Abstract
Balance and bispectral index metrics were evaluated in piglets following focal and diffuse brain injury. A significant decrease in bispectral index existed at 24 hours after diffuse brain injury, but not after focal injury. Postural sway increased at 1-6 hours after both focal and diffuse injuries.
Introduction
Objective clinical signs and noninvasive diagnostic tools to assess functional deficits and recovery over time would be valuable tools for management of traumatic brain injury (TBI), especially sports-related concussion, where there is a culture of under-reporting (Kroshus, Daneshvar, Baugh, Nowinski, & Cantu, 2014). In the present study, bispectral index (BIS) and postural sway were evaluated as potential objective diagnostic tools for identifying focal or diffuse TBI in toddler-aged piglets, as a model for the human child.
Brain injury and subsequent derangements in cerebral metabolism and signaling are known to cause changes in the electroencephalogram (EEG). We propose that EEG-based BIS measurements may aid in diagnosis of otherwise occult TBI. BIS has been evaluated for its ability to differentiate patients that are positive for head trauma by computed tomography, and also as a promising prognostic indicator (Haug, Miner, Dannehy, Seigel, & Biros, 2004; Paul & Umamaheswara Rao, 2006).
Postural sway has also shown promise as an adjunct diagnostic tool when assessing patients for the presence of sports-related TBI (Guskiewicz, 2003). Postural sway measured via accelerometry has been shown to be well correlated to the classic center of pressure assessments from force plates (Mancini et al., 2012; Whitney et al., 2011), and may be easily obtained from animal models.
We hypothesized that BIS and postural sway measures in piglets could detect a focal or diffuse traumatic brain injury.
Methods
Animals
Four-week old, female, purpose-bred Yorkshire cross piglets (N=25) weighing approximately 8-10kg were obtained from a commercial dealer and housed in an AAALAC-accredited facility. All procedures performed were approved by the University of Pennsylvania Institutional Animal Care and Use Committee.
Anesthesia and Monitoring
All pigs received ketamine (20 mg/kg) and xylazine (2 mg/kg) intramuscularly prior to induction with 3.5% isoflurane via mask, and were maintained at 1.2% isoflurane after endotracheal intubation. Animals were mechanically ventilated during their experimental procedures with a target end-tidal carbon dioxide of 35-45 mmHg. A preemptive dose of buprenorphine (0.02 mg/kg IM) was given for postoperative pain. All animals were maintained on isoflurane anesthesia with a target end tidal isoflurane concentration (EtISO) during the procedure of 1.2%. Core body temperature was maintained at 35-38°C with a circulating warm water blanket. Heart rate, pulse oximetry, respiratory rate, end tidal carbon dioxide, EtISO, rectal temperature, and indirect blood pressure were measured by a commercially available veterinary anesthetic monitor (Cardell® MAX-12 DUO HD, Midmark, Versailles, OH).
Experimental traumatic brain injury
After reaching a surgical plane of anesthesia, animals were subjected to either a focal controlled cortical impact (CCI) injury, a diffuse rapid nonimpact rotational (RNR) injury as previously described (Duhaime et al., 2000; Kilbaugh et al., 2011; Raghupathi & Margulies, 2002), or designated as an anesthetized sham. Briefly, animals undergoing CCI injury were placed in sternal recumbency and a curved skin incision was made midway between the sagittal midline and the orbit. A craniotomy and durotomy were performed along the coronal suture to access the surface of the brain parenchyma. A calibrated indenter was used to create a consistent deformation of the somatosensory cortex. After ensuring hemostasis, the skin edges were re-apposed, and a subcutaneous injection of buprivicaine/epinephrine was administered to minimize post-surgical discomfort at the incision site. The diffuse white matter damage characteristic of RNR injury was created by rotating the head 60-70 degree arc in 10-40 milliseconds in the sagittal plane, using a well-established inertial loading device. Sham animals were anesthetized without any rotational injury or surgical incision.
Bipectral index monitoring (BIS)
BIS was recorded during isofluorane anesthesia at two time points in each injured animal: prior to injury and 24 hours after injury for CCI (n=14) and RNR (n=3) using the commercially available BIS™ Monitor (A-2000™ v3.3, Aspect Medical, Newton, MA). Each animal's pre-injury measurement served as her own uninjured control. Data was collected on a laptop computer in 5 second increments. Factory default settings were used (smoothing rate 15s) and impedance was maintained below 15 kΩ. Data obtained with a signal quality index of <50 were discarded. After clipping of hair and degreasing with isopropyl alcohol solution, BIS™ Pediatric sensor electrodes were placed in a frontotemporal configuration as previously described in dogs (Campagnol, Teixeira Neto, Monteiro, Beier, & Aguiar, 2007). All BIS recordings for comparison of the effect of injury on BIS were recorded at the same time after premedication and at the same EtISO concentration. Recordings began at least 30 minutes after premedication to minimize the effect of ketamine on the readings. BISmean was calculated as the average value for a 1 minute interval. BISmean was used to minimize effect of second-to-second variation in this measurement.
Postural sway
In a separate study, nineteen piglets were divided into three groups: RNR injury (n=7), CCI injury (n=8), and anesthetized shams (n=4). Balance, via postural sway, was assessed prior to (all subjects), and 1-6 hours after anesthesia and intramuscular administration of buprenorphine (RNR and shams), or 1-6 hrs after anesthesia, intramuscular administration of buprenorphine, and local anesthetic administration (CCI). An iPod (Apple, Inc., Cupertino, CA) was affixed intrascapularly to piglets via a jacket or harness and used to measure linear acceleration in three degrees of freedom at 100Hz, similar to commercial apps available for humans (e.g. BalanceTest™, SwayBalance™). Piglets were placed in a 3 ft by 2 ft enclosure and allowed to move freely for 3 minutes while being filmed from above. The acceleration traces were exacted from segments when which the piglet's head and legs were observed to be stationary, while the piglet's trunk was allowed to sway over the legs. Analogous to the center-of-pressure algorithms, custom software was created (Mathworks, Natick, MA) to calculate the root mean square (RMS) acceleration in the anterior-posterior (AP) and medial-lateral (ML) directions during each stationary interval, and the average over all stationary intervals was calculated for each animal.
Statistical analysis
Only one animal was used for BIS and sway measurements, so separate statistical analyses were performed using SigmaPlot™ (v12, Systat Software, Inc., San Jose, CA) or JMP® 10 (SAS Institute, Inc., Cary, NC). BISmean before and after injury, as well as between injury groups, were compared using two way ANOVA with repeated measures, and a post-hoc paired t-test was used to compare pre and post injury values. Postural sway measures were compared before and after injury or sham anesthesia using a paired t-test. Relationships were determined to be statistically significant when p < 0.05.
Results
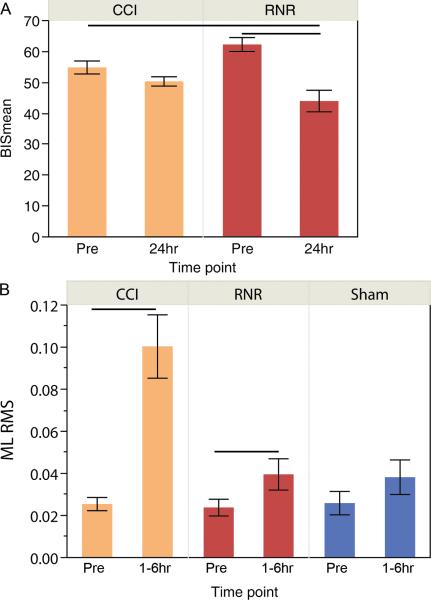
Despite a small RNR cohort, BISmean was significantly lower in the 24hr post-RNR group compared to the pre-CCI or pre-RNR groups (Figure 1a). No other significant difference was found between groups.
Figure 1.
A) BISmean prior to (Pre), and 24 hours following two different types of experimental traumatic brain injury in piglets. B) Medial-lateral root mean square of accelerometery in piglets prior to or after injury. CCI = controlled cortical impact. RNR = rapid nonimpact rotational. Lines denote significant differences between groups (p < 0.05). Mean +/− SEM shown.
The pig has a shorter ML base than AP, and would be expected to exhibit larger sway in the ML direction if balance were impaired. Within hours after injury, ML RMS acceleration was significantly increased in both CCI (p=0.0007) and RNR (p=0.02) animals, with no differences found before and after sham anesthesia (p=0.32) (Figure 1b). Interestingly, AP RMS acceleration was also significantly increased after CCI injury (p=0.0012), but not RNR injury or sham anesthesia.
Discussion
Both bispectral index monitoring and assessment of ML RMS postural sway, were able to detect post-TBI deficits in piglets, with ML RMS revealing changes following both types of injury at an earlier time point. We postulate that our frontal lobe BIS sensor location may not capture altered EEG activity from the parietal lobe focal injury. However, the area targeted by the focal injury is a region of somatosensory cortex (Craner & Ray, 1991) and likely plays a role in the proprioceptive pathways necessary for maintenance of stable posture, because we observe large difference in ML RMS between CCI injured piglets before and after injury. Interestingly, diffuse injury also disrupted the proprioceptive pathways necessary to maintain balance while standing.
Several limitations to the study may influence our conclusions. First, because of the opportunistic nature of this study design, group numbers were the result of available animals, such small groups may have limited statistical resolution of subtle differences, and we recommend further study in larger cohorts of injured and uninjured piglets. Second, although sham animals were anesthetized in the same manner as CCI and RNR injured, they did not receive the skin incision of the CCI group and may have less post-anesthesia discomfort than a CCI subject. The balance data were obtained in all groups during buprenorphine treatment to minimize the effect of this experimental factor, but the incision in the CCI group may have contributed to our observation of significantly more sway in the CCI group, despite the subcutaneous injection of anesthetic 1-6 hrs prior to measurement in the CCI group. Because RNR animals experience no cervical, scalp or skull injury, the anesthetized shams are appropriate controls for the RNR group. In contrast, the BIS measurements were obtained during anesthesia in all animals, reducing the potential influence of this experimental factor between groups.
In summary, we have found that both bispectral index and postural sway analysis can be used to make noninvasive and objective assessments of brain injury deficits in a piglet model of TBI.
Acknowledgements
The authors would like to thank Melissa Byro her technical assistance in performing the experiments. This project was partially supported by NIH grants R01N5039679, U01NS069545, and 8R25OD010986.
Abbreviations
- TBI
traumatic brain injury
- BIS
bispectral index
- EEG
electroencephalogram
- EtISO
end tidal isoflurane concentration
- CCI
controlled cortical impact
- RNR
rapid nonimpact rotational
References
- Campagnol D, Teixeira Neto FJ, Monteiro ER, Beier SL, Aguiar AJ. Use of bispectral index to monitor depth of anesthesia in isoflurane-anesthetized dogs. Am J Vet Res. 2007;68(12):1300–1307. doi: 10.2460/ajvr.68.12.1300. doi: 10.2460/ajvr.68.12.1300. [DOI] [PubMed] [Google Scholar]
- Craner SL, Ray RH. Somatosensory cortex of the neonatal pig: I. Topographic organization of the primary somatosensory cortex (SI). J Comp Neurol. 1991;306(1):24–38. doi: 10.1002/cne.903060103. doi: 10.1002/cne.903060103. [DOI] [PubMed] [Google Scholar]
- Duhaime AC, Margulies SS, Durham SR, O'Rourke MM, Golden JA, Marwaha S, Raghupathi R. Maturation-dependent response of the piglet brain to scaled cortical impact. Journal of Neurosurgery. 2000;93(3):455–462. doi: 10.3171/jns.2000.93.3.0455. doi: DOI 10.3171/jns.2000.93.3.0455. [DOI] [PubMed] [Google Scholar]
- Guskiewicz KM. Assessment of postural stability following sport-related concussion. Curr Sports Med Rep. 2003;2(1):24–30. doi: 10.1249/00149619-200302000-00006. [DOI] [PubMed] [Google Scholar]
- Haug E, Miner J, Dannehy M, Seigel T, Biros M. Bispectral electroencephalographic analysis of head-injured patients in the emergency department. Academic Emergency Medicine. 2004;11(4):349–352. doi: 10.1197/j.aem.2003.12.015. doi: DOI 10.1197/j.aem.2003.12.015. [DOI] [PubMed] [Google Scholar]
- Kilbaugh TJ, Bhandare S, Lorom DH, Saraswati M, Robertson CL, Margulies SS. Cyclosporin A Preserves Mitochondrial Function after Traumatic Brain Injury in the Immature Rat and Piglet. Journal of Neurotrauma. 2011;28(5):763–774. doi: 10.1089/neu.2010.1635. doi: DOI 10.1089/neu.2010.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroshus E, Daneshvar DH, Baugh CM, Nowinski CJ, Cantu RC. NCAA concussion education in ice hockey: an ineffective mandate. [Evaluation Studies Research Support, Non-U.S. Gov't]. Br J Sports Med. 2014;48(2):135–140. doi: 10.1136/bjsports-2013-092498. doi: 10.1136/bjsports-2013-092498. [DOI] [PubMed] [Google Scholar]
- Mancini M, Salarian A, Carlson-Kuhta P, Zampieri C, King L, Chiari L, Horak FB. ISway: a sensitive, valid and reliable measure of postural control. J Neuroeng Rehabil. 2012;9:59. doi: 10.1186/1743-0003-9-59. doi: 10.1186/1743-0003-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul DB, Umamaheswara Rao GS. Correlation of Bispectral Index with Glasgow Coma Score in mild and moderate head injuries. J Clin Monit Comput. 2006;20(6):399–404. doi: 10.1007/s10877-006-9045-9. doi: 10.1007/s10877-006-9045-9. [DOI] [PubMed] [Google Scholar]
- Raghupathi R, Margulies SS. Traumatic axonal injury after closed head injury in the neonatal pig. Journal of Neurotrauma. 2002;19(7):843–853. doi: 10.1089/08977150260190438. doi: Doi 10.1089/08977150260190438. [DOI] [PubMed] [Google Scholar]
- Whitney SL, Roche JL, Marchetti GF, Lin CC, Steed DP, Furman GR, Redfern MS. A comparison of accelerometry and center of pressure measures during computerized dynamic posturography: a measure of balance. [Comparative Study]. Gait Posture. 2011;33(4):594–599. doi: 10.1016/j.gaitpost.2011.01.015. doi: 10.1016/j.gaitpost.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]