Abstract
Marijuana policy is rapidly evolving in the United States and elsewhere, with cannabis sales fully legalized and regulated in some jurisdictions and use of the drug for medicinal purposes permitted in many others. Amidst this political change, patients and families are increasingly asking whether cannabis and its derivatives may have therapeutic utility for a number of conditions, including developmental and behavioral disorders in children and adolescents. This review examines the epidemiology of cannabis use among children and adolescents, including those with developmental and behavioral diagnoses. It then outlines the increasingly well-recognized neurocognitive changes shown to occur in adolescents who use cannabis regularly, highlighting the unique susceptibility of the developing adolescent brain and describing the role of the endocannabinoid system in normal neurodevelopment. The review then discusses some of the proposed uses of cannabis in developmental and behavioral conditions, including attention deficit hyperactivity disorder (ADHD) and autism spectrum disorder (ASD). Throughout, the review outlines gaps in current knowledge and highlights directions for future research, especially in light of a dearth of studies specifically examining neurocognitive and psychiatric outcomes among children and adolescents with developmental and behavioral concerns exposed to cannabis.
Keywords: adolescent, cannabis, marijuana abuse, attention deficit disorder with hyperactivity, child development disorders, pervasive
In the United States and throughout the world, marijuana policy is rapidly evolving.1-3 In many jurisdictions, marijuana is now decriminalized, meaning that possession of the drug does not lead to criminal charges.4 In others, its use is permitted for medical purposes if a license or permit is issued to a patient or caregiver.5 In others still, including Washington State and Colorado, as well as in the country of Uruguay, marijuana sales for recreational use among adults are now fully legal and regulated by the government.6,7
Many of these dramatic policy changes have occurred within the last decade, and amidst this shifting political landscape, patients and families are increasingly asking whether marijuana – often used interchangeably in the literature and in the present article with the term cannabis – has a role in the management of developmental and behavioral pediatric conditions, including attention deficit hyperactivity disorder (ADHD) and autism spectrum disorders (ASD), among others.8,9 This is occurring despite a dearth of scientific evidence supporting a role for cannabis in these conditions. Some of this interest in cannabis has been fueled by the lay press, which has recently showcased rare examples of children with certain medical conditions who had failed traditional pharmacologic management and for whom cannabis was seemingly the only effective treatment.9,10 Accordingly, children and adolescents are increasingly being added to medical marijuana registries by their parents for a multitude of conditions.11
Despite an absence of known efficacy of cannabis for developmental and behavioral conditions, there is indeed mounting evidence for its role in some neurological symptoms. A recent systematic review12 of adult patient trials showed that certain formulations of cannabinoids were useful for spasticity and central pain. This same review concluded that data were insufficient to conclude efficacy in a number of other conditions, including Tourette syndrome, epilepsy and dystonia. Nonetheless, anecdotal evidence suggests that certain forms of marijuana, namely those enriched with cannabidiol (one of the many cannabinoid compounds present in cannabis but which does not have psychoactive properties), reduces the frequency of seizures for certain children with intractable epilepsy.13 This anecdotal evidence is not yet supported by clinical trial data, as highlighted by a recent Cochrane review of adult studies on the subject,14 but future studies will inevitably study this further.
Clearly, some parents are already using or are considering using cannabis for treatment of a wide range of pediatric conditions. Given the increasing prevalence of adolescent cannabis misuse and dependence,15-17 as well as the growing body of literature linking cannabis use to long-term and potentially irreversible adverse physical, neurocognitive, psychiatric and psychosocial outcomes,18 it is now more important than ever for the developmental-behavioral pediatrician to understand the available evidence on cannabis. Large professional organizations, including the American Academy of Pediatrics,19 the American Medical Association,20 the American Society of Addiction Medicine,21,22 and the American Academy of Child and Adolescent Psychiatry23 all have policy statements identifying marijuana use as a public health concern and currently oppose further steps towards legalization.
Here, we begin by describing important pharmacodynamic properties of cannabinoids, and then report the epidemiology of cannabis use, including the susceptibility of youth with developmental and behavioral disorders to earlier and heavier substance use. We then describe the known adverse neurocognitive effects of cannabis, highlighting the unique vulnerability of the developing brain and emphasizing the role of the endocannabinoid system in normal neurodevelopment. We conclude by reviewing some of the proposed uses of cannabis for developmental and behavioral conditions that have recently received attention, highlighting the knowledge gap that currently exists.
Pharmacology
Marijuana, also referred to as cannabis, is traditionally derived from the plant Cannabis sativa. The dried buds and accompanying leaves of cannabis are most commonly smoked, but can also be ingested, and increasingly, youth inhale it by vaporization (a process referred to as “vaping”) through new delivery systems similar to those used for e-cigarettes.24 Hash oil, which is illegal and contains a high concentration of cannabinoids, can be extracted from cannabis plant material and also can be smoked, ingested or vaporized.25 (It is not to be confused with hemp oil, often sold legally in natural food stores, which contains very few if any cannabinoids.) Onset of physiologic and psychologic effects vary based on route of administration, with peak effects occurring 30 minutes after inhalation and two to four hours after ingestion.26 Acute effects include on the one hand relaxation, euphoria, heightened perception, sociability, sensation of time slowing, increased appetite and decreased pain, and on the other hand, paranoia, anxiety, irritability, impaired short-term memory, poor attention and judgement, and poor coordination and balance.26,27 Physiologic effects include tachycardia, hypertension, dry mouth and throat, and conjunctival injection.
Cannabis exerts its effects primarily through the compound Δ-9-tetrahydrocannabinol (THC) acting on endogenous cannabinoid receptors present through the central and peripheral nervous system.28 THC is lipophilic, and readily crosses the blood-brain barrier and placenta.29 Also owing to its lipophilicity, THC accumulates in fat and therefore has a long elimination half-life of several days to a week. Similarly, many of the byproducts of marijuana smoke are lipophilic, with as yet poorly understood effects on health and development.30 The high fat solubility of many cannabinoids results in a large volume of distribution and long half-life of elimination from the body.29 The ability of cannabinoids to cross the placenta and affect fetal neurodevelopment may underlie the observation that prenatal exposure to cannabis is associated with hyperactivity, impulsivity and inattention symptoms in childhood,31 among other adverse.cognitive and behavioral outcomes summarized in a recent review.32
Potential health effects of cannabis may be exacerbated by the doubling of THC concentration in marijuana preparations that has occurred in the last two decades.25 In recent years, numerous synthetic cannabinoids, often marketed as herbal mixtures and referred to as “Spice”, “K2” or “Kronic”, have been synthesized and sold for recreational purposes (often through the Internet), and the rapidity of their development and distribution has outpaced attempts to classify them as Schedule I substances in the US.33
Legal formulations also exist in several jurisdictions, including some in the US. Dronabinol and nabilone, both synthetic THC-based cannabinoids, are US Food and Drug Administration-approved and marketed for use for children and adults as an antiemetic in chemotherapy and as an appetite stimulant. As outlined earlier, cannabinoids without psychoactive properties, such as cannabidiol, are also increasingly receiving attention since they may impart medicinal benefits with fewer psychologic effects, but remain poorly understood and require more study prior to approval and regulation. Nabiximols represents a combined THC and cannabidiol formulation administered as an oromucosal spray available outside the United States and used for alleviation of symptoms in multiple sclerosis.
Epidemiology
In the US and other developed countries, cannabis is the second most commonly used substance among adolescents after alcohol.15-17,34 Three recurrent surveys track cannabis use in the US general adolescent population. Monitoring The Future (MTF)16 and the Youth Risk Behavior Surveillance System (YRBSS)15 are school-based surveys, and the National Survey on Drug Use and Health (NSDUH)17 is a household-based survey. Collectively, the surveys demonstrate that as many as 4 in 10 adolescents have ever used marijuana, that prevalence of marijuana use is rising even as prevalence of alcohol and tobacco are falling (indeed, in 2009 cannabis use became more prevalent than tobacco use),16 and that daily or near-daily use is becoming more common.15-17 Specifically, daily use of marijuana is reported by 6.5% of high school seniors, 3.3% by 10th graders, and 1.2% by 8th graders, all of which represent an increase in prevalence of daily use occurring since 2008, previous to which daily use had been declining.16 Use typically begins early in adolescence, with approximately 1 in 3 males and 1 in 4 females having tried marijuana by the 9th grade.15
In recent years, as the movement toward decriminalization and legalization of cannabis has progressed, adolescents' perceptions of the harms of marijuana have fallen. Indeed, since 2004, adolescents seeing “great risk” in regularly using marijuana has steadily fallen; in 2013, fewer than half of all 10th graders and high school seniors reported perceiving risk in regular use, whereas previously a majority of all adolescents had perceived risk.16 Commensurate with this, emergency department visits related to marijuana increased 52% from 2004 to 2011 in the US.35 Meanwhile, accidental ingestions by smaller children of cannabis preparations may be increasing, with ER visits at a Colorado pediatric hospital increasing from 0% (none reported) to 2.4% of all unintentional ingestions following change in state drug enforcement laws allowing possession of marijuana for medical purposes.36 Calls made to Poison Control centers in the US have also been noted to increase in states where medical marijuana policies have been implemented or are underway.37
Data suggest that certain developmental-behavioral diagnoses portend higher risk of cannabis and other substance use and dependence. ADHD is a risk factor for earlier initiation of substance use in childhood and adolescence,38-40 and may predict heavier and more problematic substance use in adolescence and adulthood.41,42 Of all ADHD symptoms, hyperactivity and impulsivity confer the greatest risk for adolescent cannabis use disorder.43 Although an early meta-analysis44 showed that stimulant treatment for adolescent ADHD reduced the risk of subsequent substance use disorders, an updated meta-analysis incorporating newer studies with null findings suggests this may not be the case.45 Oppositional defiant disorder (ODD), conduct disorder (CD), and ASD have all also been linked to problematic substance use, including of marijuana.38,40,46 Among adolescents and adults with intellectual disability (ID), prevalence of cannabis and other substance use is not higher than for the general population, but risk for problematic use may be higher.47,48 Data from an adult study suggests that those with borderline or mild ID and with a comorbid psychiatric diagnosis are at even higher risk of a substance use disorder.48
Effects of Regular Marijuana Use on Neurocognition and Brain Structure
The high prevalence of marijuana use among adolescents, including those with developmental or behavioral disorders, is concerning given the myriad long-term consequences of regular cannabis use. Because of inconsistencies in how “regular” use is defined across studies, there is no clear indication as to whether there exists a ‘safe’ amount of cannabis use for adolescents. In general, “regular” use is defined in studies as daily or near-daily use over several years.16 Regardless, in interpreting study results, it is important to recognize that because studies of cannabis use are observational in design, co-occurring use of alcohol, cigarettes or other drugs may confound reported associations, and reverse causality cannot always be excluded.49 Although chronic marijuana use is associated with a broad range of adverse physical and mental health outcomes,50-52 here we focus on the neurocognitive effects.12,53
Acute effects of marijuana intoxication vary by person and by dose. Positive effects reported by users include anxiolysis, euphoria, heightened perception, increased sociability, sensation of time slowing, increased appetite, and decreased pain.26 On the surface, some of these effects may seem desirable to an adolescent with ADHD, although it is noteworthy that a study examining whether youth with ADHD used cannabis as a form of self-medication did not find this to be the case.54 Negative effects of marijuana include paranoia, anxiety, irritability, worsened short term memory, poor attention, altered awareness of the passage of time, impaired judgement, decreased coordination and balance, and distorted spatial perception,26,55 all of which could arguably exacerbate symptoms in developmental and behavioral conditions.
Clinicians should counsel youth that many of the detrimental neurocognitive effects of acute marijuana intoxication have a ‘hangover’ effect, with effects lasting at least one day after last use and with some subtle effects even measurable one month later among adolescent users.56 Given the adverse effects of acute intoxication on attention, coordination and perception, it is perhaps unsurprising that a recent meta-analysis57 demonstrated near doubling of the odds of fatal motor vehicle accident for adolescents and adults driving under the influence of cannabis. Data suggest that youth with ADHD are already at elevated risk of motor vehicle accident compared to the general adolescent population.58-60 Therefore, counseling adolescents with ADHD to avoid driving while under the influence of marijuana is critical, particularly since many youth believe that marijuana does not affect their driving abilities.61,62
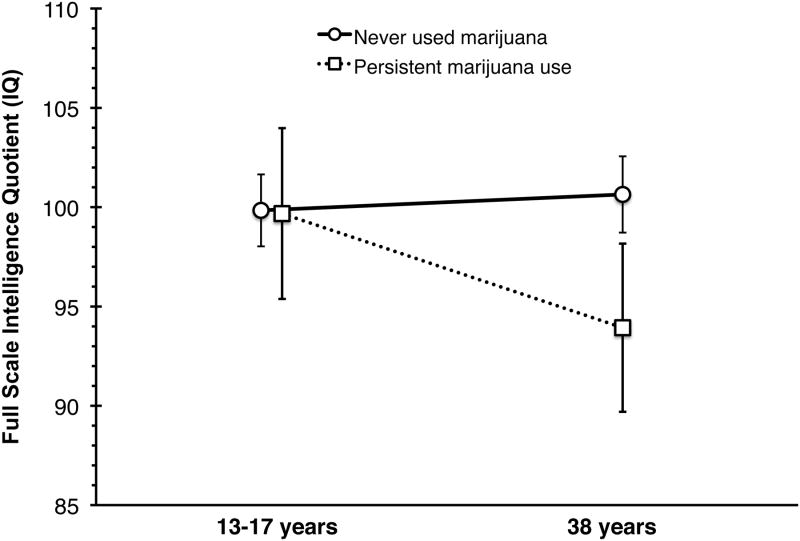
Over the long-term, adolescent cannabis use may be associated with a decline in intelligence quotient (IQ). A recent prospective study63 showed that regular cannabis use during adolescence was followed by a significant decline in IQ at age 38 years, as illustrated in Figure 1. This finding persisted after adjusting for use of alcohol or other drugs, comorbid mental illness, and educational level. Additionally, among adolescents users who later became abstinent, cessation was not associated with restoration of IQ in adulthood. These results are consistent with the possibility that cannabis impairs normal brain development during adolescence, and that heavy use may result in persistent and potentially non-reversible neurocognitive changes. A recent review53 compiled studies on changes in cognition, brain structure and brain function among adolescent cannabis users; its summary of studies demonstrating an association between earlier age of marijuana initiation and worsened outcomes is shown in Table 1.
Figure 1.
Full-scale intelligence quotient (IQ) among New Zealanders measured in childhood/adolescence (7-13 years) and adulthood (38 years). This figure highlights findings from 242 individuals who never used cannabis as compared to 38 individuals who demonstrated persistent use during study follow-up. (Persistent use was defined as reporting cannabis use ≥4 times per week at 3 or more study follow-up visits.) Error bars represent ±95% confidence intervals for the estimates. Adapted from Meier et al., 2012.63
Table 1.
Select studiesa demonstrating changes in cognition, brain structure and brain function associated with cannabis use in which adolescent onset is associated with worsened outcome.
| Reference | Cognitive | Brain Structure | Brain Function |
|---|---|---|---|
| Meier et al., 2012 | ↓ intelligence quotient (IQ) | ||
| Pope et al., 2003 | ↓ intelligence quotient (IQ) | ||
| Ehrenreich et al., 1999 | ↓ attention | ||
| Huestegge et al., 2002 | ↓ visual search | ||
| Fontes et al., 2011 | ↓ executive functioning | ||
| Solowij et al., 2012 | ↓ executive functioning | ||
| Churchwell et al., 2010 | ↓ prefrontal cortex volume | ||
| Gruber et al., 2011 | ↑ impulsivity | ↓ white matter integrity in prefrontal cortex | |
| Lopez-Larson et al., 2011 | ↓ superior prefrontal cortex thickness | ||
| Wilson et al., 2000 | ↓ total gray matter, ↑ total white matter | ||
| Becker et al., 2010a | ↑ left superior prefrontal cortex fMRIb blood oxygen level dependent (BOLD) signal during working memory task | ||
| Gruber et al., 2012 | ↓ anterior cingulate fMRIb blood oxygen level dependent (BOLD) signal during inhibition task | ||
| Jager et al., 2010 | ↑ prefrontal cortex MRIb blood oxygen level dependent (BOLD) signal during novel stimuli presentation in working memory task |
Adapted from a larger compilation of studies presented by Lisdahl et al.53
fMRI denotes functional magnetic resonance imaging.
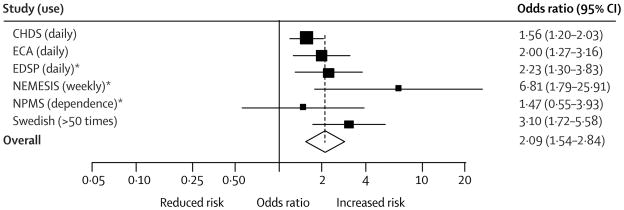
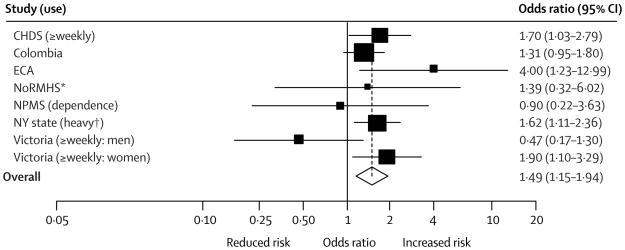
Regular cannabis use during adolescence is also associated with adverse psychiatric outcomes, although these psychiatric outcomes have not been rigorously studied among patients with developmental or behavioral concerns. A recent meta-analysis64 and large, prospective cohort study65 both reported increased odds of psychosis among adolescent cannabis users, an effect exacerbated by heavy use. Evidence linking adolescent cannabis use and depression are conflicting, with two recent systematic reviews64,66 reporting an association, but acknowledging that adjustment for confounders may reduce or eliminate this association. A more recent prospective cohort study67 of high school students demonstrated that heavy cannabis use was associated with later depression, but not suicidality. Another recent prospective study68 showed that adolescent users have nearly triple the odds of an adult anxiety disorder, although a previous systematic review64 examining adulthood anxiety among adolescent cannabis users reported conflicting data on this association. Figures 2a and 2b show the association of heavy cannabis use with psychosis and with depression, respectively, as reported by Moore et al. How the risk for subsequent psychiatric conditions differs among cannabis-using adolescents with developmental and behavioral concerns, in particular, is a critical area for further study.
Figure 2.
a: Forest plot reproduced from Moore et al.64 demonstrating adjusted odds ratios and 95% confidence intervals (CI) for association of heavy cannabis use with psychosis. Frequency of cannabis use examined in the study is reported in parentheses. Asterisks denote studies in which results were not adjusted for other drug use. (Reproduced with permission from The Lancet.)
b: Forest plot reproduced from Moore et al.64 demonstrating adjusted odds ratios and 95% confidence intervals (CI) for association of heavy cannabis use with depression. Frequency of cannabis use examined in the study is reported in parentheses. (Reproduced with permission from the Lancet.)
To understand how these neurocognitive and psychiatric effects of cannabis might arise, two concepts are critical. First, as noted above, the psychoactive compound in cannabis, Δ-9-tetrahydrocannabinol (THC), is highly lipophilic and readily crosses the blood-brain barrier as well as the placenta, with implications for normal neurodevelopment in the marijuana-using adolescent as well as the developing fetus.29 Second, the endocannabinoid system appears to play a significant role in normal neurodevelopment prenatally and extending throughout childhood and adolescence.28 Cannabinoid receptors, which are normally activated by endogenous compounds such as anandamide, appear to modulate axonal migration and long-range subcortical projections in the brain during early brain development, and affect synaptic connectivity throughout childhood and adolescence.69 Some of these developmental processes are known to occur throughout adolescence and into young adulthood, and alterations in these processes during critical windows are believed to result in permanent, irreversible deleterious effects.70
Although far from human application, data from rodents suggest that the endocannabinoid system may also be a potential target in developmental and behavioral conditions, though results remain conflicting.71 Findings from rat models of Fragile X syndrome suggest that blockade of cannabinoid receptors may normalize aberrant hippocampal development, and simultaneously correct cognitive deficits, improve seizures, and reduce pain sensitivity.72 Somewhat conflicting are additional findings from the same rat model showing that enhancing endocannabinoid signaling may correct abnormal synaptic plasticity occurring in the prefrontal cortex and ventral striatum, with simultaneous improvement in hyperlocomotion and anxiety-related behaviors.73
Alterations in neurodevelopment from chronic cannabis use may underlie several known brain changes present in heavy-using adults. Functional imaging studies (using diffusion-weighted magnetic resonance imaging and brain connectivity mapping) show that axonal connectivity is impaired in regular marijuana users, particularly with early age of onset of use in adolescence.74 Additionally, regular adult users who started cannabis use in adolescence exhibit decreased volume in the hippocampus and amygdala,74,75 which are involved in memory processing, as well in other portions of the medial temporal cortex, temporal pole, parahippocampal gyrus, insula and orbitofrontal cortex, which have high concentrations of cannabinoid receptors and are responsible for motivational, emotional and affective processing.76 The full extent of structural and functional neural changes from marijuana use is still not fully understood, and should be the focus of future study, particularly among adolescents with developmental and behavioral concerns, for whom study findings may differ from the general adolescent population.
Use of Marijuana for Pediatric Developmental and Behavioral Diagnoses
Understanding these long-term adverse consequences of cannabis use is especially important as patients and families question whether cannabis may have a role in managing pediatric conditions. Cannabis has had a broad range of proposed clinical applications (predominantly for adult conditions), including for symptomatic management of nausea, poor appetite, and pain, as well as for treatment of multiple sclerosis, spinal cord injury, glaucoma, Tourette syndrome, epilepsy and glaucoma.77 At this time, good evidence is almost entirely lacking for its application in pediatric developmental and behavioral conditions. Nonetheless, online advocacy groups that support the use of ‘medical’ marijuana for such conditions are gaining popularity, particularly on social media sites such as Facebook. At the time of press, some examples include “Mothers for Medical Marijuana Treatment for Autism”,78 “Mothers Advocating Medical Marijuana for Autism (MAMMA)”,79 and “Pediatric Cannabis Therapy”.80
Many advocates cite scientific literature regarding benefits of cannabis for the treatment of pediatric behavioral conditions, but often, data cited are from animal model-based research that does not yet have translation to human subjects. For example, a 2013 study81 from Stanford University showed that mice with a specific and rare gene mutation linked to autism showed altered endocannabinoid signaling in the central nervous system. These data were then cited by online and print media supporters of medical marijuana (for example, the High Times82) as evidence that cannabis could be used as a treatment for autism. As another example, when another recent study73 based on a mouse model of fragile X syndrome (described earlier in this review) showed alterations in endocannabinoid signaling pathways, these data were referenced (in this case, by more mainstream media outlets, such as the Huffington Post83 and Fox News84) as evidence for a promising role for cannabis as treatment. Although these and other high-impact studies share important insights into the pathogenesis of ASD and fragile X syndrome, based on their results alone, it is erroneous and potentially harmful to conclude that cannabis should be used as treatment for either of these disorders at this time.
With regard to human data on use of cannabis for developmental and behavioral conditions, to our knowledge, the only available data are from small case series or single studies. For example, one 6-year-old boy with autism was treated with daily dronabinol for six months and was noted to have improvement in hyperactivity, irritability, lethargy, stereotyped behaviors and speech, as measured by the Aberrant Behavior Checklist (ABC).85 This single case study was uncontrolled and unblinded. In another single case study86 of a cannabis-using adult male with ADHD off stimulants, the subject's driving skills in a simulated test during a time of abstinence improved after smoking marijuana. (What is unclear is whether this subject may have actually been experiencing cannabis withdrawal from his abstinence, with alleviation of his symptoms through subsequent use of marijuana.87) Another small case series88 showed an improvement in self-injurious behaviors among adolescents following dronabinol therapy, but to date, the study has not been published, leaving protocol details scarce. In sum, none of these studies provide sufficient, high-quality data to suggest that cannabis should be recommended for treatment of ASD or ADHD at this time.
Nonetheless, these data have prompted patient and family groups to advocate for the use of cannabis in children,89 occasionally even partnering with private, for-profit organizations who may stand to gain financially from such arrangements.90 This movement is coupled by a possibly increasing willingness of physicians to prescribe cannabis for medicinal purposes.91 Given the significant adverse health effects of cannabis, these two forces may result in issuing of medical marijuana permits for developmental and behavioral diagnoses for which no data on efficacy, safety or tolerability exist. Even if and when studies on cannabis for developmental and behavioral conditions are conducted, they will likely use formulations of oral dronabinol or cannabidiol, both of which can be administered with a known dose and predictable schedule; at this time, the bulk of medical marijuana is sold in plant form, which results in a highly variable dose of active compound and with less predictable onset of effect based on whether it is inhaled or ingested.
Conclusion
Given the current scarcity of data, cannabis cannot be safely recommended for the treatment of developmental or behavioral disorders at this time. At best, some might consider its use as a last-line therapy when all other conventional therapies have failed.92,93 As marijuana policy evolves and as the drug becomes more readily available, it is important that practicing clinicians recognize the long-term health and neuropsychiatric consequences of regular use. Although a decades-long public health campaign has showcased the harms of cigarette smoking, similar movements to illustrate the hazards of cannabis use have not been as rigorous or successful. As a result, accurate information on regular cannabis use remains poorly disseminated to patients, families and physicians. Further, there are especially few studies examining neurocognitive and psychiatric outcomes among children and adolescents with developmental or behavioral concerns who are exposed to cannabis, and this remains a critical area for future study. In coming to the decision to use marijuana for medicinal purposes, all parties should be fully aware of the long-term hazards of regular cannabis use, recognize the lack of evidence on its efficacy in developmental and behavioral conditions, and incorporate this information into a careful risk-benefit analysis.
Acknowledgments
Drs. Hadland and Harris are supported by the Division of Adolescent and Young Adult Medicine at Boston Children's Hospital and the Leadership Education in Adolescent Health Training Program T71 MC00009 (MCH/HRSA). Drs. Knight and Harris are supported by the National Institute on Alcohol Abuse and Alcoholism (1R01AA021904).
Table 1 adapted from Frontiers in Psychiatry, Vol. 4, by KM Lisdahl, ER Gilbart, NE Wright, S Shollenbarger, “Dare to Delay? The impacts of adolescent alcohol and marijuana use onset on cognition, brain structure, and function”, doi: 10.3389/fpsyt.2013.00053, copyright 2013. Figure 1 adapted from Proceedings of the National Academy of Science of the United States of America, Vol. 110, by MH Meier, A Caspi, A Ambler, H Harrington, R Houts, RS Keefe, K McDonald, A Ward, R Poulton, TE Moffitt, “Persistent cannabis users show neuropsychological decline from childhood to midlife”, pages e2657-2664, copyright 2012. Figures 2a and 2b reprinted from The Lancet, Vol. 370, by TH Moore, S Zammit, A Lingford-Hughes, TR Barnes, PB Jones, M Burke, G Lewis, “Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review”, pages 319-328, copyright 2012, with permission from Elsevier.”
Footnotes
Conflict of Interest Statement: The authors have no conflicts of interest to disclose.
References
- 1.GCODP. War on Drugs: Report of the Global Commission on Drug Policy. [Accessed 6 October, 2013];2011 http://www.globalcommissionondrugs.org/wp-content/themes/gcdp_v1/pdf/Global_Commission_Report_English.pdf.
- 2.Kilmer B, Caulkins JP, Pacula RL, Reuter PH. The U.S. Drug Policy Landscape: Insights and Opportunities for Improving the View. [Accessed March 26, 2014];2012 http://www.rand.org/pubs/occasional_papers/OP393.html. [PMC free article] [PubMed]
- 3.Richter KP, Levy S. Big marijuana--lessons from big tobacco. N Engl J Med. 2014 Jul 31;371(5):399–401. doi: 10.1056/NEJMp1406074. [DOI] [PubMed] [Google Scholar]
- 4.Reinarman C, Cohen PD, Kaal HL. The limited relevance of drug policy: cannabis in Amsterdam and in San Francisco. Am J Public Health. 2004 May;94(5):836–842. doi: 10.2105/ajph.94.5.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lynne-Landsman SD, Livingston MD, Wagenaar AC. Effects of state medical marijuana laws on adolescent marijuana use. Am J Public Health. 2013 Aug;103(8):1500–1506. doi: 10.2105/AJPH.2012.301117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arie S. Uruguay legalises sale and production of marijuana. BMJ. 2013;347:f7499. doi: 10.1136/bmj.f7499. [DOI] [PubMed] [Google Scholar]
- 7.Levy S. Effects of marijuana policy on children and adolescents. JAMA Pediatr. 2013 Jul 1;167(7):600–602. doi: 10.1001/jamapediatrics.2013.2270. [DOI] [PubMed] [Google Scholar]
- 8.Miles K. Marijuana-Like Chemical May Help Autism And Fragile X Syndrome Symptoms. [Accessed March 26, 2014];2012 http://www.huffingtonpost.com/2012/09/27/marijuana-chemical-autism-fragile-x_n_1920320.html.
- 9.Ellison K. Medical Marijuana: No Longer Just for Adults. [Accessed March 26, 2014];2009 http://www.nytimes.com/2009/11/22/health/22sfmedical.html.
- 10.Young S. Marijuana stops child's severe seizures. [Accessed March 26, 2014];2013 http://www.cnn.com/2013/08/07/health/charlotte-child-medical-marijuana/
- 11.Ferner M. Number Of Children Seeking Medical Marijuana Soars In Colorado. [Accessed April 16, 2014];2014 http://www.huffingtonpost.com/2014/02/13/medical-marijuana-children_n_4768219.html.
- 12.Koppel BS, Brust JC, Fife T, et al. Systematic review: Efficacy and safety of medical marijuana in selected neurologic disorders: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2014 Apr 29;82(17):1556–1563. doi: 10.1212/WNL.0000000000000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Porter BE, Jacobson C. Report of a parent survey of cannabidiol-enriched cannabis use in pediatric treatment-resistant epilepsy. Epilepsy Behav. 2013 Dec;29(3):574–577. doi: 10.1016/j.yebeh.2013.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gloss D, Vickrey B. Cannabinoids for epilepsy. Cochrane Database Syst Rev. 2014;3:CD009270. doi: 10.1002/14651858.CD009270.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eaton DK, Kann L, Kinchen S, et al. Youth risk behavior surveillance - United States, 2011. MMWR Surveill Summ. 2012 Jun 8;61(4):1–162. [PubMed] [Google Scholar]
- 16.Johnston LD, O'Mally PM, Bachman JG, Schulenberg JE. Monitoring the Future national results on drug use: 2013 Overview, Key Findings on Adolescent Drug Use. [Accessed 30 December, 2013];2013 http://www.monitoringthefuture.org/data/13data.html.
- 17.SAMHSA. Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings. (NSDUH Series H-46). [Accessed Accessed 19 September, 2013];2013 HHS Publication No. (SMA) 13-4795. http://www.samhsa.gov/data/NSDUH/2012SummNatFindDetTables/NationalFindings/NSDUHresults2012.htm.
- 18.Hall W, Degenhardt L. Adverse health effects of non-medical cannabis use. Lancet. 2009 Oct 17;374(9698):1383–1391. doi: 10.1016/S0140-6736(09)61037-0. [DOI] [PubMed] [Google Scholar]
- 19.Joffe A. Legalization of marijuana: potential impact on youth. Pediatrics. 2004 Jun;113(6):1825–1826. [PubMed] [Google Scholar]
- 20.AMA. H-95.998 AMA Policy Statement on Cannabis (Marijuana) [Accessed 24 January, 2014];2012 http://www.ama-assn.org/resources/doc/PolicyFinder/policyfiles/HnE/H-95.998.HTM.
- 21.ASAM. Public Policy Statement on Marijuana. [Accessed 24 January, 2014];2006 http://www.asam.org/docs/publicy-policy-statements/1marijuana-5-062.pdf.
- 22.ASAM. White Paper on State-Level Proposals to Legalize Marijuana. [Accessed 24 January, 2014];2012 http://www.asam.org/docs/publicy-policy-statements/state-level-proposals-to-legalize-marijuana-final2773DD668C2D.pdf.
- 23.AACAP. AACAP Medical Marijuana Policy Statement. [Accessed 24 January, 2014];2012 http://www.aacap.org/AACAP/Policy_Statements/2012/AACAP_Medical_Marijuana_Policy_Statement.aspx.
- 24.Abrams DI, Vizoso HP, Shade SB, Jay C, Kelly ME, Benowitz NL. Vaporization as a smokeless cannabis delivery system: a pilot study. Clin Pharmacol Ther. 2007 Nov;82(5):572–578. doi: 10.1038/sj.clpt.6100200. [DOI] [PubMed] [Google Scholar]
- 25.Mehmedic Z, Chandra S, Slade D, et al. Potency trends of Delta9-THC and other cannabinoids in confiscated cannabis preparations from 1993 to 2008. J Forensic Sci. 2010 Sep;55(5):1209–1217. doi: 10.1111/j.1556-4029.2010.01441.x. [DOI] [PubMed] [Google Scholar]
- 26.Winstock AR, Ford C, Witton J. Assessment and management of cannabis use disorders in primary care. BMJ. 2010;340:c1571. doi: 10.1136/bmj.c1571. [DOI] [PubMed] [Google Scholar]
- 27.Bramness JG, Khiabani HZ, Morland J. Impairment due to cannabis and ethanol: clinical signs and additive effects. Addiction. 2010 Jun;105(6):1080–1087. doi: 10.1111/j.1360-0443.2010.02911.x. [DOI] [PubMed] [Google Scholar]
- 28.Harkany T, Guzmán M, Hurd YL. Endocannabinoid Functions in Neurogenesis, Neuronal Migration, and Specification. In: Köfalvi A, editor. Cannabinoids and the Brain. New York, NY: Springer Science + Business Media, LLC; 2008. pp. 237–256. [Google Scholar]
- 29.Grotenhermen F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet. 2003;42(4):327–360. doi: 10.2165/00003088-200342040-00003. [DOI] [PubMed] [Google Scholar]
- 30.Moir D, Rickert WS, Levasseur G, et al. A comparison of mainstream and sidestream marijuana and tobacco cigarette smoke produced under two machine smoking conditions. Chem Res Toxicol. 2008 Feb;21(2):494–502. doi: 10.1021/tx700275p. [DOI] [PubMed] [Google Scholar]
- 31.Goldschmidt L, Day NL, Richardson GA. Effects of prenatal marijuana exposure on child behavior problems at age 10. Neurotoxicol Teratol. 2000 May-Jun;22(3):325–336. doi: 10.1016/s0892-0362(00)00066-0. [DOI] [PubMed] [Google Scholar]
- 32.Wu CS, Jew CP, Lu HC. Lasting impacts of prenatal cannabis exposure and the role of endogenous cannabinoids in the developing brain. Future Neurol. 2011 Jul 1;6(4):459–480. doi: 10.2217/fnl.11.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sacco LN, Finklea K. Synthetic Drugs: Overview and Issues for Congress. [Accessed 7 October, 2013];2013 https://www.fas.org/sgp/crs/misc/R42066.pdf.
- 34.UNODC. World Drug Report 2013. [Accessed 7 October, 2013];2013 http://www.unodc.org/unodc/secured/wdr/wdr2013/World_Drug_Report_2013.pdf.
- 35.Substance Abuse and Mental Health Services Administration, Center for Behavioral Statistics and Quality. The DAWN Report: Highlights of the 2011 Drug Abuse Warning Network (DAWN) Findings on Drug-Related Emergency Department Visits. [Accessed September 3, 2014]; http://www.samhsa.gov/data/2k13/DAWN127/sr127-DAWN-highlights.htm. [PubMed]
- 36.Wang GS, Roosevelt G, Heard K. Pediatric marijuana exposures in a medical marijuana state. JAMA Pediatr. 2013 Jul 1;167(7):630–633. doi: 10.1001/jamapediatrics.2013.140. [DOI] [PubMed] [Google Scholar]
- 37.Wang GS, Roosevelt G, Le Lait MC, et al. Association of Unintentional Pediatric Exposures With Decriminalization of Marijuana in the United States. Ann Emerg Med. 2014 Feb 3; doi: 10.1016/j.annemergmed.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 38.Creemers HE, van Lier PA, Vollebergh WA, Ormel J, Verhulst FC, Huizink AC. Predicting onset of cannabis use in early adolescence: the interrelation between high-intensity pleasure and disruptive behavior. The TRAILS Study. J Stud Alcohol Drugs. 2009 Nov;70(6):850–858. doi: 10.15288/jsad.2009.70.850. [DOI] [PubMed] [Google Scholar]
- 39.Milberger S, Biederman J, Faraone SV, Chen L, Jones J. Further evidence of an association between attention-deficit/hyperactivity disorder and cigarette smoking. Findings from a high-risk sample of siblings. Am J Addict. 1997 Summer;6(3):205–217. [PubMed] [Google Scholar]
- 40.Molina BS, Pelham WE., Jr Childhood predictors of adolescent substance use in a longitudinal study of children with ADHD. J Abnorm Psychol. 2003 Aug;112(3):497–507. doi: 10.1037/0021-843x.112.3.497. [DOI] [PubMed] [Google Scholar]
- 41.Charach A, Yeung E, Climans T, Lillie E. Childhood attention-deficit/hyperactivity disorder and future substance use disorders: comparative meta-analyses. J Am Acad Child Adolesc Psychiatry. 2011 Jan;50(1):9–21. doi: 10.1016/j.jaac.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 42.Groenman AP, Oosterlaan J, Rommelse N, et al. Substance use disorders in adolescents with attention deficit hyperactivity disorder: a 4-year follow-up study. Addiction. 2013 Aug;108(8):1503–1511. doi: 10.1111/add.12188. [DOI] [PubMed] [Google Scholar]
- 43.Elkins IJ, McGue M, Iacono WG. Prospective effects of attention-deficit/hyperactivity disorder, conduct disorder, and sex on adolescent substance use and abuse. Arch Gen Psychiatry. 2007 Oct;64(10):1145–1152. doi: 10.1001/archpsyc.64.10.1145. [DOI] [PubMed] [Google Scholar]
- 44.Wilens TE, Faraone SV, Biederman J, Gunawardene S. Does stimulant therapy of attention-deficit/hyperactivity disorder beget later substance abuse? A meta-analytic review of the literature. Pediatrics. 2003 Jan;111(1):179–185. doi: 10.1542/peds.111.1.179. [DOI] [PubMed] [Google Scholar]
- 45.Humphreys KL, Eng T, Lee SS. Stimulant medication and substance use outcomes: a meta-analysis. JAMA Psychiatry. 2013 Jul;70(7):740–749. doi: 10.1001/jamapsychiatry.2013.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Alwis D, Agrawal A, Reiersen AM, et al. ADHD symptoms, autistic traits, and substance use and misuse in adult Australian twins. J Stud Alcohol Drugs. 2014 Mar;75(2):211–221. doi: 10.15288/jsad.2014.75.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carroll Chapman SL, Wu LT. Substance abuse among individuals with intellectual disabilities. Res Dev Disabil. 2012 Jul-Aug;33(4):1147–1156. doi: 10.1016/j.ridd.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chaplin E, Gilvarry C, Tsakanikos E. Recreational substance use patterns and co-morbid psychopathology in adults with intellectual disability. Res Dev Disabil. 2011 Nov-Dec;32(6):2981–2986. doi: 10.1016/j.ridd.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 49.Hall W, Degenhardt L. The adverse health effects of chronic cannabis use. Drug Test Anal. 2014 Jan-Feb;6(1-2):39–45. doi: 10.1002/dta.1506. [DOI] [PubMed] [Google Scholar]
- 50.Hadland SE, Kerr T, Li K, Montaner JS, Wood E. Access to drug and alcohol treatment among a cohort of street-involved youth. Drug Alcohol Depend. 2009 Apr 1;101(1-2):1–7. doi: 10.1016/j.drugalcdep.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hall W, Degenhardt L. The adverse health effects of chronic cannabis use. Drug Test Anal. 2013 Jul 8; doi: 10.1002/dta.1506. [DOI] [PubMed] [Google Scholar]
- 52.Volkow ND, Compton WM, Weiss SR. Adverse health effects of marijuana use. N Engl J Med. 2014 Aug 28;371(9):879. doi: 10.1056/NEJMc1407928. [DOI] [PubMed] [Google Scholar]
- 53.Lisdahl KM, Gilbart ER, Wright NE, Shollenbarger S. Dare to delay? The impacts of adolescent alcohol and marijuana use onset on cognition, brain structure, and function. Front Psychiatry. 2013;4:53. doi: 10.3389/fpsyt.2013.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilens TE, Adamson J, Sgambati S, et al. Do individuals with ADHD self-medicate with cigarettes and substances of abuse? Results from a controlled family study of ADHD. Am J Addict. 2007;16(Suppl 1):14–21. doi: 10.1080/10550490601082742. quiz 22-13. [DOI] [PubMed] [Google Scholar]
- 55.Crean RD, Crane NA, Mason BJ. An evidence based review of acute and long-term effects of cannabis use on executive cognitive functions. J Addict Med. 2011 Mar;5(1):1–8. doi: 10.1097/ADM.0b013e31820c23fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Medina KL, Hanson KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Neuropsychological functioning in adolescent marijuana users: Subtle deficits detectable after a month of abstinence. J Int Neuropsychol Soc. 2007;13(05):807–820. doi: 10.1017/S1355617707071032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Asbridge M, Hayden JA, Cartwright JL. Acute cannabis consumption and motor vehicle collision risk: systematic review of observational studies and meta-analysis. BMJ. 2012;344:e536. doi: 10.1136/bmj.e536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barkley RA, Murphy KR, Kwasnik D. Motor vehicle driving competencies and risks in teens and young adults with attention deficit hyperactivity disorder. Pediatrics. 1996 Dec;98(6 Pt 1):1089–1095. [PubMed] [Google Scholar]
- 59.Jerome L, Segal A, Habinski L. What we know about ADHD and driving risk: a literature review, meta-analysis and critique. J Can Acad Child Adolesc Psychiatry. 2006 Aug;15(3):105–125. [PMC free article] [PubMed] [Google Scholar]
- 60.Schubiner H, Tzelepis A, Milberger S, et al. Prevalence of attention-deficit/hyperactivity disorder and conduct disorder among substance abusers. J Clin Psychiatry. 2000 Apr;61(4):244–251. doi: 10.4088/jcp.v61n0402. [DOI] [PubMed] [Google Scholar]
- 61.SADD LM. Hazy Logic: Liberty Mutual Insurance / SADD Study Finds Driving Under the Influence of Marijuana a Greater Threat to Teen Drivers than Alcohol. [Accessed 30 Dec, 2013];2012 http://www.sadd.org/press/presspdfs/Marijuana%20Teen%20Release.pdf.
- 62.Whitehill JM, Rivara FP, Moreno MA. Marijuana-using drivers, alcohol-using drivers, and their passengers: prevalence and risk factors among underage college students. JAMA Pediatr. 2014 Jul 1;168(7):618–624. doi: 10.1001/jamapediatrics.2013.5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meier MH, Caspi A, Ambler A, et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci U S A. 2012 Oct 2;109(40):E2657–2664. doi: 10.1073/pnas.1206820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moore TH, Zammit S, Lingford-Hughes A, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007 Jul 28;370(9584):319–328. doi: 10.1016/S0140-6736(07)61162-3. [DOI] [PubMed] [Google Scholar]
- 65.Manrique-Garcia E, Zammit S, Dalman C, Hemmingsson T, Andreasson S, Allebeck P. Cannabis, schizophrenia and other non-affective psychoses: 35 years of follow-up of a population-based cohort. Psychol Med. 2012 Jun;42(6):1321–1328. doi: 10.1017/S0033291711002078. [DOI] [PubMed] [Google Scholar]
- 66.Horwood LJ, Fergusson DM, Coffey C, et al. Cannabis and depression: an integrative data analysis of four Australasian cohorts. Drug Alcohol Depend. 2012 Dec 1;126(3):369–378. doi: 10.1016/j.drugalcdep.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 67.Rasic D, Weerasinghe S, Asbridge M, Langille DB. Longitudinal associations of cannabis and illicit drug use with depression, suicidal ideation and suicidal attempts among Nova Scotia high school students. Drug Alcohol Depend. 2013 Apr 1;129(1-2):49–53. doi: 10.1016/j.drugalcdep.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 68.Degenhardt L, Coffey C, Romaniuk H, et al. The persistence of the association between adolescent cannabis use and common mental disorders into young adulthood. Addiction. 2013 Jan;108(1):124–133. doi: 10.1111/j.1360-0443.2012.04015.x. [DOI] [PubMed] [Google Scholar]
- 69.Galve-Roperh I, Palazuelos J, Aguado T, Guzman M. The endocannabinoid system and the regulation of neural development: potential implications in psychiatric disorders. Eur Arch Psychiatry Clin Neurosci. 2009 Oct;259(7):371–382. doi: 10.1007/s00406-009-0028-y. [DOI] [PubMed] [Google Scholar]
- 70.Schneider M. Puberty as a highly vulnerable developmental period for the consequences of cannabis exposure. Addict Biol. 2008 Jun;13(2):253–263. doi: 10.1111/j.1369-1600.2008.00110.x. [DOI] [PubMed] [Google Scholar]
- 71.Busquets-Garcia A, Maldonado R, Ozaita A. New insights into the molecular pathophysiology of fragile X syndrome and therapeutic perspectives from the animal model. Int J Biochem Cell Biol. 2014 Aug;53:121–126. doi: 10.1016/j.biocel.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 72.Busquets-Garcia A, Gomis-Gonzalez M, Guegan T, et al. Targeting the endocannabinoid system in the treatment of fragile X syndrome. Nat Med. 2013 May;19(5):603–607. doi: 10.1038/nm.3127. [DOI] [PubMed] [Google Scholar]
- 73.Jung KM, Sepers M, Henstridge CM, et al. Uncoupling of the endocannabinoid signalling complex in a mouse model of fragile X syndrome. Nat Commun. 2012;3:1080. doi: 10.1038/ncomms2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zalesky A, Solowij N, Yucel M, et al. Effect of long-term cannabis use on axonal fibre connectivity. Brain. 2012 Jul;135(Pt 7):2245–2255. doi: 10.1093/brain/aws136. [DOI] [PubMed] [Google Scholar]
- 75.Schacht JP, Hutchison KE, Filbey FM. Associations between cannabinoid receptor-1 (CNR1) variation and hippocampus and amygdala volumes in heavy cannabis users. Neuropsychopharmacology. 2012 Oct;37(11):2368–2376. doi: 10.1038/npp.2012.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Battistella G, Fornari E, Annoni JM, et al. Long-term effects of cannabis on brain structure. Neuropsychopharmacology. 2014 Aug;39(9):2041–2048. doi: 10.1038/npp.2014.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ben Amar M. Cannabinoids in medicine: A review of their therapeutic potential. J Ethnopharmacol. 2006 Apr 21;105(1-2):1–25. doi: 10.1016/j.jep.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 78.Mothers for Medical Marijuana Treatment for Autism. [Accessed March 30, 2014]; (No date) https://www.facebook.com/pages/Mothers-for-medical-marijuana-treatment-for-autism/236681176392778.
- 79.MAMMA - Mothers Advocating Medical Marijuana for Autism. [Accessed Accessed March 30, 2014]; (No date) https://www.facebook.com/TexasMammas.
- 80.Pediatric Cannabis Therapy. [Accessed Accessed March 30, 2014]; (No date) https://www.facebook.com/groups/151947154925108/
- 81.Foldy C, Malenka RC, Sudhof TC. Autism-associated neuroligin-3 mutations commonly disrupt tonic endocannabinoid signaling. Neuron. 2013 May 8;78(3):498–509. doi: 10.1016/j.neuron.2013.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Adams M. Marijuana May Be Used to Treat Autism. [Accessed March 26, 2014];2013 http://www.hightimes.com/read/marijuana-may-be-used-treat-autism.
- 83.Miles K. Marijuana-Like Chemical May Help Autism And Fragile X Syndrome Symptoms. [Accessed March 31, 2014];2012 http://www.huffingtonpost.com/2012/09/27/marijuana-chemical-autism-fragile-x_n_1920320.html.
- 84.Grush L. Marijuana-Like Brain Chemicals Could Be Key to Treating Fragile X Syndrome. [Accessed March 31, 2014];2012 http://www.foxnews.com/health/2012/09/25/marijuana-like-brain-chemicals-could-be-key-to-treating-fragile-x-syndrome/
- 85.Kurz R, Blaas K. Use of dronabinol (delta-9-THC) in autism: A prospective single-case-study with an early infantile autistic child. Cannabinoids. 2010;5(4):4–6. [Google Scholar]
- 86.Strohbeck-Kuehner P, Skopp G, Mattern R. Cannabis improves symptoms of ADHD. Cannabinoids. 2008;3(1):1–3. [Google Scholar]
- 87.Budney AJ, Hughes JR, Moore BA, Vandrey R. Review of the validity and significance of cannabis withdrawal syndrome. Am J Psychiatry. 2004 Nov;161(11):1967–1977. doi: 10.1176/appi.ajp.161.11.1967. [DOI] [PubMed] [Google Scholar]
- 88.Kruger T, Christophersen E. An open label study of the use of dronabinol (Marinol) in the management of treatment-resistant self-injurious behavior in 10 retarded adolescent patients. J Dev Behav Pediatr. 2006;27(5):433. [Google Scholar]
- 89.Cannabis Science and The Unconventional Foundation for Autism (UF4A) Partner to Advance Successful Cannabis-based Autism Treatments. [Accessed March 30, 2014];2011 http://www.reuters.com/article/2011/03/17/idUS143853+17-Mar-2011+BW20110317.
- 90.Cannabis Science, Inc. (CBIS) Announces Submission of Cannabinoid-Based Patent Application N2010968 Titled ‘Composition for the Treatment of Neurobehavioral Disorders’. [Accessed March 30, 2014];2013 http://www.cannabisscience.com/index.php/news-media/news-releases/321-cannabis-science-inc-cbis-announces-submission-of-cannabinoid-based-patent-application-n2010968-titled-composition-for-the-treatment-of-neurobehavioral-disorders.
- 91.Adler JN, Colbert JA. Clinical decisions. Medicinal use of marijuana--polling results. N Engl J Med. 2013 May 30;368(22):e30. doi: 10.1056/NEJMclde1305159. [DOI] [PubMed] [Google Scholar]
- 92.Bostwick JM, Reisfield GM, DuPont RL. Clinical decisions. Medicinal use of marijuana. N Engl J Med. 2013 Feb 28;368(9):866–868. doi: 10.1056/NEJMclde1300970. [DOI] [PubMed] [Google Scholar]
- 93.Turcotte D, Le Dorze JA, Esfahani F, Frost E, Gomori A, Namaka M. Examining the roles of cannabinoids in pain and other therapeutic indications: a review. Expert Opin Pharmacother. 2010 Jan;11(1):17–31. doi: 10.1517/14656560903413534. [DOI] [PubMed] [Google Scholar]