 7 µm sections and stained with freshly prepared Masson’s Trichrome. Masson Trichrome stains nuclei black, cytoplasm in muscle fibers red, and collagen blue. The stained sections were imaged with an Olympus BX-51 Light Microscope equipped with a DP-71 digital camera. The images were printed onto paper and overlaid with a transparency imprinted with a counting grid containing points evenly spaced according to the rules outlined previously [20]. The space between these course points was used to calculate area of fibrosis. This was done for each section stained. Six to eight sections from each mouse were analyzed, and the average percent fibrosis was calculated for each mouse. In addition, muscle sections were stained with primary antibodies for collagen III (Abcam #ab7778) or fibronectin (Abcam #ab6328) and hematoxylin, and followed by detection with chromagen anti-Mouse HRP/DAB kit (Vector #SK4100). The reagents in the HRP/DAB (horseradish peroxidase/3,3’-diaminobenzidine) kit constitute a labeled streptavidin-biotin immunoenzymatic antigen detection system that involves sequential incubation of the specimen with primary antibody specific to the target antigen, a biotinylated secondary antibody, enzyme-labeled streptavidin, and substrate-chromogen. The enzymatic reaction produces a dark brown color. The stained sections were imaged with an Olympus BX-51 Light Microscope equipped with a DP-71 digital camera.
(ii) Immunofluorescence
For immunostaining, the tissues were embedded with OCT compound and rapidly frozen in isopropanol-chilled liquid nitrogen. They were stored at −80°C until 7 µm sections were made using a cryostat. The primary antibody for detection of AT1R-immunoreactivity was a rabbit polyclonal antibody to the AT1R (1:100 dilution; catalog no. sc-1173, Santa Cruz). Mas-immunoreactivity was detected using anti-Mas antibody (1:60 dilution; AAR-013 Alomone Labs). The sections were incubated with a secondary antibody, Alexa Fluor 488 goat, anti-rabbit IgG and DAPI to counterstain cell nuclei. Immunostaining was visualized with a confocal microscope (Zeiss 710).
(iii) Oxidative Stress
Superoxide (O2•−) levels measured by dihydroethidium (DHE, Sigma) fluorescence were used as a marker of oxidative stress in skeletal muscle. DHE stock solution (20 nM) was prepared by dissolving DHE (catalog D1168, Invitrogen) in dimethyl-sulphoxide (DMSO) under argon vapor. 8 µl of the DHE stock solution was diluted into 20 ml of PBS which was then applied onto quadriceps sections for 15 min at 37°C and protected from light. Images were captured on a confocal microscope (BioRad 1024) at excitation 488 nm and emission wavelength of 568 nm. An average of six (7 µm) sections of skeletal muscle were obtained from each mouse. Intensity of fluorescence was quantified using NIH ImageJ software and normalized to a percentage of the average fluorescence intensity measured in untreated control mice.
(iv) Western Blot
For Western blot analysis of AT1R and Mas protein expression, quadriceps muscles were directly snap frozen without OCT and stored at −80°C. The samples were subsequently ground on a mortar and pestle. The tissue was added to 500 ul of homogenization buffer (100 mM Hepes, 320 mM sucrose, 1 mM PMSF with 1% protein inhibitor cocktail (Sigma P8340) and given twenty strokes in a glass dounce homogenizer. The sample was spun at 4000 g for 10 min. The supernatant was removed, and the tissue was resuspended in 500 ul of buffer and spun again. The pooled supernatants were spun at 100,000g for 60 minutes at 4 degrees C. The supernatant was removed and the separated membrane fraction was suspended in 250 ul of homogenization buffer. Protein content was determined using a Bradford protein assay (Bio-Rad). The membranes were blocked in Tris-Buffered Saline containing 0.1 % Tween-20 (TBS-T) with 5 % non-fat dried milk for 3 hours at room temperature, then incubated overnight at 4 degrees C in anti-AT1R antibody (sc-1173, Santa Cruz) diluted 1:5000 in TBS-T with 3 % BSA. For Mas receptors, we used anti-Ang-(1-7) Mas receptor antibody (AAR-013, Alomone Labs) diluted 1:7500 in TBS-T with 3 % BSA. The membranes were rinsed and then incubated in peroxidase-labeled goat-anti-rabbit IgG (Jackson) diluted 1:120,000 in TBS-T. The bands were visualized with an ECL reagent (Amersham). The membranes were then stripped, blocked, and incubated with peroxidase-labeled anti-GAPDH (NB-110, Novus Bio) diluted 1:250,000 in TBS-T with 3 % BSA, and visualized with ECL as a loading control. The films were scanned and analyzed using NIH ImageJ software.
Data Analysis
The results are expressed as means ± SEM. Significant differences were defined at P < 0.05. Statistical evaluation was performed using unpaired t-test to compare between experimental groups (StatView SAS Institute, Cary, NC).
RESULTS
CD-Ang-(1-7) Prevents Autonomic Dysfunction and Increases Locomotor Activity in Sgcd−/− Mice without Lowering Blood Pressure
Mean arterial pressure (MAP, 24 hr avg) was significantly lower in Sgcd−/− mice compared with control mice, whereas mean HR did not differ between the genotypes (Fig 1A, 1B). Sgcd−/− mice exhibited significant decreases in spontaneous BRS (Fig 1C) and vagal tone (Fig 1D), and an increase in sympathetic vasomotor tone (Fig 1F). The increase in cardiac sympathetic tone did not reach statistical significance (Fig 1E). Locomotor activity was markedly decreased in Sgcd−/− mice (Fig 1G). We have reported similar findings in our earlier studies of young Sgcd−/− mice (12, 13).
Figure 1. Blood pressure, HR autonomic indices and locomotor activity in untreated and CD-Ang-(1-7) treated control and Sgcd−/− mice.
Sgcd−/− mice exhibited lower mean blood pressure and locomotor activity, and severe autonomic dysfunction at a young age compared with control mice. CD-Ang-(1-7) prevented the autonomic dysfunction, reduced heart rate without further lowering blood pressure, and markedly improved locomotor activity in Sgcd−/− mice. Results from untreated control (n=5, black bars), treated control (n=6, gray bars), untreated Sgcd−/− (n=7, black bars), and treated Sgcd−/− (n=8, gray bars) mice are shown. *P < 0.05, Sgcd−/− vs. C57BL/6 mice; †P < 0.05, vs. untreated mice (unpaired t-test). Panel A. 24 hr average of mean blood pressure. Panel B. 24 hr average of heart rate. Panel C. Baroreflex Sensitivity. Panel D. Cardiac vagal tone measured as change in heart rate in response to methylatropine. Panel E. Cardiac sympathetic tone measured as a change in heart rate in response to propranolol. Panel F. Vasomotor sympathetic tone measured as a change in blood pressure in response to chlorisondamine. Panel G. 24 hr average of spontaneous locomotor activity.
Oral administration of CD-Ang-(1-7) for 8–9 wks reduced HR, increased BRS and cardiac vagal tone, and decreased cardiac and vasomotor sympathetic tone without further lowering MAP in Sgcd−/− mice (Fig 1A-1F). CD-Ang-(1-7) also increased locomotor activity in Sgcd−/− mice (Fig 1G). Each of these measurements in treated Sgcd−/− mice were restored to levels not different than those measured in control mice (Figs 1B-1G). The beneficial effects of CD-Ang- (1-7) were selective in that the treatment had essentially no effect on these variables in control mice (Figs 1A-1G).
CD-Ang-(1-7) Decreases Fibrosis and Attenuates Oxidative Stress in Skeletal Muscle of Sgcd−/− Mice
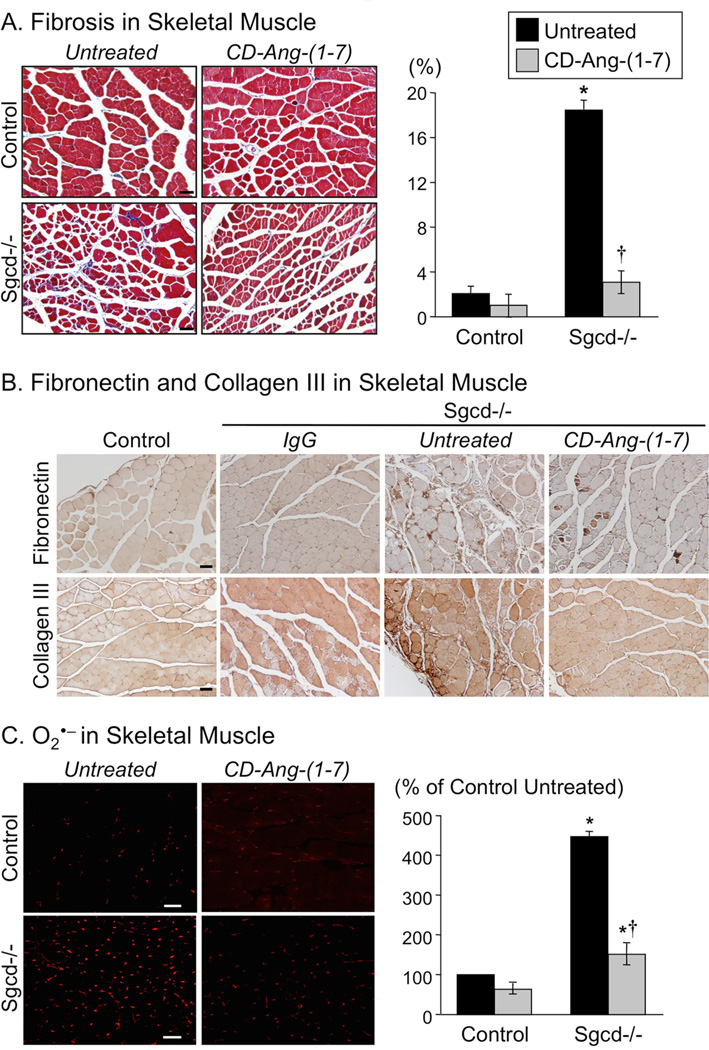
Skeletal muscle fibrosis and oxidative stress are hallmark pathological features in muscular dystrophy [16, 21, 22, 23]. As expected, total fibrosis, collagen III, fibronectin and superoxide levels were elevated in skeletal muscle of Sgcd−/− mice (Figs 2A-2C). Treatment with CD-Ang- (1-7) markedly reduced fibrosis and superoxide in Sgcd−/− mice to levels near those observed in control mice (Figs 2A-2C). CD-Ang-(1-7) treatment had essentially no effect on fibrosis or superoxide in control mice (Figs 2A-2C).
Figure 2. Fibrosis, fibronectin, collagen III, and superoxide (O2•−) in skeletal muscle of untreated and Ang-(1-7)-treated control and Sgcd−/− mice.
Panel A. Left: Fibrosis indicated by blue staining (Masson Trichrome) in tissue sections of quadriceps muscle from control and Sgcd−/− mice (magnification 20X; scale bar 50 microns). Right: Fibrosis is expressed as a percentage of the total area examined in quadriceps from untreated control (n=6), treated control (n=5), untreated Sgcd−/− (n=9), and treated Sgcd−/− (n=5) mice. Panel B. Fibronectin and collagen III indicated by dark brown color (HRP/DAB kit) in quadriceps skeletal muscle from control and Sgcd−/− mice (magnification 20X; scale bar 50 microns). A negative control is shown for tissue of Sgcd−/− mouse exposed to secondary antibody (IgG). Similar results of fibronectin and collagen III staining were obtained from other untreated control (n=4), treated control (n=4), untreated Sgcd−/− (n=5), and treated Sgcd−/−(n=4) mice. Panel C. Left: O2•− indicated by dihydroethidium (DHE) fluorescence (red) in 7µm sections of quadriceps muscle from control and Sgcd−/− mice (magnification, 20X; scale bar, 50 microns). Right: Quantification of O2•− in muscle from untreated control (n=5), treated control (n=4), untreated Sgcd−/− (n=4), and treated Sgcd−/− (n=4) mice. Fluorescence is expressed as a percentage of the mean level of fluorescence measured in muscle from untreated control mice. CD-Ang-(1-7) markedly attenuated fibrosis, fibronectin, collagen III and O2•− levels in Sgcd−/− mice, with no effect on either measurement in control mice. Data in this figure are from untreated (black bars), and CD-Ang-(1-7)-treated (gray bars) groups. *P < 0.05, Sgcd−/− vs. control mice; †P < 0.05, vs. untreated mice (unpaired t-test).
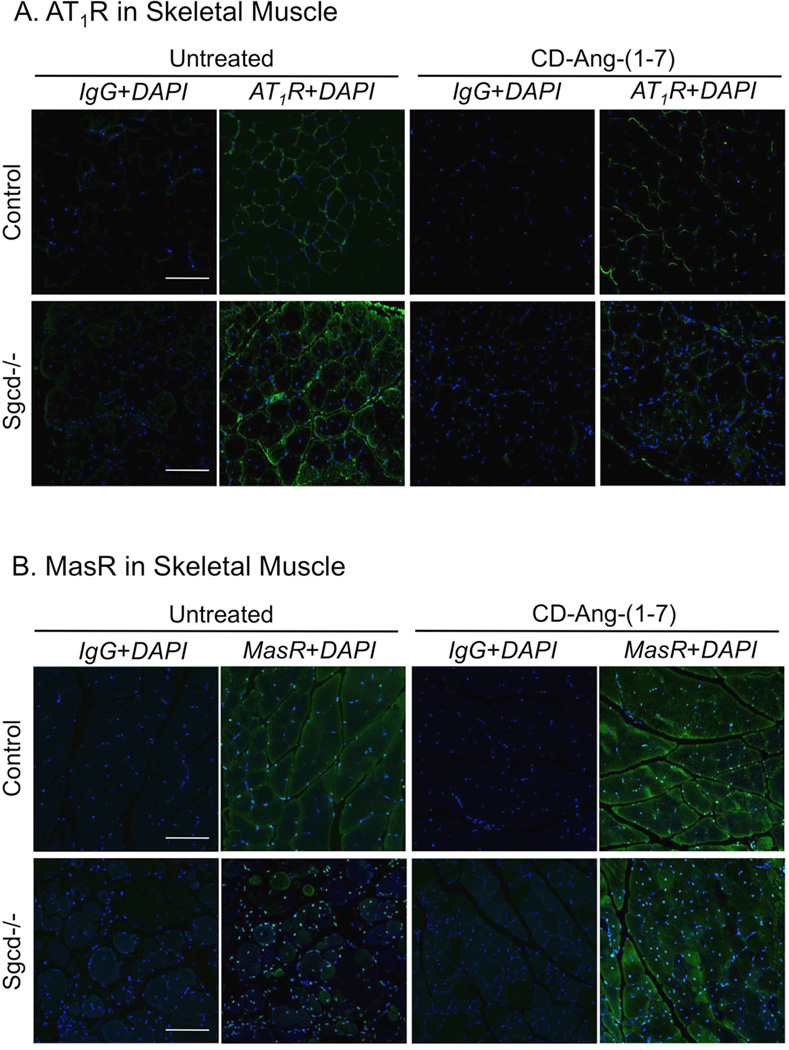
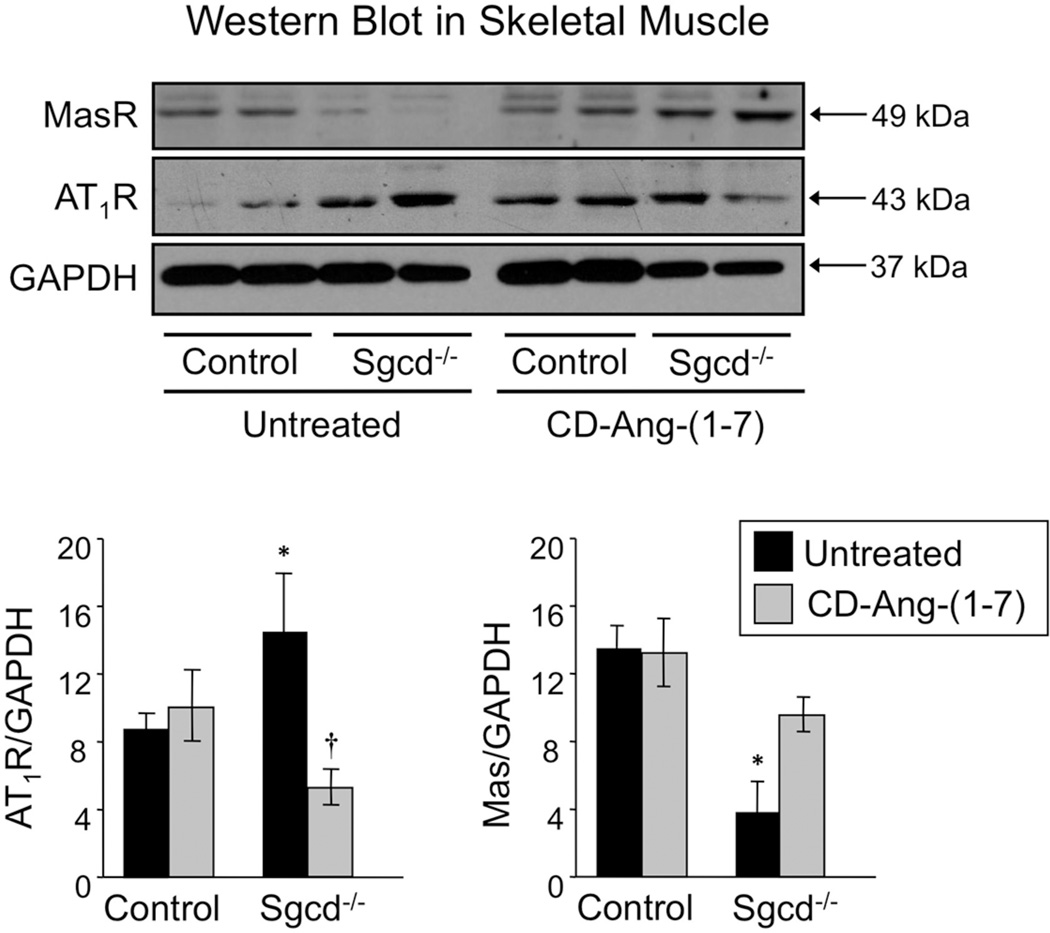
CD-Ang-(1-7) Alters AT1R and Mas Protein Expression in Dystrophic Skeletal Muscle
Ang II binding to AT1R generates superoxide and upregulates AT1R expression in states of chronic RAS activation [24, 25]. As we predicted, AT1R expression measured by Western blot and immunofluorescence was significantly increased in quadriceps muscle of Sgcd−/− mice, while Mas expression was significantly reduced (Figs 3, 4). Treatment of Sgcd−/− mice with CDAng-(1-7) abolished these differences (Figs 3, 4). CD-Ang-(1-7) did not alter the expression of AT1R or Mas in control mice (Fig 3, 4). AT1R immunofluorescence in skeletal muscle averaged 15 ± 3, 13 ± 2, 25 ± 4, and 15 ± 3 units in untreated C57BL/6, treated C57BL/6, untreated Sgcd- /-, and treated Sgcd−/− mice, respectively. Conversely, Mas immunofluorescence averaged 10 ± 4, 14 ± 5, 3 ± 1, and 14 ± 3 units in these same groups of mice.
Figure 3. Expression of AT1R and Mas receptors in skeletal muscle of untreated and CD-Ang-(1-7)-treated control and Sgcd−/− mice.
Panel A. Immunofluorescence showing increased AT1R-immunoreactivity in quadriceps muscle of untreated Sgcd−/− vs. control mice, but normal expression in CD-Ang-(1-7)-treated Sgcd−/− mice. Confocal image of muscle with AT1R staining in green (magnification, 20X; scale bar, 100 microns). Panel B. Immunofluorescence showing reduced Mas-immunoreactivity in quadriceps muscle of untreated Sgcd−/− vs. control mice, but normal expression in Ang-(1-7)-treated Sgcd−/− mice. Confocal images of muscle with Mas staining in green and nuclei staining in blue (magnification, 20X; scale bar, 100 microns). A negative control is shown for quadriceps muscle exposed to secondary antibody (IgG) and nuclear stain DAPI (4',6-diamidino-2-phenylindole) in control and Sgcd−/− mice.
Figure 4. Western Blot Analysis of AT1R and Mas receptors in skeletal muscle of untreated and CD-Ang-(1-7)-treated control and Sgcd−/− mice.
Western blots showing increased AT1R and reduced Mas protein expression in skeletal muscle (quadriceps) of untreated Sgcd−/− vs. control mice. CD-Ang-(1-7) treatment increased Mas and reduced AT1R expression in Sgcd−/− mice. Bottom: Group data obtained from untreated control (n=4, black bar), treated control (n=6, gray), untreated Sgcd−/− (n=4, black) and treated Sgcd−/− (n=6, gray) mice. *P < 0.05, Sgcd−/− vs. control mice; †P < 0.05, vs. untreated mice (unpaired t-test).
DISCUSSION
Muscular dystrophies are a group of heterogeneous genetic disorders that cause progressive muscle weakness and wasting, cardiac and respiratory abnormalities, loss of mobility and early mortality. Although each of the individual muscular dystrophies is relatively rare, they collectively affect millions of people worldwide and share a synergistic, pathological pathway of myofiber degeneration and atrophy. There is no cure for muscular dystrophy and current therapies are limited.
The major findings of the present study are that treatment of Sgcd−/− mice with CD-Ang-(1-7): (1) Markedly reduces fibrosis and oxidative stress in skeletal muscle accompanied by restoration of the balance between AT1R and Mas expression; (2) Reverses autonomic dysregulation, i.e., increases cardiac vagal tone and BRS, and decreases cardiac and vasomotor sympathetic tone; and (3) Restores spontaneous locomotor activity to levels similar to that measured in control mice. The results suggest that these beneficial effects of Ang-(1-7) are mediated at least in part by inhibition of the Ang II/AT1R axis.
The importance of activation of skeletal muscle RAS and autonomic dysregulation in the pathogenesis of multiple types of muscular dystrophy is now widely recognized [4, 5, 14–16, 27–28]. We recently demonstrated that Sgcd−/− mice exhibit skeletal muscle RAS activation and severe autonomic dysregulation at a young age before onset of cardiac dysfunction [12, 13], results that were confirmed in the present study. In addition, we showed that levels of Ang II are increased in both skeletal muscle and plasma in young Sgcd−/− mice [13]. The increased circulating levels of Ang II may contribute to the autonomic dysregulation. Ang II exerts actions at multiple central and peripheral nervous system sites that facilitate an increase in sympathetic tone and decreases in vagal tone and BRS [18, 30–32].
In addition, oxidative stress, acidic pH and/or inflammation in dystrophic muscle may cause autonomic dysregulation by activating sensory nerves in muscle and enhancing sympathoexcitatory reflexes [33–36]. AT1Rs are expressed in sensory nerve terminals in muscle [37, 38]. Furthermore, AT1Rs may function as mechanosensors and contribute to activation of mechanosensitive nerve terminals [39]. Ang II/AT1R promotes fibrosis by increasing extracellular proteins collagen III and fibronectin, oxidative stress and TGFβ expression in the skeletal muscles [25].
Deleterious effects of activation of Ang II/AT1R are countered by protective effects mediated by the Ang-(1-7)/Mas axis in a variety of pathological states [7, 8, 31,40–43]. We found that oral administration of Ang-(1-7) encapsulated in hydroxypropyl β-cyclodextrin to Sgcd−/− mice for 8–9 wks significantly reduced fibrosis and oxidative stress in skeletal muscle, improved autonomic regulation (increased BRS and cardiac vagal tone, decreased sympathetic tone and HR), and normalized locomotor activity. Importantly, these beneficial effects of Ang-(1-7) were achieved through oral administration and occurred at a dose that did not lower MAP. The use of β- cyclodextrin to facilitate absorption and the half-life of Ang-(1-7) is of key importance to the effectiveness of the compound administered orally [17], and increases the potential to translate this therapy to patients.
As Ang-(1-7) was administered systemically in vivo, the cellular and molecular targets responsible for mediating its beneficial effects on skeletal muscle, autonomic regulation and locomotor activity in Sgcd−/− mice remain to be determined. Ang-(1-7) has been shown to exert protective effects in numerous organs/tissues including blood vessels, heart, brain, lungs, liver, kidneys, adipose tissue and skeletal muscle [7, 8, 40–42]. In an elegant study published recently, Acuna et al. showed that CD-Ang-(1-7) restored muscle strength and reduced fibrosis by inhibiting TGF-β signaling in the gastronemius, tibialis anterior, and diaphragm skeletal muscles of mdx mice, a model of Duchenne muscular dystrophy [44]. Ang-(1-7) has also been shown to improve insulin sensitivity in skeletal muscle of fructose-fed rats, a model of metabolic syndrome, and to attenuate Ang II-induced inhibition of insulin signaling in a Mas receptor-dependent manner, both in vivo and in vitro [45, 46]. Chronic treatment with Ang-(1-7) has been reported to decrease plasma renin activity and plasma aldosterone concentration in fructose-fed rats [47]. Such an effect could contribute to changes in autonomic regulation and control of body fluid balance. We suspect that Ang-(1-7) may exert protective actions at multiple sites including those within skeletal muscle and the brain, reflecting the numerous, documented sites of actions of Ang II and Ang-(1-7) that mediate their effects on skeletal muscle, blood vessels, heart and the autonomic nervous system [8, 11, 41, 42, 47–50]. Future studies are needed to determine the key sites of action of Ang-(1-7) in mediating its protective effects in muscular dystrophy.
A chronic peptide imbalance in which the Ang II/AT1R axis is increased and the Ang-(1- 7)/Mas axis is decreased is evident in aging, hypertension, and other pathological states [32, 43]. These findings predict that a balance between Ang II/AT1R and Ang-(1-7)/Mas is essential for normal organ system function. The decreased expression of Mas and increased expression of AT1R in skeletal muscle of untreated Sgcd−/− mice along with restoration of the peptide balance after treatment with Ang-(1-7) support this concept.
Previously, we reported that the degree of autonomic dysfunction in Sgcd−/− mice at a young age predicts the severity of cardiac dysfunction and mortality at a later age [12]. We now speculate that improving autonomic and skeletal muscle function at a young age by treatment of Sgcd−/− mice with Ang-(1-7) will delay the onset of DCM and prolong lifespan.
CLINICAL PERSPECTIVES.
Muscular dystrophy is a catastrophic disease that is in need of new therapies to delay pathogenicity of disease and improve quality of life in the afflicted population. In the present study, we show that Sgcd−/− mice (model of LGMD2F) exhibit severe autonomic and skeletal muscle dysfunction at a young age. Chronic administration of Ang-(1-7) in Sgcd−/− mice (before onset of cardiac dysfunction) restores autonomic function, increases locomotor activity, and attenuates skeletal muscle fibrosis and oxidative stress. These protective benefits arise from restoration of balance between the increased Ang II/AT1R and reduced Ang-(1-7)/Mas axes. Thus, our results provide strong support for the use of Ang-(1-7) or enhancement of its endogenous production as a novel therapeutic approach to correct the early autonomic dysfunction and delay the pathological consequences in muscular dystrophy.
SUMMARY STATEMENT.
Muscular dystrophy is a devastating disease that causes muscular weakness, heart problems and early mortality. Currently, there is no cure for it. Our study provides strong evidence for a novel therapeutic target that can delay pathogenesis of this disease.
ACKNOWLEDGEMENTS
We acknowledge members of the Central Microscopy Research Facility, (University of Iowa) for providing assistance with histology and immunohistochemistry. The authors also thank Dr. Kevin Campbell (University of Iowa) for providing the breeding pairs of Sgcd−/− mice.
FUNDING
This project was funded in part by the NIH (HL14388) and the US Department of Veterans Affairs (1 I01 BX001414).
ABBREVIATIONS FOOTNOTE
- LGMD2F
Limb girdle muscular dystrophy-2F
- Sgcd
sarcoglycan delta
- Ang II
angiotensin II
- AT1R
angiotensin II type 1 receptor
- AT2R
angiotensin II type 2 receptor
- Ang-(1-7)
angiotensin-(1-7)
- Mas
Mas receptor
- ACE2
angiotensin converting enzyme 2
- ACE
angiotensin converting enzyme
- TGFβ
transforming growth factor beta
- BP
blood pressure
- MAP
mean arterial pressure
- HR
heart rate
- BRS
baroreflex sensitivity
- HRP
horseradish peroxidase
- DAB
3,3’-diaminobenzidine
- O2•−
superoxide
- DAPI
4',6-diamidino-2-phenylindole
- DHE
dihydroethidium
Footnotes
AUTHOR CONTRIBUTIONS
Rasna Sabharwal conceived and designed the study, performed the experiments, analyzed the data, interpreted the results and wrote the paper. Michael Cicha carried out Western blot analysis. Robson Santos and collaborators from UFMG provided the CD-Ang-(1-7) compound used in this study. Mark Chapleau was involved in interpretation of results and wrote the paper. All authors have read and approved the final paper.
DISCLOSURES
Drs. Sabharwal, Santos and Chapleau are collaborating with Tarix Pharmaceuticals in developing novel therapies for muscular dystrophy.
REFERENCES
- 1.Heydemann A, McNally EM. Consequences of disrupting the dystrophin-sarcoglycan complex in cardiac and skeletal myopathy. Trends. Cardiovasc. Med. 2007;17(2):55–59. doi: 10.1016/j.tcm.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Coral-Vazquez R, Cohn RD, Moore SA, Hill JA, Weiss RM, Davisson RL, Straub V, Barresi R, Bansal D, Hrstka RF, Williamson R, Campbell KP. Disruption of the sarcoglycan-sarcospan complex in vascular smooth muscle: a novel mechanism for cardiomyopathy and muscular dystrophy. Cell. 1999;98:465–474. doi: 10.1016/s0092-8674(00)81975-3. [DOI] [PubMed] [Google Scholar]
- 3.Lapidos KA, Kakkar R, McNally EM. The dystrophin glycoprotein complex: signaling strength and integrity for the sarcolemma. Circ. Res. 2004;94(8):1023–1031. doi: 10.1161/01.RES.0000126574.61061.25. [DOI] [PubMed] [Google Scholar]
- 4.Politano L, Palladino A, Nigro G, Scutifero M, Cozza V. Usefulness of heart rate variability as a predictor of sudden cardiac death in muscular dystrophies. Acta. Myol. 2008;27:114–122. [PMC free article] [PubMed] [Google Scholar]
- 5.Inoue M, Mori K, Hayabuchi Y, Tatara K, Kagami S. Autonomic function in patients with Duchenne muscular dystrophy. Pediatr. Int. 2009;51:33–40. doi: 10.1111/j.1442-200X.2008.02656.x. [DOI] [PubMed] [Google Scholar]
- 6.Matsusaka T, Ichikawa I. Biological functions of angiotensin and its receptors. Annu. Rev. Physiol. 1997;59:395–412. doi: 10.1146/annurev.physiol.59.1.395. [DOI] [PubMed] [Google Scholar]
- 7.Ferreira AJ, Santos RA, Bradford CN, Mecca AP, Sumners C, Katovich MJ, Raizada MK. Therapeutic implications of the vasoprotective axis of the renin-angiotensin system in cardiovascular diseases. Hypertension. 2010;55:207–213. doi: 10.1161/HYPERTENSIONAHA.109.140145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferreira AJ, Santos RA. Cardiovascular actions of angiotensin-(1–7) Braz. J. Med. Biol. Res. 2005;38:499–507. doi: 10.1590/s0100-879x2005000400003. [DOI] [PubMed] [Google Scholar]
- 9.Santos RA, Simoes e Silva AC, Maric C, Silva DM, Machado RP, de Buhr I, Heringer-Walther S, Pinheiro SV, Lopes MT, Bader M, Mendes EP, Lemos VS, Campagnole-Santos MJ, Schultheiss HP, Speth R, Walther T. Angiotensin-(1–7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci U S A. 2003;100:8258–8263. doi: 10.1073/pnas.1432869100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrario CM, Chappell MC, Tallant EA, Brosnihan KB, Diz DI. Counterregulatory actions of angiotensin-(1–7) Hypertension. 1997;30:535–541. doi: 10.1161/01.hyp.30.3.535. [DOI] [PubMed] [Google Scholar]
- 11.Santos RA, Ferreira AJ, Simoes ESAC. Recent advances in the angiotensin-converting enzyme 2-angiotensin(1–7)-Mas axis. Exp. Physiol. 2008;93:519–527. doi: 10.1113/expphysiol.2008.042002. [DOI] [PubMed] [Google Scholar]
- 12.Sabharwal R, Weiss RM, Chapleau MW. Dysautonomia precedes cardiomyopathy in a mouse model of muscular dystrophy. Clin. Auton. Res. (abstract) 2010;20:51–52. [Google Scholar]
- 13.Sabharwal R, Weiss RM, Zimmerman K, Chapleau MW. Angiotensin II contributes to skeletal muscle fibrosis, reduced locomotor activity and autonomic dysfunction in δ-sarcoglycan deficient mice with muscular dystrophy. Hypertension. (abstract) 2010;56:E103. [Google Scholar]
- 14.Sun G, Haginoya K, Dai H, Chiba Y, Uematsu M, Hino-Fukuyo N, Onuma A, Iinuma K, Tsuchiya S. Intramuscular renin-angiotensin system is activated in human muscular dystrophy. J. Neurol. Sci. 2009;280:40–48. doi: 10.1016/j.jns.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 15.Cabello-Verrugio C, Morales MG, Cabrera D, Vio CP, Brandan E. Angiotensin II receptor type 1 blockade decreases CTGF/CCN2-mediated damage and fibrosis in normal and dystrophic skeletal muscles. J. Cell. Mol. Med. 2012;16:752–764. doi: 10.1111/j.1582-4934.2011.01354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohn RD, van Erp C, Habashi JP, Soleimani AA, Klein EC, Lisi MT, Gamradt M, ap Rhys CM, Holm TM, Loeys BL, Ramirez F, Judge DP, Ward CW, Dietz HC. Angiotensin II type 1 receptor blockade attenuates TGFbeta-induced failure of muscle regeneration in multiple myopathic states. Nat. Med. 2007;13:204–210. doi: 10.1038/nm1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lula I, Denadai AL, Resende JM, de Sousa FB, de Lima GF, Pilo-Veloso D, Heine T, Duarte HA, Santos RA, Sinisterra RD. Study of angiotensin-(1–7) vasoactive peptide and its beta-cyclodextrin inclusion complexes: complete sequence-specific NMR assignments and structural studies. Peptides. 2007;28:2199–2210. doi: 10.1016/j.peptides.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Sabharwal R, Zhang Z, Lu Y, Abboud FM, Russo AF, Chapleau MW. Receptor activity-modifying protein 1 increases baroreflex sensitivity and attenuates angiotensin-induced hypertension. Hypertension. 2010;55:627–635. doi: 10.1161/HYPERTENSIONAHA.109.148171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu Y, Ma X, Sabharwal R, Snitsarev V, Morgan D, Rahmouni K, Drummond HA, Whiteis CA, Costa V, Price M, Benson C, Welsh MJ, Chapleau MW, Abboud FM. The ion channel ASIC2 is required for baroreceptor and autonomic control of the circulation. Neuron. 2009;64:885–897. doi: 10.1016/j.neuron.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gundersen HJ, Jensen EB. Stereological estimation of the volumeweighted mean volume of arbitrary particles observed on random sections. J. Microsc. 1985;138:127–142. doi: 10.1111/j.1365-2818.1985.tb02607.x. [DOI] [PubMed] [Google Scholar]
- 21.Klingler W, Jurkat-Rott K, Lehmann-Horn F, Schleip R. The role of fibrosis in Duchenne muscular dystrophy. Acta. Myol. 2012;31:184–195. [PMC free article] [PubMed] [Google Scholar]
- 22.Lorts A, Schwanekamp JA, Baudino TA, McNally EM, Molkentin JD. Deletion of periostin reduces muscular dystrophy and fibrosis in mice by modulating the transforming growth factor-beta pathway. Proc. Natl. Acad. Sci. U S A. 2012;109:10978–10983. doi: 10.1073/pnas.1204708109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tidball JG, Wehling-Henricks M. The role of free radicals in the pathophysiology of muscular dystrophy. J. Appl. Physiol. 2007;102:1677–1686. doi: 10.1152/japplphysiol.01145.2006. [DOI] [PubMed] [Google Scholar]
- 24.Gao L, Wang W, Li Y, Schultz HD, Liu D, Cornish KG, Zucker IH. Sympathoexcitation by central ANG II: Roles forAT1 receptor upregulation and NAD(P)H oxidase in RVLM. Am. J. Physiol. Heart. Circ. Physiol. 2005;288:H2271–H2279. doi: 10.1152/ajpheart.00949.2004. [DOI] [PubMed] [Google Scholar]
- 25.Morales MG, Vazquez Y, Acuna MJ, Rivera JC, Simon F, Salas JD, Alvarez Ruf J, Brandan E, Cabello-Verrugio C. Angiotensin II-induced pro-fibrotic effects p38MAPK activity and transforming growth factor beta 1 expression in skeletal muscle cells. Int. J. Biochem. Cell. Biol. 2012;44(11):1993–2002. doi: 10.1016/j.biocel.2012.07.028. [DOI] [PubMed] [Google Scholar]
- 26.Thomas GD, Sander M, Lau KS, Huang PL, Stull JT, Victor RG. Impaired metabolic modulation of alpha-adrenergic vasoconstriction in dystrophindeficient skeletal muscle. Proc. Natl. Acad. Sci. U S A. 1998;95:15090–15095. doi: 10.1073/pnas.95.25.15090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Della Marca G, Frusciante R, Scatena M, Dittoni S, Testani E, Vollono C, Losurdo A, Scarano E, Colicchio S, Farina B, Gnoni V, Mazza S, Tonali PA, Ricci E. Heart rate variability in facioscapulohumeral muscular dystrophy. Funct. Neurol. 2010;25:211–216. [PubMed] [Google Scholar]
- 28.Hampton TG, Kale A, McCue S, Bhagavan HN, Vandongen C. Developmental changes in the ECG of a hamster model of muscular dystrophy and heart failure. Front. Pharmacol. 2012;3:80. doi: 10.3389/fphar.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duboc D, Meune C, Lerebours G, Devaux JY, Vaksmann G, Becane HM. Effect of perindopril on the onset and progression of left ventricular dysfunction in Duchenne muscular dystrophy. J. Am. Coll. Cardiol. 2005;45:855–857. doi: 10.1016/j.jacc.2004.09.078. [DOI] [PubMed] [Google Scholar]
- 30.Reid IA. Interactions between ANG II, sympathetic nervous system, and baroreceptor reflexes in regulation of blood pressure. Am. J. Physiol. 1992;262:E763–E778. doi: 10.1152/ajpendo.1992.262.6.E763. [DOI] [PubMed] [Google Scholar]
- 31.Ferguson AV, Bains JS. Actions of angiotensin in the subfornical organ and area postrema: implications for long term control of autonomic output. Clin. Exp. Pharmacol. Physiol. 1997;24:96–101. doi: 10.1111/j.1440-1681.1997.tb01790.x. [DOI] [PubMed] [Google Scholar]
- 32.Diz DI, Kasper SO, Sakima A, Ferrario CM. Aging and the brain renin-angiotensin system: insights from studies in transgenic rats. Cleve. Clin. J. Med. 2007;74(Suppl 1):S95–S98. doi: 10.3949/ccjm.74.suppl_1.s95. [DOI] [PubMed] [Google Scholar]
- 33.Delliaux S, Brerro-Saby C, Steinberg JG, Jammes Y. Reactive oxygen species activate the group IV muscle afferents in resting and exercising muscle in rats. Pflugers. Arch. 2009;459:143–150. doi: 10.1007/s00424-009-0713-8. [DOI] [PubMed] [Google Scholar]
- 34.Kaufman MP. The exercise pressor reflex in animals. Exp Physiol. 2012;97:51–58. doi: 10.1113/expphysiol.2011.057539. [DOI] [PubMed] [Google Scholar]
- 35.Piepoli MF, Dimopoulos K, Concu A, Crisafulli A. Cardiovascular and ventilatory control during exercise in chronic heart failure: role of muscle reflexes. Int. J. Cardiol. 2008;130:3–10. doi: 10.1016/j.ijcard.2008.02.030. [DOI] [PubMed] [Google Scholar]
- 36.Koba S, Gao Z, Sinoway LI. Oxidative stress and the muscle reflex in heart failure. J. Physiol. 2009;587.21:5227–5237. doi: 10.1113/jphysiol.2009.177071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patil J, Schwab A, Nussberger J, Schaffner T, Saavedra JM, Imboden H. Intraneuronal angiotensinergic system in rat and human dorsal root ganglia. Regul. Pept. 2010;162:90–98. doi: 10.1016/j.regpep.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pavel J, Tang H, Brimijoin S, Moughamian A, Nishioku T, Benicky J, Saavedra JM. Expression and transport of Angiotensin II AT1 receptors in spinal cord, dorsal root ganglia and sciatic nerve of the rat. Brain. Res. 2008;1246:111–122. doi: 10.1016/j.brainres.2008.09.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mederos y Schnitzler M, Storch U, Gudermann T. AT1 receptors as mechanosensors. Curr. Opin. Pharmacol. 2011;11:112–116. doi: 10.1016/j.coph.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Katovich MJ, Grobe JL, Raizada MK. Angiotensin-(1–7) as an antihypertensive, antifibrotic target. Curr. Hypertens. Rep. 2008;10:227–232. doi: 10.1007/s11906-008-0043-9. [DOI] [PubMed] [Google Scholar]
- 41.Shenoy V, Ferreira AJ, Qi Y, Fraga-Silva RA, Diez-Freire C, Dooies A, Jun JY, Sriramula S, Mariappan N, Pourang D, Venugopal CS, Francis J, Reudelhuber T, Santos RA, Patel JM, Raizada MK, Katovich MJ. The angiotensin-converting enzyme 2/angiogenesis-(1–7)/Mas axis confers cardiopulmonary protection against lung fibrosis and pulmonary hypertension. Am. J. Respir. Crit. Care. Med. 2010;182:1065–1072. doi: 10.1164/rccm.200912-1840OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCollum LT, Gallagher PE, Ann Tallant E. Angiotensin-(1–7) attenuates angiotensin II-induced cardiac remodeling associated with upregulation of dual-specificity phosphatase 1. Am. J. Physiol. Heart. Circ. Physiol. 2012;302:H801–H810. doi: 10.1152/ajpheart.00908.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shaltout HA, Westwood BM, Averill DB, Ferrario CM, Figueroa JP, Diz DI, Rose JC, Chappell MC. Angiotensin metabolism in renal proximal tubules, urine, and serum of sheep: evidence for ACE2-dependent processing of angiotensin II. Am. J. Physiol. Renal. Physiol. 2007;292:F82–F91. doi: 10.1152/ajprenal.00139.2006. [DOI] [PubMed] [Google Scholar]
- 44.Acuna MJ, Pessina P, Olguin H, Cabrera D, Vio CP, Bader M, Munoz-Canoves P, Cabello-Verrugio C, Brandan E. Restoration of muscle strength in dystrophic muscle by angiotensin-1–7 through inhibition of TGF-β signalling. Hum. Mol. Genet. 2013 doi: 10.1093/hmg/ddt514. [DOI] [PubMed] [Google Scholar]
- 45.Munoz MC, Giani JF, Burghi V, Mayer MA, Carranza A, Taira CA, Dominici FP. The Mas receptor mediates modulation of insulin signaling by angiotensin-(1–7) Regul. Pept. 2012;177:1–11. doi: 10.1016/j.regpep.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 46.Prasannarong M, Santos FR, Henriksen EJ. ANG-(1–7) reduces ANG IIinduced insulin resistance by enhancing Akt phosphorylation via a Mas receptordependent mechanism in rat skeletal muscle. Biochem. Biophys. Res. Commun. 2012;426:369–373. doi: 10.1016/j.bbrc.2012.08.093. [DOI] [PubMed] [Google Scholar]
- 47.Marcus Y, Shefer G, Sasson K, Kohen F, Limor B, Pappo O, Nevo N, Biton I, Bach M, Berkutzki T, Fridkin M, Benayahu D, Shechter Y, Stern N. Angiotensin 1–7 as means to prevent the metabolic syndrome: Lessons from the fructosefed rat model. Diabetes. 2013;62:1121–1130. doi: 10.2337/db12-0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Benter IF, Yousif MH, Cojocel C, Al-Maghrebi M, Diz DI. Angiotensin-(1–7) prevents diabetes-induced cardiovascular dysfunction. Am. J. Physiol. Heart. Circ. Physiol. 2007;292:H666–H672. doi: 10.1152/ajpheart.00372.2006. [DOI] [PubMed] [Google Scholar]
- 49.Gironacci MM, Vatta M, Rodriguez-Fermepin M, Fernandez BE, Pena C. Angiotensin-(1–7) reduces norepinephrine release through a nitric oxide mechanism in rat hypothalamus. Hypertension. 2000;35:1248–1252. doi: 10.1161/01.hyp.35.6.1248. [DOI] [PubMed] [Google Scholar]
- 50.Guimaraes PS, Santiago NM, Xavier CH, Velloso EP, Fontes MA, Santos RA, Campagnole-Santos MJ. Chronic infusion of angiotensin-(1–7) into the lateral ventricle of the brain attenuates hypertension in DOCA-salt rats. Am. J. Physiol. Heart. Circ. Physiol. 2012;303:H393–H400. doi: 10.1152/ajpheart.00075.2012. [DOI] [PubMed] [Google Scholar]