Abstract
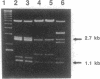
Dolichol in the form of dolichyl phosphate participates in the synthesis of N- and O-linked glycoproteins and phosphatidylinositol-linked proteins in the yeast Saccharomyces cerevisiae. In this organism, as well as in higher eukaryotes, a number of the enzymes in the polyisoprenoid and glycoprotein biosynthetic pathways have not been identified. In this study, we have developed a convenient, highly sensitive assay that uses one of the end products of the dolichylphosphate synthetic pathway, oligosaccharide-diphosphodolichol, and a 125I-labeled peptide substrate for N-linked glycosylation to screen a collection of temperature-sensitive yeast mutants for defects in protein glycosylation. By using a combination of biochemical and genetic procedures, the defective mutants were grouped into three categories: those containing defects in dolichyl-phosphate synthesis (class 1), lipid-linked oligosaccharide assembly (class 2), or oligosaccharide transferase activity (class 3). Among the mutants identified by this screen were sec59 (which encodes dolichol kinase) and a mutant that affects the activity of the ALG1-encoded mannosyltransferase that forms dolichol-PP-(GlcNAc)2Man1. Of particular interest was a mutant that exhibits a temperature-sensitive defect in oligosaccharide transferase activity. This mutant, meg1 (microsomal protein essential for glycosylation 1) assembles a complete oligosaccharide chain and, therefore, is likely to be a class 3 mutant. We report the cloning of MEG1, the gene that rescues the oligosaccharide transferase activity defect in this mutant. A number of criteria distinguish this gene from previously described genes in this pathway.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Geetha-Habib M., Park H. R., Lennarz W. J. In vivo N-glycosylation and fate of Asn-X-Ser/Thr tripeptides. J Biol Chem. 1990 Aug 15;265(23):13655–13660. [PubMed] [Google Scholar]
- Hartwell L. H. Macromolecule synthesis in temperature-sensitive mutants of yeast. J Bacteriol. 1967 May;93(5):1662–1670. doi: 10.1128/jb.93.5.1662-1670.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J., Donald K. A., Griffiths D. E., Donald G. DMSO-enhanced whole cell yeast transformation. Nucleic Acids Res. 1991 Oct 25;19(20):5791–5791. doi: 10.1093/nar/19.20.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffaker T. C., Robbins P. W. Temperature-sensitive yeast mutants deficient in asparagine-linked glycosylation. J Biol Chem. 1982 Mar 25;257(6):3203–3210. [PubMed] [Google Scholar]
- Huffaker T. C., Robbins P. W. Yeast mutants deficient in protein glycosylation. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7466–7470. doi: 10.1073/pnas.80.24.7466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan H. A., Naider F., Lennarz W. J. Partial characterization and purification of the glycosylation site recognition component of oligosaccharyltransferase. J Biol Chem. 1988 Jun 5;263(16):7814–7820. [PubMed] [Google Scholar]
- Kelleher D. J., Kreibich G., Gilmore R. Oligosaccharyltransferase activity is associated with a protein complex composed of ribophorins I and II and a 48 kd protein. Cell. 1992 Apr 3;69(1):55–65. doi: 10.1016/0092-8674(92)90118-v. [DOI] [PubMed] [Google Scholar]
- Kukuruzinska M. A., Bergh M. L., Jackson B. J. Protein glycosylation in yeast. Annu Rev Biochem. 1987;56:915–944. doi: 10.1146/annurev.bi.56.070187.004411. [DOI] [PubMed] [Google Scholar]
- Noiva R., Freedman R. B., Lennarz W. J. Peptide binding to protein disulfide isomerase occurs at a site distinct from the active sites. J Biol Chem. 1993 Sep 15;268(26):19210–19217. [PubMed] [Google Scholar]
- Rose M. D., Broach J. R. Cloning genes by complementation in yeast. Methods Enzymol. 1991;194:195–230. doi: 10.1016/0076-6879(91)94017-7. [DOI] [PubMed] [Google Scholar]
- Rothblatt J. A., Meyer D. I. Secretion in yeast: reconstitution of the translocation and glycosylation of alpha-factor and invertase in a homologous cell-free system. Cell. 1986 Feb 28;44(4):619–628. doi: 10.1016/0092-8674(86)90271-0. [DOI] [PubMed] [Google Scholar]
- Rush J. S., Shelling J. G., Zingg N. S., Ray P. H., Waechter C. J. Mannosylphosphoryldolichol-mediated reactions in oligosaccharide-P-P-dolichol biosynthesis. Recognition of the saturated alpha-isoprene unit of the mannosyl donor by pig brain mannosyltransferases. J Biol Chem. 1993 Jun 25;268(18):13110–13117. [PubMed] [Google Scholar]
- Silberstein S., Kelleher D. J., Gilmore R. The 48-kDa subunit of the mammalian oligosaccharyltransferase complex is homologous to the essential yeast protein WBP1. J Biol Chem. 1992 Nov 25;267(33):23658–23663. [PubMed] [Google Scholar]
- Tanner W., Lehle L. Protein glycosylation in yeast. Biochim Biophys Acta. 1987 Apr 27;906(1):81–99. doi: 10.1016/0304-4157(87)90006-2. [DOI] [PubMed] [Google Scholar]
- Waechter C. J., Lennarz W. J. The role of polyprenol-linked sugars in glycoprotein synthesis. Annu Rev Biochem. 1976;45:95–112. doi: 10.1146/annurev.bi.45.070176.000523. [DOI] [PubMed] [Google Scholar]
- Welply J. K., Kaplan H. A., Shenbagamurthi P., Naider F., Lennarz W. J. Studies on properties of membrane-associated oligosaccharyltransferase using an active site-directed photoaffinity probe. Arch Biochem Biophys. 1986 May 1;246(2):808–819. doi: 10.1016/0003-9861(86)90337-1. [DOI] [PubMed] [Google Scholar]
- Welply J. K., Shenbagamurthi P., Lennarz W. J., Naider F. Substrate recognition by oligosaccharyltransferase. Studies on glycosylation of modified Asn-X-Thr/Ser tripeptides. J Biol Chem. 1983 Oct 10;258(19):11856–11863. [PubMed] [Google Scholar]
- te Heesen S., Janetzky B., Lehle L., Aebi M. The yeast WBP1 is essential for oligosaccharyl transferase activity in vivo and in vitro. EMBO J. 1992 Jun;11(6):2071–2075. doi: 10.1002/j.1460-2075.1992.tb05265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Heesen S., Knauer R., Lehle L., Aebi M. Yeast Wbp1p and Swp1p form a protein complex essential for oligosaccharyl transferase activity. EMBO J. 1993 Jan;12(1):279–284. doi: 10.1002/j.1460-2075.1993.tb05654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]