Abstract
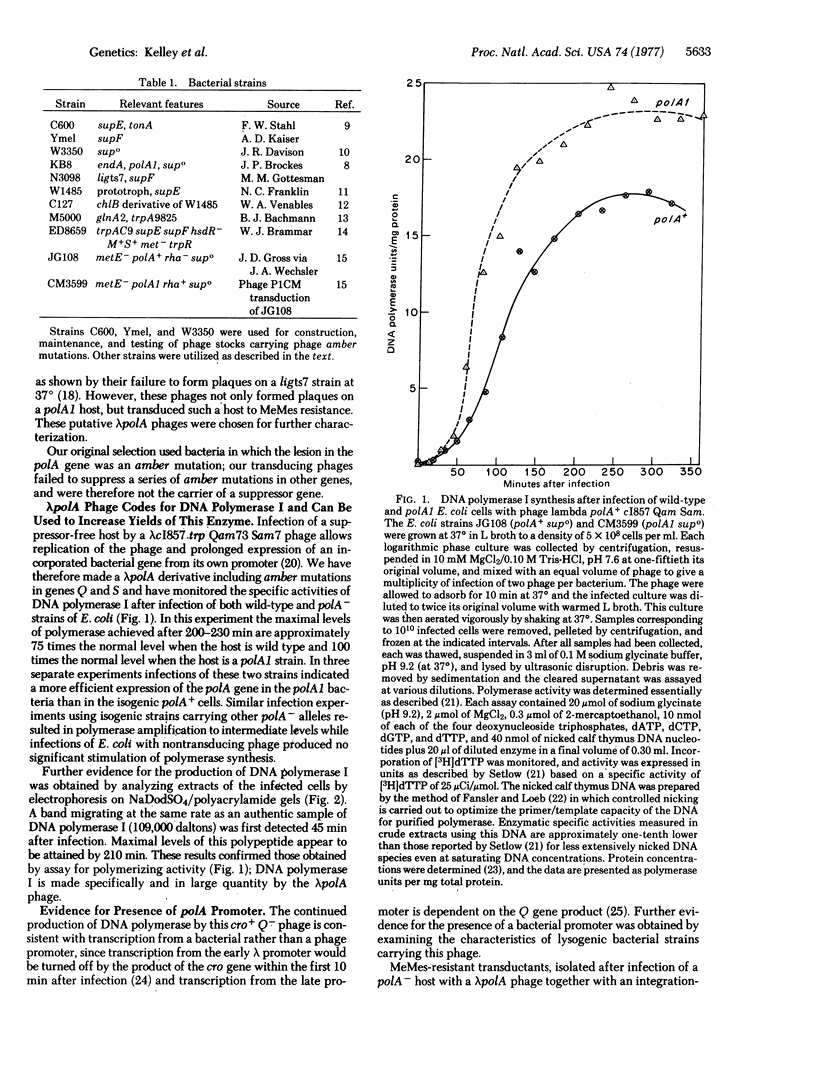
A plaque-forming lambdapolA phage was isolated from a population of transducing phage made in vitro from Escherichia coli DNA and a phage vector digested with restriction endonuclease HindIII. Amber mutations, in genes whose products are necessary for late protein synthesis (Q) and cell lysis (S), were crossed into the lambdapolA phage. Infection of either polA+ or polA- bacteria with this phage, under conditions permitting DNA replication but preventing phage production and lysis, elevated the levels of DNA polymerase I to between 75- and 100-fold that detected in a wild-type strain. The kinetics of enzyme production suggest that the polA gene is transcribed from its own promoter rather than from any of the well-characterized phage promoters. The fragment of E. coli DNA within the lambdapolA phage comprises approximately 5000 base pairs, sufficient to accommodate the polA gene and one, or two, coding sequences for smaller proteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleyard R K. Segregation of New Lysogenic Types during Growth of a Doubly Lysogenic Strain Derived from Escherichia Coli K12. Genetics. 1954 Jul;39(4):440–452. doi: 10.1093/genetics/39.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borck K., Beggs J. D., Brammar W. J., Hopkins A. S., Murray N. E. The construction in vitro of transducing derivatives of phage lambda. Mol Gen Genet. 1976 Jul 23;146(2):199–207. doi: 10.1007/BF00268089. [DOI] [PubMed] [Google Scholar]
- CAMPBELL A. Sensitive mutants of bacteriophage lambda. Virology. 1961 May;14:22–32. doi: 10.1016/0042-6822(61)90128-3. [DOI] [PubMed] [Google Scholar]
- Cameron J. R., Davis R. W. The effects of Escherichia coli and yeast DNA insertions on the growth of lambda bacteriophage. Science. 1977 Apr 8;196(4286):212–215. doi: 10.1126/science.322285. [DOI] [PubMed] [Google Scholar]
- Couturier M., Dambly C., Thomas R. Control of development in temperate bacteriophages. V. Sequential activation of the viral functions. Mol Gen Genet. 1973 Feb 2;120(3):231–252. doi: 10.1007/BF00267155. [DOI] [PubMed] [Google Scholar]
- Grindley N. D., Kelley W. S. Effects of different alleles of the E. coli K12 pol A gene on the replication of non-transferring plasmids. Mol Gen Genet. 1976 Feb 2;143(3):311–318. doi: 10.1007/BF00269409. [DOI] [PubMed] [Google Scholar]
- Gross J., Gross M. Genetic analysis of an E. coli strain with a mutation affecting DNA polymerase. Nature. 1969 Dec 20;224(5225):1166–1168. doi: 10.1038/2241166a0. [DOI] [PubMed] [Google Scholar]
- Hopkins A. S., Murray N. E., Brammar W. J. Characterization of lambdatrp-transducing bacteriophages made in vitro. J Mol Biol. 1976 Nov 15;107(4):549–569. doi: 10.1016/s0022-2836(76)80082-4. [DOI] [PubMed] [Google Scholar]
- Jovin T. M., Englund P. T., Bertsch L. L. Enzymatic synthesis of deoxyribonucleic acid. XXVI. Physical and chemical studies of a homogeneous deoxyribonucleic acid polymerase. J Biol Chem. 1969 Jun 10;244(11):2996–3008. [PubMed] [Google Scholar]
- Kelley W. S., Grindley N. D. Mapping of the polA locus of Escherichia coli K12: orientation in the amino- and carboxy-termini of the cistron. Mol Gen Genet. 1976 Sep 23;147(3):307–314. doi: 10.1007/BF00582882. [DOI] [PubMed] [Google Scholar]
- Kelly W. S., Grindley N. D. polA6, A mutation affecting the DNA binding capacity of DNA polymerase I. Nucleic Acids Res. 1976 Nov;3(11):2971–2984. doi: 10.1093/nar/3.11.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lehman I. R., Uyemura D. G. DNA polymerase I: essential replication enzyme. Science. 1976 Sep 10;193(4257):963–969. doi: 10.1126/science.781842. [DOI] [PubMed] [Google Scholar]
- Mayer E. P., Smith O. H., Fredricks W. W., McKinney M. A. The isolation and characterization of glutamine-requiring strains of Escherichia coli K12. Mol Gen Genet. 1975;137(2):131–142. doi: 10.1007/BF00341679. [DOI] [PubMed] [Google Scholar]
- Moir A., Brammar W. J. The use of specialised transducing phages in the amplification of enzyme production. Mol Gen Genet. 1976 Nov 24;149(1):87–99. doi: 10.1007/BF00275963. [DOI] [PubMed] [Google Scholar]
- Murray K., Murray N. E. Phage lambda receptor chromosomes for DNA fragments made with restriction endonuclease III of Haemophilus influenzae and restriction endonuclease I of Escherichia coli. J Mol Biol. 1975 Nov 5;98(3):551–564. doi: 10.1016/s0022-2836(75)80086-6. [DOI] [PubMed] [Google Scholar]
- Murray N. E., Brammar W. J., Murray K. Lambdoid phages that simplify the recovery of in vitro recombinants. Mol Gen Genet. 1977 Jan 7;150(1):53–61. doi: 10.1007/BF02425325. [DOI] [PubMed] [Google Scholar]
- Murray N. E., De Ritis P. M., Foster L. A. DNA targets for the Escherichia coli K restriction system analysed genetically in recombinants between phages phi80 and lambda. Mol Gen Genet. 1973 Feb 2;120(3):261–281. doi: 10.1007/BF00267157. [DOI] [PubMed] [Google Scholar]
- Panasenko S. M., Cameron J. R., Davis R. W., Lehman I. R. Five hundredfold overproduction of DNA ligase after induction of a hybrid lambda lysogen constructed in vitro. Science. 1977 Apr 8;196(4286):188–189. doi: 10.1126/science.322281. [DOI] [PubMed] [Google Scholar]
- Sato K., Matsushiro A. The tryptophan operon regulated by phage immunity. J Mol Biol. 1965 Dec;14(2):608–610. doi: 10.1016/s0022-2836(65)80212-1. [DOI] [PubMed] [Google Scholar]
- Schrenk W. J., Weisberg R. A. A simple method for making new transducing lines of coliphage lambda. Mol Gen Genet. 1975;137(2):101–107. doi: 10.1007/BF00341676. [DOI] [PubMed] [Google Scholar]
- Thomas M., Davis R. W. Studies on the cleavage of bacteriophage lambda DNA with EcoRI Restriction endonuclease. J Mol Biol. 1975 Jan 25;91(3):315–328. doi: 10.1016/0022-2836(75)90383-6. [DOI] [PubMed] [Google Scholar]
- Venables W. A., Guest J. R. Transduction of nitrate reductase loci of Escherichia coli by phages P-1 and lambda. Mol Gen Genet. 1968;103(2):127–140. doi: 10.1007/BF00427140. [DOI] [PubMed] [Google Scholar]




