Abstract
To study central nervous system airborne PM related subchronic toxicity, SD male rats were exposed for eight weeks to either coarse (32 µg/m3), fine (178 µg/m3) or ultrafine (107 µg/m3) concentrated PM or filtered air. Different brain regions (olfactory bulb, frontal cortex, striatum and hippocampus), were harvested from the rats following exposure to airborne PM. Subsequently, prooxidant (HO-1 and SOD-2), and inflammatory markers (IL-1β and TNFα), apoptotic (caspase 3), and unfolded protein response (UPR) markers (XBP-1S and BiP), were also measured using real-time PCR. Activation of nuclear transcription factors Nrf-2 and NF-κB, associated with antioxidant and inflammation processes, respectively, were also analyzed by GSMA. Ultrafine PM increased HO-1 and SOD-2 mRNA levels in the striatum and hippocampus, in the presence of Nrf-2 activation. Also, ultrafine PM activated NF-κB and increased IL-1β and TNFα in the striatum. Activation of UPR was observed after exposure to coarse PM through the increment of XBP-1S and BiP in the striatum, accompanied by an increase in antioxidant response markers HO-1 and SOD-2. Our results indicate that exposure to different size fractions of PM may induce physiological changes (in a neuroanatomical manner) in the central nervous system (CNS), specifically within the striatum, where inflammation, oxidative stress and UPR signals were effectively activated.
Keywords: Particulate matter, Central nervous system, Striatum, Oxidative stress, Inflammation, Unfolded protein response
1. Introduction
The deleterious effects of air pollution, and especially particulate air pollution, on the lungs and the heart have been well established. Oxidative stress and inflammation have been suggested as some of the underlying mechanisms involved in the toxicity on the cardiopulmonary system induced by exposure to particulate matter (PM) (Katsouyanni et al., 1997; Pope and Dockery, 2006; Araujo, 2011). Exposure to environmental stressors has been reported to also influence the development of neurodegenerative diseases (Dosunmu et al., 2007; Migliore and Coppedè, 2009; Ranft et al., 2009). However the involvement of free radical production and inflammatory processes as stressors in the central nervous system (Abbott, 2011; Block and Calderon-Garciduenas, 2009; Calderon-Garciduenas et al., 2004; Landrigan et al., 2005) is still an area of needing intense research.
Reports have documented increase in neuroinflammation and accumulations of misfolded proteins: β-amyloid and α-synuclein, in the brains of residents living in highly polluted cities (Calderon-Garciduenas et al., 2004). The same authors showed higher particulate deposition (<100 nm) in olfactory bulb neurons and alterations in the blood–brain barrier in the brain of children and young adults living in cities with high air pollution vs. those living in conditions of low air pollution (Calderon-Garciduenas et al., 2008). Inhaled particles, or components, that deposit in the nose can access the brain, either by transport along olfactory nerves or possibly by penetration of the blood–brain barrier through systemic distribution of PM (Oberdorster et al., 2002, 2004). Neuroinflammatory events associated with exposure to PM10 (particles with mass median diameters ≤ 10 µm) and PM2.5 (particles ≤ 2.5 µm mass median diameter), have been observed in experimental murine models as well as in case-control studies from human tissue, where an increase of pro-inflammatory cytokines in the presence of nuclear factor kappa B (NF-κB) activation was observed (Calderon- Garciduenas et al., 2004; Campbell et al., 2005; Gerlofs-Nijland et al., 2010; Kleinman et al., 2008). Additionally, oxidative stress and cerebral vascular damage have also been described as relevant effects induced by exposure to PM, biological effects that may be systemic and local in the different brain regions (Block and Calderon-Garciduenas, 2009).
While production of free radicals and oxidant molecules are part of normal metabolic and inflammatory processes, excessive production of these molecules can lead to cell death and tissue injury. Homeostatic control of these compounds is maintained through the activation of the antioxidant response element (ARE), which is under control of nuclear (erythroid-derived 2)-like 2 (Nrf-2) transcription factor. Nrf-2 activates the transcription of antioxidant enzymes such as SOD and HO-1 in tissues, including the brain (Ghosh et al., 2011). The possibility that Nrf-2 in the brain may be a molecule targeted by exposure to PM has not been extensively explored. A possible consequence of oxidative stress or inflammation in the brain is the accumulation of oxidized proteins in the CNS cells. Such accumulations can produce endoplasmic reticulum (ER) stress. Under normal conditions, ER stress is regulated by the unfolded protein response (UPR), largely through PERK, IRE1α and ATF6 signaling. In addition, under normal physiological conditions, these three molecules, that reside in the ER are kept inactive by BiP chaperone proteins (Kaufman, 2002; Malhotra and Kaufman, 2007; Ron and Walter, 2007; Zhang and Kaufman, 2008). When unfolded or misfolded proteins accumulate, BiP associates with these proteins, thereby allowing them to become active. In addition to their specific functions, these three components stimulate the production of proteins, which control protein folding (Kaufman, 2002; Malhotra and Kaufman, 2007; Ron and Walter, 2007; Zhang and Kaufman, 2008). The presence of protein aggregates in the interior of neurons, largely as a result of oxidation of cytoskeletal and mitochondrial respiratory chain proteins, in patients with neurodegenerative diseases such as Alzheimer’s (AD) and Parkinson’s disease (PD) has also been documented (Ferrer, 2009; Martínez et al., 2010).
Several PM components induce oxidative stress and inflammation. The pro-oxidant effects of transition metals contained in PM have been extensively described (Valko et al., 2005; Mazzoli-Rocha et al., 2010). In addition, Li et al. (2004) proposed that polycyclic aromatic hydrocarbons (PAH) contained in PM can also induce oxidative stress through quinone and oxygenated PAH metabolism. We, and others, have previously reported that PM collected from the air in Mexico City characteristically contain higher concentrations of minerals, metals and carbonaceous compounds, all which are toxic to cells, capable of inducing apoptosis and DNA damage in vitro (De Vizcaya-Ruiz et al., 2006; Mugica et al., 2009), that might be found in ambient air of other cities. There has been a great deal of emphasis given to the role of PM2.5 and ultrafine PM (UFP; particles ≤ 100 nm) with respect to human health effects, and less attention has been paid to the effects of coarse PM (PM2.5–10). However all three particle size fractions contain components that are cytotoxic and capable of inducing tissue injury. In addition coarse PM has a strong tendency to deposit in the nose, as does UFP. The goal of this project is to examine whether PM-induced oxidative stress and inflammatory processes in the brain, that adversely impact the CNS, acts through mechanisms that lead to ER stress by modifying elements of the unfolded protein response.
Accordingly, we exposed rats to concentrate coarse, fine, and ultrafine PM sampled in Mexico City ambient air and evaluated the effects on the CNS using methods from molecular biology.
2. Materials and methods
2.1. Animal exposure
Six-week-old male Sprague-Dawley rats (purchased from Harlan®, Mexico) were administered food and water ad libitum. Animals were housed under barrier conditions using a vented isolation caging system (OneCage® Labproducts, FL) and kept on a strict 12-h light/12-h dark cycle in the animal facility at CINVESTAV-IPN according to institutional guidelines.
2.1.1. Ethics
Experiments described in this study were carried out according to the “Principles of Laboratory Animal Care” (NIH publication #85–23, revised 1985) guidelines and to the “Norma Oficial Mexicana de la Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación” (SAGARPA), subsection: “Especificaciones técnicas para la producción, cuidado y uso de los animales de laboratorio” (Clave NOM-062-ZOO-1999). Animal handling and exposure was described in protocol ID. 363-06, which was approved by the Institutional Internal Committee for the Use and Care of Laboratory Animals (Comité Interno para el Cuidado y Uso de los Animales de Laboratorio).
2.1.2. Exposure conditions
Animals were exposed to concentrated ambient particulates from Mexico City ambient air by means of size-selective inlets during the warm–dry season between May and June of 2009, 5 h per day, 4 days per week for a total of eight weeks (Table 1). The mass concentration for coarse, fine and ultrafine in the exposure chambers were estimated by using the mass determination of the concentrated PM and of the parallel outside ambient samples collected (collection and mass determination is described in Section 2.2). Outside coarse mass determination was obtained using PM10 and PM2.5 mass levels, and PM2.5 levels from its mass determination, no data for outdoor ultrafine mass levels was available (see Table 1).
Table 1.
Experimental exposure conditions to concentrated ambient particulates.
| Exposure condition | MMAD (GSD) (µm)b |
Concentration ambient air (µg/m3) |
Concentration exposure chamber (µg/m3) |
Number of animals per group |
|---|---|---|---|---|
| Coarse | 10–2.5 | 22 | 32 | 6 |
| Fine | ≤2.5 | 28 | 178 | 6 |
| Ultrafine | ≤0.1 | NMa | 107 | 6 |
NM, not measured.
MMAD is the mass median aerodynamic diameter measured with the Microorifice Uniform Deposit Impactor (MOUDI). GSD is the geometric standard deviation (Kim et al., 2001a,b; Kleinman et al., 2005).
Concentrated ambient particles used for the animal exposures were obtained using an inertial particle separator system (Kim et al., 2001a). This system is capable of enriching the concentration of particles by drawing outside ambient air into whole-body animal exposure chambers (Kleinman et al., 2008). Ambient air for the exposures was drawn at a flow rate of 150 L per minute (Kim et al., 2001a,b) through an aluminum duct measuring 2 m in length and 7.5 cm in diameter in order to minimize particle loss due to electrostatic deposition. The duct intake was situated about 3 m above ground level. To control particle flow inline calibrated rotameters were used (Campbell et al., 2005; Kleinman et al., 2005). Concentrated aerosols were delivered to whole-body animal exposure chambers, and each exposure chamber was a sealed unit, sectioned for housing three rats per chamber, and two groups of three rats were exposed to each type of concentrated PM (Kleinman et al., 2005). Temperature and airflow were controlled during the exposures to ensure adequate ventilation, minimize buildup of animal-generated contaminants (dander, ammonia and CO2) and to avoid thermal stresses.
Control animals were exposed to air purified through scrubbers containing permanganate-impregnated alumina spheres and activated carbon for oxidation and adsorption of organic vapors and filtered through a high efficiency particle HEPA-filter (Mautz and Kleinman, 1997).
2.2. Particle mass determination and chemical characterization
To determine mass concentration in the exposure chambers concentrated PM were collected on weighed and equilibrated 37 mm Teflon filters (PTFE 2 µm pore, Gelman Science, Ann Arbor, MI). The filters were mounted in cassettes and connected to the inlet of the rat exposure chambers. Filters were removed at the end of each exposure day and remounted at the beginning of the next exposure day. At the end of each four-day exposure period, filters were removed from their cassettes and stored at constant humidity and temperature for 24 h prior to reweighing to ensure removal of particle bound water, and weighed after equilibration to calculate mass. To measure outdoor ambient PM levels and its elemental composition parallel PM10 and PM2.5 samples were collected on teflon filters using MiniVol samplers (Airmetrics, Eugene, OR) at a flow rate of 5.0 L/min and in quartz filters using High Volume samplers (Tisch Environmental®).
Teflon filters were treated as described above for particle mass determination; quartz filters were baked for 12 h at 500°C prior to sampling to reduce residual carbon levels associated with new filters. Conditioning of filters was performed in a heat chamber (40 ± 5% humidity and 20 ± 2°C) 48 h before sampling.
Duplicate fractions of each filter were analyzed using an Atom Advantage Thermo Jarrel Ash Inductively Coupled Plasma-Atomic Emission Spectrometry (ICPAES) to determine the elemental components of the PM collected. Each section of each filter was digested with suprapure hydrochloric and nitric acids according to the microwave program established by Method IO 3.1 (USEPA, 1999) (Mugica et al., 2009).
PAHs were extracted from the filters in an ultrasonic bath using acetonitrile/dichloromethane 1/1, v/v, for three 10 min periods adding Pyrene d10 as surrugate. The extracts were concentrated down to 5 mL with a rotary evaporator followed by evaporation under purified nitrogen to near dryness and reconstitution with acetonitrile. The resulting solutions were filtered to remove insoluble residues. Finally the extracts were transferred to small amber glass vials, sealed and stored in darkness at −18°C until analysis. Identification and quantification of PAH was performed by GC/MS (GC model HP 6890, MS model 5973 equipped with a quadrupole mass filter and autosampler) using a 60-m 0.25 mm diameter HP-1701 capillary column (0.25 m film thickness HP). The temperature program applied was 65°C from 2 min, then 8°C/min to 320°C, held for 10 min. Fluoranthene d10 was added as internal standard (EPA, Method TO-13). A standard PAHs mixture was used for quantification (Valle-Hernández et al., 2010).
Reference material for quality control of organic and inorganic species used was urban dust SRM 1649a from the National Institute of Standards and Technology (NIST).
2.3. Sample preparation
Rats were euthanized 24 h after the last exposure (using sodium phentobarbital 50–100 mg/kg intraperitoneally), and brain tissue was harvested for subsequent bioassays. Olfactory bulb, corpus striatum, frontal cortex and hippocampus were harvested and quickly frozen in liquid nitrogen.
2.4. Real-time polymerase chain reaction (PCR-QT)
RNA was isolated using the TRIzol method (Invitrogen, Carlsbad, CA). The purity and amount of RNA were determined by spectrophotometry at 260–280 nm, and integrity was evaluated in 1% agarose gels. DNase treatment (Turbo DNA-Free, Ambion, Austin, Texas) was used to avoid DNA contamination. Reverse transcriptase (TaqMan Reverse, Roche, Branchburg, NJ) from 2 µg of RNA was used to produce cDNA. PCR reactions were performed in a final volume of 20 µL using Universal PCR master mix on a StepOne Plus thermocycler (Applied Biosystems, Carlsbad, CA) with predesigned oligonucleotides: SOD2 (Rn00566942 gl); HO-1 (Rn01536933 ml); IL1-β(Rn00580432_ml); TNFα (Rn99999017_ml); XBP-1S (Rn01752572_gl); BiP (Rn00565250_ml); and caspase 3 (Rn00563902_ml) (Applied Biosystems, Carlsbad, CA). Hypoxanthine–guanine phosphoribosyl transferase (HPRT) was used as a reference gene, sense GTCCATTCCTATGACTGTAGATTTTATCAGA; antisense AATAACTTTTATGTCCCCCGTTGACT (Applied Biosystems, Carlsbad, CA) was used has an internal standard. IL-1β, TNFα, XBP-1S, BiP and caspase 3 mRNA levels were measured trough Δ ΔCt quantification method (Livak and Schmittgen, 2001). The standard curve method was performed on SOD-2 and HO-1 data.
Samples carrying the isolated RNA (non-reversed transcribed) or water were used as a control of specificity.
2.5. Gel shift mobility assay (GSMA)
Nuclear fractions were prepared using Pierce NE-PER kit (Rockford, IL). GSMA was used to determine the extent of Nrf-2 and NF-κB activation in brain tissue nuclear fractions, with the consensus oligonucleotide sequences GTGCTGAGTCA and CACGACTCAGT, respectively, in the nuclear fractions using a protocol developed by Promega (Madison, WI). The amount of protein was determined using the Bradford assay (Bradford, 1976), and 25 µg of protein extract per each sample, incubated with 32P-labeled oligonucleotides, containing the Nrf-2 or NF-κB specific consensus sequence was loaded onto a gel. A simultaneous negative control, containing no cell extract, and competitor reactions were run. The specific competitor contained unlabeled consensus nucleotides, while the nonspecific competitor contained unlabeled SP-1 consensus oligonucleotides (CGCTTGATGAGTCAGCCGGAA). All oligonucleotide sequences were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The competitor reactions also contained 25 µg of protein per sample. X-ray films were exposed to gels and then manually developed. The intensity of each band was measured and quantified using an image analyzer (Vision Works LS, UVP, Upland, CA).
2.6. Statistical analysis
The values for gene expression markers are reported as the mean ± standard error (S.E.), (n = 6). Standardization of real-time PCR showed accuracy at around 90% for tested genes. Data represent a normal distribution. Differences between exposure conditions were tested using one-way analysis of variance (ANOVA), and Student’s t test was further applied to look for differences between groups for real-time PCR (n = 6) and GSMA (n = 3) (JMP 6.0, SAS Institute Inc., Cary, NC). Only significant differences in neurotoxic biomarkers are presented in Figs. 2–5 (all other comparisons, e.g., caspase 3, or other brain regions, accordingly, were not significant). Values were accepted as significant if p ≤ 0.05.
3. Results
3.1. PM levels and chemical speciation
The estimated average concentration of PM exposure in ambient air during the 8-week exposure period was 22 µg/m3 for PM10–2.5 and 28 µg/m3 for PM2.5, for technical reasons we were not able to determine the concentration of PM0.1. Chemical analysis revealed high levels of metals, including Mn, Fe, Al, Cu and Zn (Table 2), which are known to be involved in neurodegenerative processes. PAH concentrations in collected PM were 12.85 ng/m3 PM10 and 10 ng/m3 for PM2.5.
Table 2.
Chemical composition of PM10 and PM2.5 collected during the sampling period. Values are representative of n = 8 filters and are given as ng/m3.
| ng/m3 | PM10 | PM2.5 | ||
|---|---|---|---|---|
| Metals | Mean | SD | Mean | SD |
| Cd | 37.19 | 12.54 | 35.72 | 15.51 |
| Co | 47.20 | 22.30 | 37.07 | 22.92 |
| Cr | 311.61 | 181.30 | 50.81 | 19.55 |
| Cu | 526.07 | 164.88 | 308.50 | 84.58 |
| Fe | 3561.44 | 787.96 | 1447.67 | 871.97 |
| Mn | 607.76 | 127.25 | 331.69 | 118.06 |
| Ni | 114.63 | 33.45 | 20.90 | 23.50 |
| Pb | 628.26 | 260.37 | 247.58 | 67.76 |
| V | 202.90 | 87.40 | 148.87 | 59.34 |
| Zn | 2443.94 | 588.81 | 363.30 | 89.88 |
| Total | 8491.01 | 2992.12 | ||
| ng/m3 | PM10 | PM2.5 | ||
|---|---|---|---|---|
| PAH species | Mean | SD | Mean | SD |
| Naphtalene | 0.346 | 0.052 | 0.173 | 0.034 |
| Dimethylnaphtalenes | 0.113 | 0.010 | 0.078 | 0.024 |
| Acenaphthylene | 0.098 | 0.0003 | 0.023 | 0.008 |
| Acenaphthene | 0.0035 | 0.002 | 0.0028 | 0.001 |
| Fluorene | 0.000 | 0.000 | 0.000 | 0.00 |
| Phenanthrene | 0.241 | 0.022 | 0.154 | 0.022 |
| Anthrancene | 0.000 | 0.000 | 0.000 | 0.00 |
| Fluoranthene | 0.417 | 0.025 | 0.333 | 0.024 |
| Pyrene | 0.589 | 0.038 | 0.460 | 0.037 |
| Benzo(a)anthracene | 0.237 | 0.008 | 0.205 | 0.016 |
| Chrysene | 1.061 | 0.053 | 0.824 | 0.048 |
| Benzo(b)fluoranthene | 2.049 | 0.090 | 1.674 | 0.078 |
| Benzo(k)fluoranthene | 0.859 | 0.044 | 0.711 | 0.035 |
| Benzo(a)pyrene | 1.118 | 0.044 | 0.902 | 0.043 |
| Indenol(1,2,3-cd)pyrene | 2.255 | 0.089 | 1.729 | 0.065 |
| Dibenzo(a,h)anthracene | 0.716 | 0.124 | 0.529 | 0.096 |
| Benzo(ghi)perylene | 2.749 | 0.131 | 2.281 | 0.113 |
| Total | 12.85 | 10.0 | ||
3.2. Antioxidant response
No weight loss or deterioration of health was observed in the animals during the exposure period (data not shown).
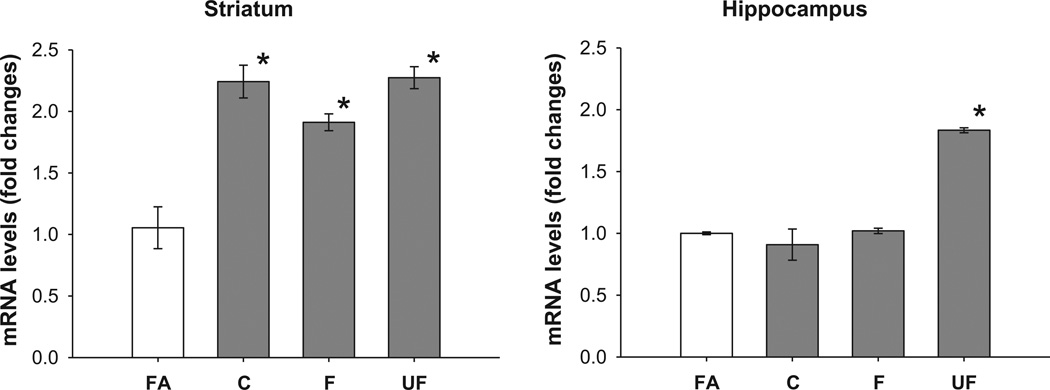
To investigate the induction of oxidative stress from the exposure to coarse, fine and ultrafine particles in different regions of the brain after eight weeks of exposure, transcript levels of a high sensitivity marker, HO-1, were evaluated. Compared with filtered air, a generalized statistically significant HO-1 increase in transcript levels throughout the brain regions was observed, regardless of the PM aerodynamic size (Fig. 1), except for the coarse fraction in the frontal cortex and hippocampus.
Fig. 1.
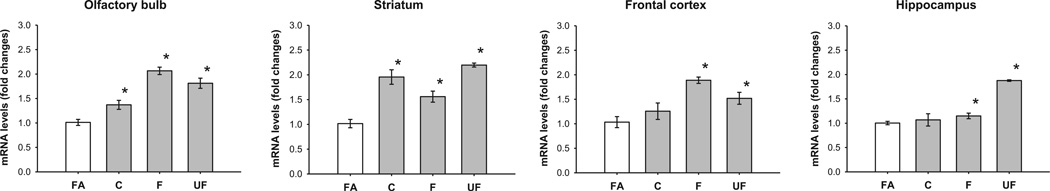
Expression of mRNA levels of HO-1 in olfactory bulb, striatum, frontal cortex and hippocampus. Data represent mean of 6 samples of mRNA fold changes ± S.E. FA, filtered air; C, coarse; F, fine; UF, ultrafine. *Value is significantly different (p ≤ 0.05) from filtered air.
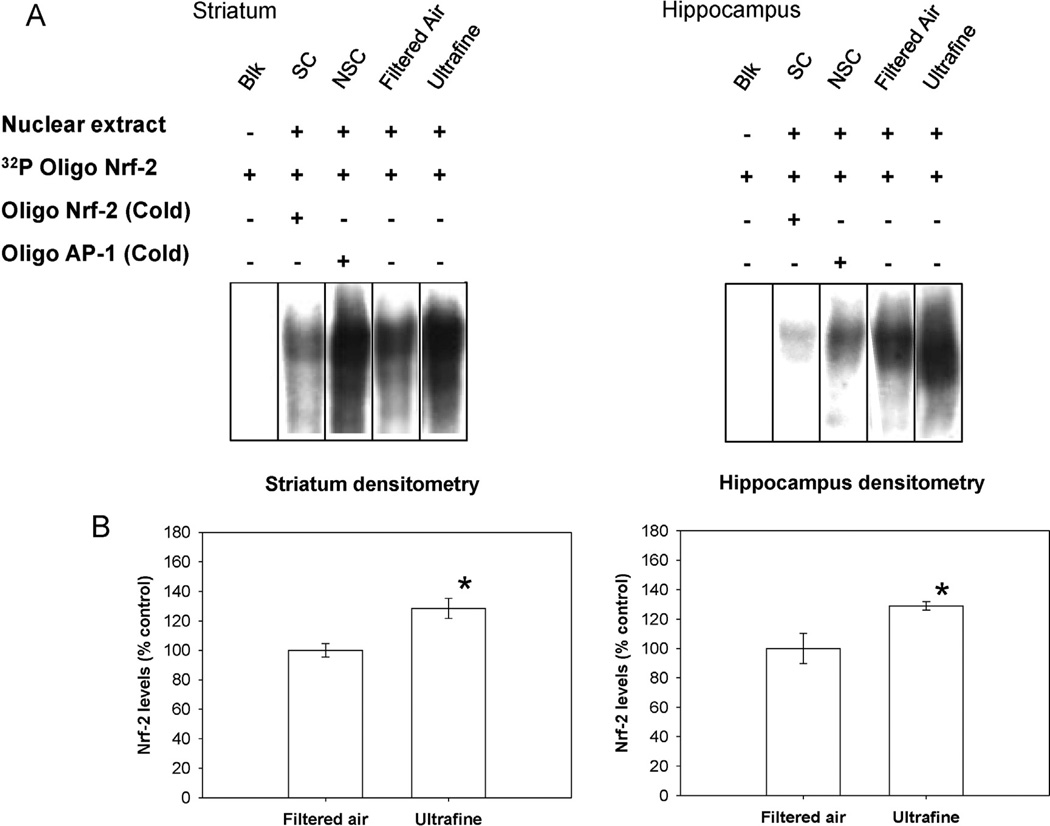
In order to evaluate the extent of the antioxidant response, we endeavored to determine the activation of Nrf-2 transcription factor as well as the transcript levels of the mitochondrial superoxide dismutase (SOD-2). The group exposed to ultrafine PM exhibited activation of Nrf-2 in striatum (p = 0.04) and hippocampus (p = 0.03) in comparison to the control group exposed to filtered air (Fig. 2). This event was accompanied by an increase of transcript levels of SOD-2 in striatum (p = 0.006); and in the hippocampus (p < 0.0001). The striatum sample also showed a significant increase in SOD-2 transcript levels for the group exposed to coarse and fine PM (p = 0.008) compared to filtered air (Fig. 3). Only significant changes are presented. They were more evident in the striatum and hippocampus when animals were exposed to ultrafine PM, suggesting susceptibility of these brain regions.
Fig. 2.
Gel shift mobility analysis of levels of activated Nrf-2 in the striatum and hippocampus. Panel A: Gel shift mobility analysis of levels of activated Nrf-2 (SC, specific competitor containing sample and unlabeled Nrf-2 consensus oligonucleotide; NSC, non-specific competitor containing sample and unlabeled SP1 consensus oligonucleotide). Integrated density of the shifted band. *Value is significantly different (p ≤ 0.05) from filtered air. Bars represent mean of 3 samples ± S.E. (B).
Fig. 3.
Expression of mRNA of SOD2 in striatum and hippocampus. Bars represent mean of 6 samples ± S.E. *Value is significantly different to filtered air.
3.3. Inflammation markers
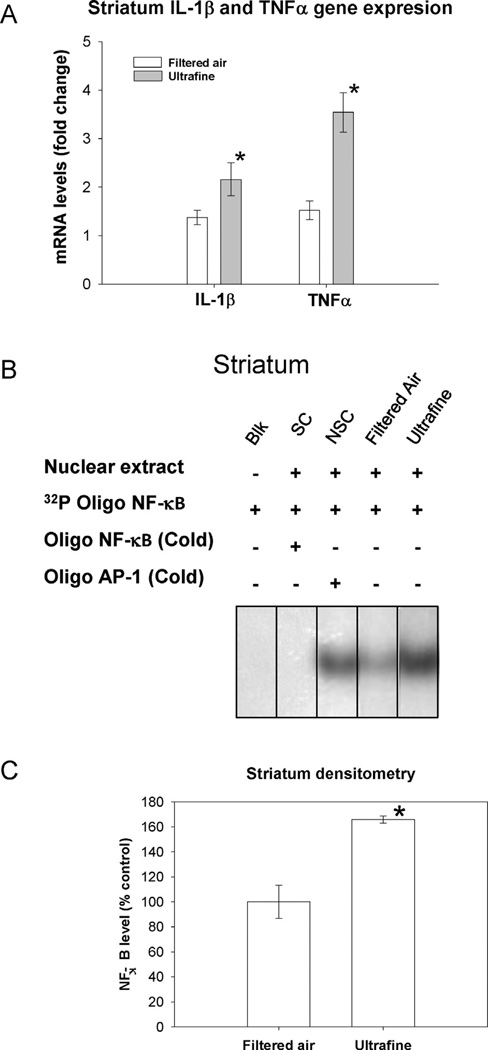
After the observed activation of the antioxidant response in striatum and hippocampus, we decided to explore markers of inflammation by determining the activation of the nuclear factor NF-κB and the transcript levels of two cytokines which trigger the inflammatory process, IL-1β and TNFα, The group exposed to ultrafine PM, showed an increase in IL-1β and TNFα transcript levels (p = 0.04 and 0.05, respectively), in the striatum, which was accompanied by an activation of NF-κB (p = 0.01) when compared to the filtered air group (Fig. 4).
Fig. 4.
Expression of mRNA of IL-1β and TNFα and gel shift mobility assay of NF-κB. Panel A: Bars represent mean of 6 samples ± S.E. Panel B: Gel shift mobility analysis of levels of activated NF-κB (SC, specific competitor containing sample and unlabelled NF-κB consensus oligonucleotide; NSC, non-specific competitor containing sample and unlabelled SP1 consensus oligonucleotide). Integrated density of the shifted band. *Value is significantly different (p ≤ 0.05) from the control. Panel C: Bars represent mean of 3 individuals ± S.E.
3.4. Unfolded protein response markers
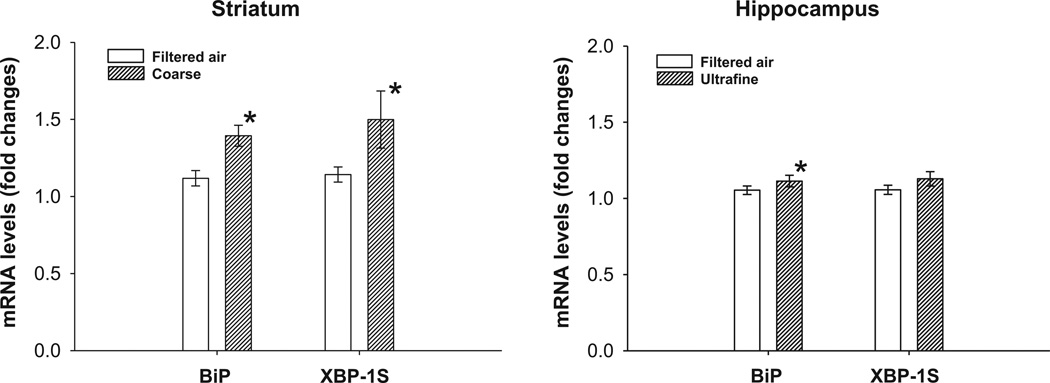
The antioxidant response and the inflammatory process are strongly associated with the presence of misfolded protein aggregation. For this reason we conducted the evaluation of two UPR markers via an assessment of nuclear factor XBP-1S and by measuring the transcript levels of the chaperone protein associated with the detection of misfolded protein accumulation BiP. In the group exposed to coarse PM, in the striatum an increase of XBP-1S levels (p = 0.03) accompanied with an increase of BiP transcript levels (p = 0.005) versus the filtered air exposed group, was observed. The hippocampus sample also showed an increase in BiP transcript levels (p = 0.04) in the group exposed to ultrafine PM versus the filtered air exposure group (Fig. 5).
Fig. 5.
RNA expression of BiP and XBP-1S. Bars represent mean of 6 individuals ± S.E. *Value is significantly different (p ≤ 0.05) from filtered air.
4. Discussion
The prevalence of high levels of PM in the air is a serious health issue, evidenced by the association of exposures to elevated concentrations of PM with increased incidences of illnesses and deaths caused by cardiac and respiratory problems (Pope, 2000). There is growing evidence showing that PM exerts deleterious effects on the central nervous system, starting with the observation of inflammation markers and β-amyloid aggregates in the brain of people who live in cities with high PM levels (Block and Calderon-Garciduenas, 2009). Moreover, the persistence of this high levels of airborne PM may be involved in the onset of neurodegenerative diseases, which are a common phenomena in large cities (Abbott, 2011). In the current study we searched for the induction of early markers of oxidative stress, inflammation and unfolded protein as a response to the exposure to coarse, fine and ultrafine concentrated particulate matter, compared to filtered, in a murine model, to define if different size fractions of PM could induce physiological changes in the central nervous system (CNS).
The main mechanism by which PM produces toxic effects has been described as a function of the generation of oxidative stress resulting from the high concentration of metals and PAH (Block and Calderon-Garciduenas, 2009). Li et al. (2003) found that PM is a powerful inducer for the expression of the antioxidant enzyme HO-1. In a later study, these same researchers showed that the induction of HO-1, in conjunction with other antioxidant enzymes, is carried out by means of activation of nuclear factor Nrf-2 through the oxidative elements present in PM (Li et al., 2004). Because of the high sensitivity to HO-1 induction resulting from the presence of PM, we decided to assess its expression in order to measure the pro-oxidative effect on the cerebral structures that are most frequently subject to neurodegenerative disease through exposure to coarse, fine and ultrafine PM. The increase in the transcription of the HO-1 in the group exposed to coarse PM that we observed suggests that some metal components, or a consequential response to their interaction, may enter the brain through this transneural transport mechanism. We propose that components adsorbed in PM, like metals and minerals, can reach the striatum and hippocampus from the olfactory bulb directly through the olfactory tract and to the frontal cortex by transneuronal transport (Tjälve and Henriksson, 1999; Elder et al., 2006) and can trigger inflammatory and oxidant responses. Studies of the exposure to specific elements, such as Mn show a higher accumulation in the olfactory bulb than other regions when administered individually (Dorman et al., 2002). Also, some elements like Mn content are historically high in PM from Mexico City. A study evaluating PM-related elemental distribution could help understand the influence of elemental distribution in brain regions and corresponding neurotoxic responses related to PM exposure. Fine and ultrafine PM, as well as cytokines produced secondarily for PM exposure, can also reach brain regions (Kleinman et al., 2008). Although not much has been reported on the effects of coarse PM in the brain, we observed that either in its entirety or its components reach brain tissues, possibly through the respiratory or circulatory systems, even though that before entering the lower respiratory system these particles are detained by physical barriers (Foster and Costa, 2005). Metals and minerals existing on the surface of PM, however, may well go into solution in the nasal cavity and thereby be transported through olfactory nerve endings. The hippocampus and frontal cortices assessed (those regions farthest from the olfactory bulb nerve endings) of the coarse-PM exposure group (see Fig. 6) did not exhibit changes in the expression of the HO-1 enzyme (see Fig. 1); however, the exposure to fine and ultrafine PM did exhibit increased transcription in all regions. Even though the induction of HO-1 is a highly sensitive biomarker of the antioxidant response, Kooter et al. (2006) observed that this response does not keep to a monotonic order, i.e., with high concentrations of PM, the expression of HO-1 tends to decrease. Because of this, the response we observed is very likely driven by a small amount of pro-oxidative elements that were sufficiently reactive to induce a proinflammatory response in the corpus striatum. The results obtained regarding the expression of HO-1 suggests to us that the aerodynamic size of the PM and the anatomic and physiological situation of the brain regions examined determines their pro-oxidative effects. This supposition can be seen more clearly in the results obtained from evaluations of other oxidative stress markers, inflammation and the UPR.
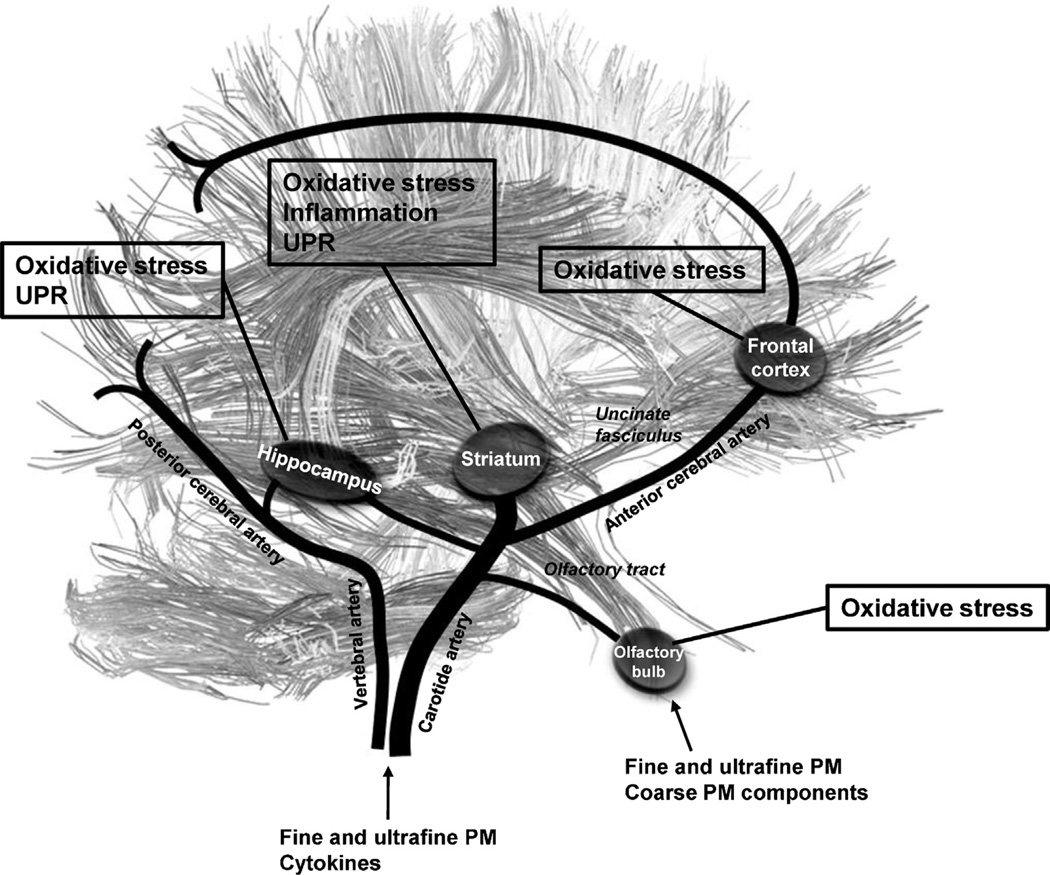
Fig. 6.
Schematic representation of the matter and blood vessels networks of brain regions evaluated. We propose that some components of PM like metals can reach the striatum and hippocampus from the olfactory bulb directly through the olfactory tract, and to the frontal cortex through the uncinate fasciculus by transneuronal transport, previously reported for nanoparticles of Mn, Al, Fe, Zn, Co, Ni. Fine and ultrafine PM, as well as cytokines produced secondarily for PM exposure, can also reach brain regions.
Previous studies have described the corpus striatum as the brain structure most susceptible to oxidative effects of PM (Block et al., 2004). In addition to having a high metabolic rate as it regulates the body’s movement, it also receives a more generous supply of blood than other brain regions. Moreover, the corpus striatum has direct links to nerves belonging to the limbic system coming from the olfactory bulb (Haines, 2012): as such, some components can be carried there easily by trans-neural means or in the blood flow (Fig. 6). In vitro studies (Block et al., 2004) and models using previously sensitized murine models (Campbell et al., 2005; Gerlofs-Nijland et al., 2010; Kleinman et al., 2008) have observed the ultrafine PM in ambient air and in diesel emissions produces oxidative stress and inflammation in this brain structure. We observed for the first time that the brains of non-sensitized rats subchronically exposed to ultrafine particles respond to oxidative stress through the activation of nuclear factor Nrf-2 accompanied by an increase in the HO-1 and SOD2 anti-oxidative enzymes transcripts. Moreover, we observed the presence of a pro-inflammatory response through the activation of nuclear factor NF-κB in conjunction with an increase in IL-1β and TNFαcytokine transcripts. Similarly, the hippocampus, a brain region providing spatial orientation and learning memory, exhibits a high metabolic rate. As such and in view of its particular anatomic situation (Fig. 6), we can see that it receives a relatively high blood supply and, like the corpus striatum, it has nerve endings from limbic system nerves extending from the olfactory bulb (Haines, 2012). These factors suggest these regions, striatum and hippocampus, as to be susceptible to the effects of PM-induced oxidative stress and inflammation, possibly due to their neuroanatomical position.
Furthermore, after eight weeks of exposure to ultrafine PM, the group exhibited an anti-oxidant response characterized by activation of nuclear factor Nrf-2, in conjunction with increased transduction of antioxidant enzymes HO-1 and SOD-2. This suggests that the oxidative effect may have been arrested through this means, thereby preventing alterations in the inflammatory markers. The antioxidant response, however, came in conjunction with a modest increase in the level of BiP chaperone protein transcription that is involved in the process of protein unfolding and the detection of accumulated defective proteins. The accumulation of unfolded proteins in this brain region is associated with the physical pathology of Alzheimer’s disease, characterized by the accumulation of β-amyloid tissue plaques (Ferrer, 2009).
The coarse PM exposure group exhibited an increase in HO-1 and SOD-2 transcripts in the absence of Nrf-2 activation; however, we observed the presence of UPR through the activation of nuclear factor XBP-1 (by means of the increase in its edited form, i.e., XBP-1S) in conjunction with increased expression of the BiP chaperone protein. It has been observed that by means of the IRE1α, which activates nuclear factor XBP-1S, it is also involved in activation of the anti-oxidant response (Schroder and Kaufman, 2005). The activation of UPR in the corpus striatum of the coarse PM exposure group could be enough to counteract the oxidant damage caused by chemical elements in PM; however, the ultrafine PM exposure group, as has been reported earlier, exhibits greater reactivity than others because of the small size of PM and its high content of PAH. Consequently, the antioxidant and pro-inflammatory responses were most prevalent. The accumulation of unfolded proteins in this region of the brain is associated with the physical pathology of Parkinson’s disease, a condition characterized by the presence of Lewy bodies that consist of cytoplasmic deposits of presynaptic protein α-sinuclein (Bandopadhyay and de Belleroche, 2010).
In addition, our findings show that coarse PM exposure can also alter gene expression in the central nervous system in a fashion similar to that seen in fine and ultrafine PM exposure. We are also reporting for the first time that the unfolded protein response is activated by exposure to PM. The brain region most susceptible to the deleterious effect of PM is the corpus striatum. The effects observed from the exposure to coarse PM involved the activation of the unfolded protein response, and could possibly be followed by a homeostatic effect through modulation of the inflammatory response. Further research into whether the stress on the endoplasmic reticulum might be a factor that contributes to the aggregation of proteins in the brain is needed. On the other hand, the ultrafine PM exposure group exhibited activation of the inflammatory response without activation of the unfolded protein response. This suggests an unfavorable prognosis for that region of the brain.
Moreover, activation of biomarkers related to oxidative stress and inflammation was mainly observed in the striatum and hippocampus, where learning, motor control, planning, decision-making and several other cognitive processes are controlled (Johnson et al., 2007). Alterations in these regions have been associated with progressive brain disorders like, Parkinson’s, Alzheimer’s and Huntington’s disease. However, in order to assess the significance of our observations in the disease process of those conditions, additional experimentation targeting specific neurobehavioral tests in exposed animals still are required need to be carried out to evaluate the impact of PM exposures on brain function.
Certain limitations were encountered in this study, sample quantities for each brain region studied were only sufficient for molecular analysis, yet future studies to also assess histological changes are underway, to evaluate the translocation of PM or its components into the brain regions, as well as the presence of protein aggregates, such as α-sinuclein y β-amyloid. Since the striatum was the brain region (out of four regions studied) that showed more positive markers of toxicity, we can possibly suggest that the striatum is a susceptible region to be affected after PM exposure.
The central nervous system is a target of the exposure to PM, affecting several regions and contributing to neurotoxic responses. The exposure to different size fractions of PM activated inflammation, oxidative stress and UPR signals in the brain, specifically within the striatum; thus persistent exposure to airborne particulate matter may contribute to the progression of neurological disorders associated with inflammation and oxidative stress.
HIGHLIGHTS.
Ultrafine PM induce HO-1 and SOD-2 expression and Nrf-2 activation in CNS.
Ultrafine PM induce activation of NF-κB and increase of IL-1β and TNFα in striatum.
Presence of UPR increase of XBP-1S and BiP in striatum after exposure to coarse PM.
Acknowledgements
This work was supported by Conacyt Grant 57752 and NIH Fogarty Grant 5D43TW000623. The authors wish to acknowledge the support of CENICA-INE and SIMAT-SMA-GDF for gravimetrical analysis.
Abbreviations
- ATF6
activating transcription factor 6
- BiP
heat shock 70 kDa protein 5 (glucose-regulated protein, 78 kDa)
- GSMA
gel shift mobility assay
- HO-1
heme oxygenase 1
- IL-1β
interleukin 1 beta
- IRE1α
inositol-requiring protein 1
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- Nrf-2
nuclear factor (erythroid-derived 2)-like 2
- PAHs
polycyclic aromatic hydrocarbons
- PERK
eukaryotic translation initiation factor 2-alpha kinase 3
- PM
particulate matter
- SOD
superoxide dismutase
- TNFα
tumor necrosis factor-alpha
- UPR
unfolded protein response
- VACES
versatile aerosol concentrator system
- XBP-1
X-box binding protein 1
- XBP-1S
XBP-1 spliced form
- XBP-1U
XBP-1 unspliced form.
Footnotes
Conflict of interest
None declared.
References
- Abbott A. Dementia: a problem for our age. Nature. 2011;475:S2–S4. doi: 10.1038/475S2a. [DOI] [PubMed] [Google Scholar]
- Araujo SA. Particulate air pollution, systemic oxidative stress, inflammation, and atherosclerosis. Air Quality, Atmosphere and Health. 2011;4:79–93. doi: 10.1007/s11869-010-0101-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandopadhyay R, de Belleroche J. Pathogenesis of Parkinson’s disease: emerging role of molecular chaperones. Trends in Molecular Medicine. 2010;16:27–36. doi: 10.1016/j.molmed.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Block ML, Calderon-Garciduenas L. Air pollution: mechanisms of neuroinflammation and CNS disease. Trends in Neuroscience. 2009;32:506–516. doi: 10.1016/j.tins.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block ML, Wu X, Pei Z, Li G, Wang T, Qin L, Wilson B, Yang J, Hong JS, Veronesi B. Nanometer size diesel exhaust particles are selectively toxic to dopaminergic neurons: the role of microglia, phagocytosis, and NADPH oxidase. FASEB Journal. 2004;18:1618–1620. doi: 10.1096/fj.04-1945fje. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Reed W, Maronpot RR, Henriquez-Roldan C, Delgado-Chavez R, Calderon-Garciduenas A, Dragustinovis I, Franco-Lira M, Aragon-Flores M, Solt AC, Altenburg M, Torres-Jardon R, Swenberg JA. Brain inflammation and Alzheimer’s-like pathology in individuals exposed to severe air pollution. Toxicologic Pathology. 2004;32:650–658. doi: 10.1080/01926230490520232. [DOI] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Solt AC, Henriquez-Roldan C, Torres-Jardon R, Nuse B, Herritt L, Villarreal-Calderon R, Osnaya N, Stone I, Garcia R, Brooks DM, Gonzalez-Maciel A, Reynoso-Robles R, Delgado-Chavez R, Reed W. Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood–brain barrier, ultrafine particulate deposition, and accumulation of amyloid beta-42 and alpha-synuclein in children and young adults. Toxicologic Pathology. 2008;36:289–310. doi: 10.1177/0192623307313011. [DOI] [PubMed] [Google Scholar]
- Campbell A, Oldham M, Becaria A, Bondy SC, Meacher D, Sioutas C, Misra C, Mendez LB, Kleinman M. Particulate matter in polluted air may increase biomarkers of inflammation in mouse brain. Neurotoxicology. 2005;26:133–140. doi: 10.1016/j.neuro.2004.08.003. [DOI] [PubMed] [Google Scholar]
- De Vizcaya-Ruiz A, Gutiérrez-Castillo ME, Uribe-Ramirez M, Cebrián ME, Mugica-Alvarez V, Sepúlveda J, Rosas I, Salinas E, Garcia-Cuéllar C, Martínez F, Alfaro-Moreno E, Torres-Flores V, Osornio-Vargas A, Sioutas C, Fine PM, Singh M, Geller MD, Kuhn T, Miguel AH, Eiguren-Fernandez A, Schiestl RH, Reliene R, Froines J. Characterization and in vitro biological effects of concentrated particulate matter from Mexico city. Atmospheric Environment. 2006;40:583–592. [Google Scholar]
- Dorman DC, Struve MF, Wong BA. Brain manganese concentrations in rats following manganese tetroxide inhalation are unaffected by dietary manganese intake. Neurotoxicology. 2002;23:185–195. doi: 10.1016/s0161-813x(01)00075-4. [DOI] [PubMed] [Google Scholar]
- Dosunmu R, Wu J, Basha MR, Zawia NH. Environmental and dietary risk factors in Alzheimer’s disease. Expert Review of Neurotherapeutics. 2007;7:887–900. doi: 10.1586/14737175.7.7.887. [DOI] [PubMed] [Google Scholar]
- Elder A, Gelein R, Silva V, Feikert T, Opanashuk L, Carter J, Potter R, Maynard A, Ito Y, Finkelstein J, Oberdörster G. Translocation of inhaled ultrafine manganese oxide particles to the central nervous system. Environmental Health Perspectives. 2006;114:1172–1178. doi: 10.1289/ehp.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I. Altered mitochondria, energy metabolism, voltage-dependent anion channel, and lipid rafts converge to exhaust neurons in Alzheimer’s disease. Journal of Bioenergetics and Biomembranes. 2009;41:425–431. doi: 10.1007/s10863-009-9243-5. [DOI] [PubMed] [Google Scholar]
- Foster WM, Costa DL. Air Pollutants and the Respiratory Tract. Boca Raton, FL: Taylor & Francis; 2005. [Google Scholar]
- Gerlofs-Nijland ME, van Berlo D, Cassee FR, Schins RP, Wang K, Campbell A. Effect of prolonged exposure to diesel engine exhaust on proinflammatory markers in different regions of the rat brain. Particle and Fibre Toxicology. 2010;7:12. doi: 10.1186/1743-8977-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh N, Ghosh R, Mandal SC. Antioxidant protection: a promising therapeutic intervention in neurodegenerative disease. Free Radical Research. 2011;45:888–905. doi: 10.3109/10715762.2011.574290. [DOI] [PubMed] [Google Scholar]
- Haines DE. Neuroanatomy: An Atlas of Structures, Sections, and Systems. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2012. p. xi.p. 332. [Google Scholar]
- Johnson A, van der Meer MAA, Redish AD. Integrating hippocampus and striatum in decision-making. Current Opinion in Neurobiology. 2007;17:692–697. doi: 10.1016/j.conb.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsouyanni K, Touloumi G, Spix C, Schwartz J, Balducci F, Medina S, Rossi G, Wojtyniak B, Sunyer J, Bacharova L, Schouten JP, Ponka A, Anderson HR. Short-term effects of ambient sulphur dioxide and particulate matter on mortality in 12 European cities: results from time series data from the APHEA project. Air Pollution and Health: a European Approach. British Medical Journal. 1997;314:1658–1663. doi: 10.1136/bmj.314.7095.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman RJ. Orchestrating the unfolded protein response in health and disease. Journal of Clinical Investigation. 2002;110:1389–1398. doi: 10.1172/JCI16886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Jaques PA, Chang M, Barone T, Xiong C, Friedlander SK, Sioutas C. Versatile aerosol concentration enrichment system (VACES) for simultaneous in vivo and in vitro evaluation of toxic effects of ultrafine, fine and coarse ambient particles: part II. Field evaluation. Journal of Aerosol Science. 2001a;32:1299–1314. [Google Scholar]
- Kim S, Jaques PA, Chang M, Froines JR, Sioutas C. Versatile aerosol concentration enrichment system (VACES) for simultaneous in vivo and in vitro evaluation of toxic effects of ultrafine, fine and coarse ambient particles: part I. Development and laboratory characterization. Journal of Aerosol Science. 2001b;32:1281–1297. [Google Scholar]
- Kleinman MT, Araujo JA, Nel A, Sioutas C, Campbell A, Cong PQ, Li H, Bondy SC. Inhaled ultrafine particulate matter affects CNS inflammatory processes and may act via MAP kinase signaling pathways. Toxicology Letters. 2008;178:127–130. doi: 10.1016/j.toxlet.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman MT, Hamade A, Meacher D, Oldham M, Sioutas C, Chakrabarti B, Stram D, Froines JR, Cho AK. Inhalation of concentrated ambient particulate matter near a heavily trafficked road stimulates antigen-induced airway responses in mice. Journal of the Air & Waste Management Association. 2005;55:1277–1288. doi: 10.1080/10473289.2005.10464727. [DOI] [PubMed] [Google Scholar]
- Kooter IM, Boere AJ, Fokkens PH, Leseman DL, Dormans JA, Cassee FR. Response of spontaneously hypertensive rats to inhalation of fine and ultrafine particles from traffic: experimental controlled study. Particle and Fibre Toxicology. 2006;3:7. doi: 10.1186/1743-8977-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrigan PJ, Sonawane B, Butler RN, Trasande L, Callan R, Droller D. Early environmental origins of neurodegenerative disease in later life. Environmental Health Perspectives. 2005;113:1230–1233. doi: 10.1289/ehp.7571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Sioutas C, Cho A, Schmitz D, Misra C, Sempf J, Wang M, Oberley T, Froines J, Nel A. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environmental Health Perspectives. 2003;111:455–460. doi: 10.1289/ehp.6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Alam J, Venkatesan MI, Eiguren-Fernandez A, Schmitz D, Di Stefano E, Slaughter N, Killeen E, Wang X, Huang A, Wang M, Miguel AH, Cho A, Sioutas C, Nel AE. Nrf2 is a key transcription factor that regulates antioxidant defense in macrophages and epithelial cells: protecting against the proinflammatory and oxidizing effects of diesel exhaust chemicals. Journal of Immunology. 2004;173:3467–3481. doi: 10.4049/jimmunol.173.5.3467. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods (San Diego, CA) 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Malhotra JD, Kaufman RJ. The endoplasmic reticulum and the unfolded protein response. Seminars in Cell & Developmental Biology. 2007;18:716–731. doi: 10.1016/j.semcdb.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez A, Portero-Otin M, Pamplona R, Ferrer I. Protein targets of oxidative damage in human neurodegenerative diseases with abnormal protein aggregates. Brain Pathology. 2010;20:281–297. doi: 10.1111/j.1750-3639.2009.00326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mautz WJ, Kleinman MT. Animal quality and protocol approval. In: Phalen RF, editor. Methods in Inhalation Toxicology. Boca Raton, FL: CRC Press; 1997. p. 1616. [Google Scholar]
- Mazzoli-Rocha F, Fernandes S, Einicker-Lamas M, Araújo Zin W. Roles of oxidative stress in signaling and inflammation induced by particulate matter. Cell Biology and Toxicology. 2010;26:481–498. doi: 10.1007/s10565-010-9158-2. [DOI] [PubMed] [Google Scholar]
- Migliore L, Coppedè F. Environmental-induced oxidative stress in neurodegenerative disorders and aging. Mutation Research. 2009;674:73–84. doi: 10.1016/j.mrgentox.2008.09.013. [DOI] [PubMed] [Google Scholar]
- Mugica V, Ortiz E, Molina L, De Vizcaya-Ruiz A, Nebot A, Quintana R, Aguilar J, Alcántara E. PM composition and source reconciliation in Mexico city. Atmospheric Environment. 2009;43:5068–5074. [Google Scholar]
- Oberdorster G, Sharp Z, Atudorei V, Elder A, Gelein R, Lunts A, Kreyling W, Cox C. Extrapulmonary translocation of ultrafine carbon particles following whole-body inhalation exposure of rats. Journal of Toxicology and Environmental Health, Part A. 2002;65:1531–1543. doi: 10.1080/00984100290071658. [DOI] [PubMed] [Google Scholar]
- Oberdorster G, Sharp Z, Atudorei V, Elder A, Gelein R, Kreyling W, Cox C. Translocation of inhaled ultrafine particles to the brain. Inhalation Toxicology. 2004;16:437–445. doi: 10.1080/08958370490439597. [DOI] [PubMed] [Google Scholar]
- Pope CA., 3rd Epidemiology of fine particulate air pollution and human health: biologic mechanisms and who’s at risk? Environmental Health Perspectives. 2000;108(Suppl. 4):713–723. doi: 10.1289/ehp.108-1637679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope CA, 3rd, Dockery DW. Health effects of fine particulate air pollution: lines that connect. Journal of the Air & Waste Management Association. 2006;56:709–742. doi: 10.1080/10473289.2006.10464485. [DOI] [PubMed] [Google Scholar]
- Ranft U, Schikowski T, Sugiri D, Krutmann J, Krämer U. Long-term exposure to traffic-related particulate matter impairs cognitive function in the elderly. Environmental Research. 2009;109:1004–1011. doi: 10.1016/j.envres.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nature Reviews Molecular Cell Biology. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annual Review of Biochemistry. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- Tjälve H, Henriksson J. Uptake of metals in the brain via olfactory pathways. Neurotoxicology. 1999;20:181–195. [PubMed] [Google Scholar]
- USEPA. Compendium Method TO-13A. United States Environmental Protection Agency; 1999. Compendium of Methods for the determination of toxic organic compounds in ambient air. EPA-625/R-96-010b. [Google Scholar]
- Valko M, Morris H, Cronin MT. Metals, toxicity and oxidative stress. Current Medicinal Chemistry. 2005;12:1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- Valle-Hernández BL, Mugica-Álvarez V, Salinas-Talavera E, Amador-Muñoz O, Murillo-Tovar MA, Villalobos-Pietrini R, De Vizcaya-Ruiz A. Temporal variation of nitro-polycyclic aromatic hydrocarbons in PM10 and PM2.5 collected in northern Mexico city. Science of the Total Environment. 2010;408:5429–5438. doi: 10.1016/j.scitotenv.2010.07.065. [DOI] [PubMed] [Google Scholar]
- Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454:455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]