Abstract
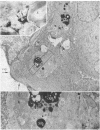
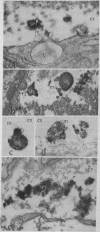
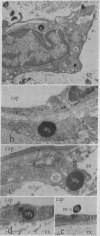
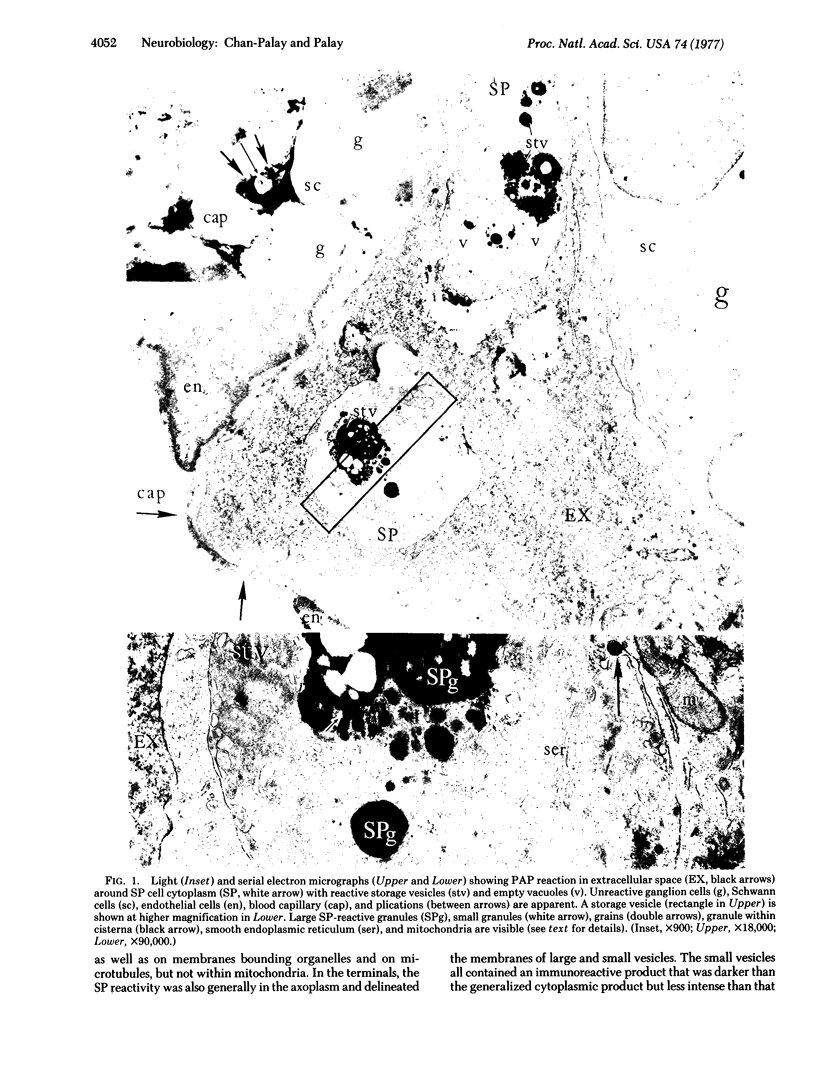
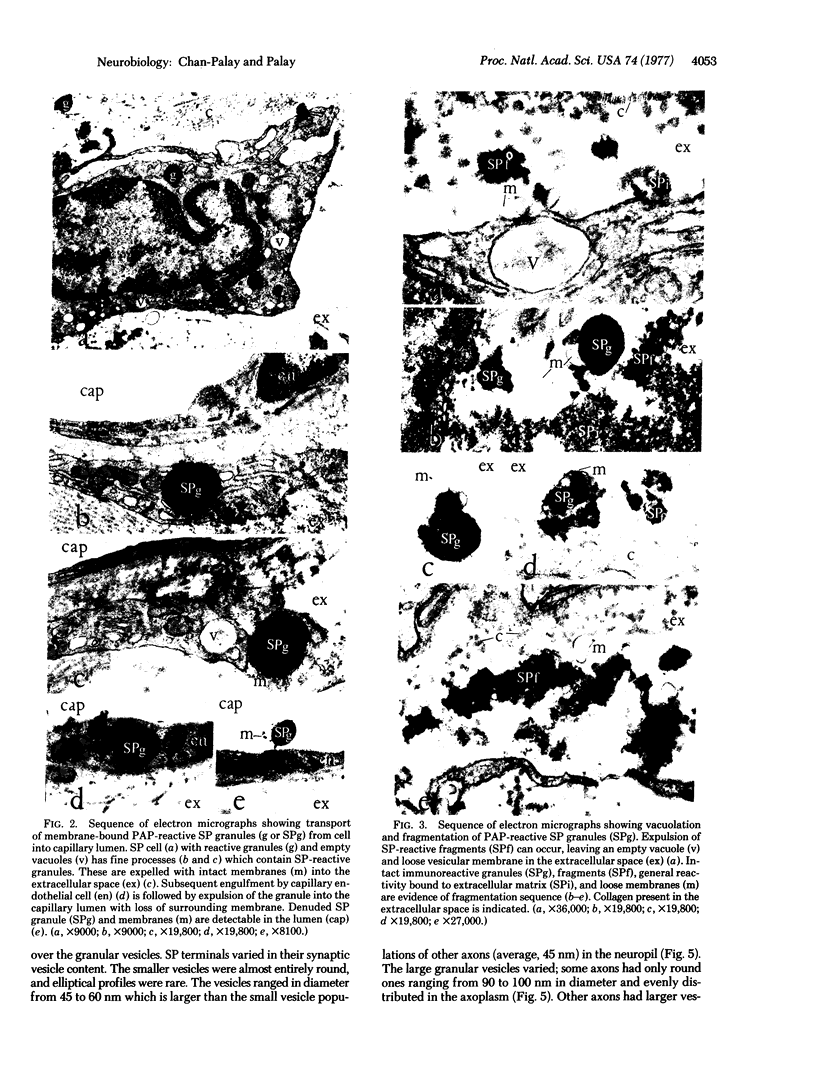
The unlabeled substance P (SP) antibody-peroxidase-antiperoxidase reaction was used on tissue prior to embedding in epoxy reins for ultrastructural identification of the SP cell and its immunoreactive granules. The SP cell is 10-20 μm in diameter and has sparse cytoplasm with numerous intensely reactive SP granules 100-300 nm across, large clear vacuoles, elaborate smooth endoplasmic reticulum, fragmentary rough endoplasmic reticulum, dispersed ribosomes, few mitochondria, and a modest Glogi apparatus. The large SP-reactive granules are discharged into the extracellular space, either with cell membrane intact or as unbound dense material. The membrane-bound dense granucles are transported intact through endothelial cells into the blood or are picked up by Schwann cells and fibroblasts. Other SP-reactive granules lose their limiting membranes, fragment, and then disperse into fine immunoreactive grains that bind to the extracellular matrix and to collagen. Dispersed SP-reactive granules are transported within myriad pinocytotic vesicles across endothelial cells with numerous luminal plications and are discharged into the blood. Pinocytosis of dispersed SP-reactive material, that can be detected intracellularly, also occurs in Schwann cells and fibroblasts. The SP axons to the substantia gleatinosa are unmyelinated or finely myelinated. Their synaptic varicosities display a generalized axoplasmic immunoreactivity, which also occurs in and around small vesicles. The larger SP synaptic vesicles are intensely reactive.
Keywords: granules, storage, release, extracellular, synaptic
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chan-Palay V., Palay S. L. Immunocytochemical identification of substance P cells and their processes in rat sensory ganglia and their terminals in the spinal cord: light microscopic studies. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3597–3601. doi: 10.1073/pnas.74.8.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy M. J., Mulhall D., Powell D. Subcellular distribution of substance P in bovine hypothalamus and substantia nigra. J Neurochem. 1975 Sep;25(3):305–307. doi: 10.1111/j.1471-4159.1975.tb06971.x. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Kellerth J. O., Nilsson G., Pernow B. Experimental immunohistochemical studies on the localization and distribution of substance P in cat primary sensory neurons. Brain Res. 1975 Dec 19;100(2):235–252. doi: 10.1016/0006-8993(75)90481-3. [DOI] [PubMed] [Google Scholar]
- KATAOKA K. The subcellular distribution of substance P in the nervous tissues. Jpn J Physiol. 1962 Feb 15;12:81–96. doi: 10.2170/jjphysiol.12.81. [DOI] [PubMed] [Google Scholar]
- Otsuka M., Konishi S. Substance P and excitatory transmitter of primary sensory neurons. Cold Spring Harb Symp Quant Biol. 1976;40:135–143. doi: 10.1101/sqb.1976.040.01.015. [DOI] [PubMed] [Google Scholar]
- Pickel V. M., Reis D. J., Leeman S. E. Ultrastructural localization of substance P in neurons of rat spinal cord. Brain Res. 1977 Feb 25;122(3):534–540. doi: 10.1016/0006-8993(77)90463-2. [DOI] [PubMed] [Google Scholar]
- RYALL R. W. THE SUBCELLULAR DISTRIBUTIONS OF ACETYLCHOLINE, SUBSTANCE P, 5-HYDROXYTRYPTAMINE, GAMMA-AMINOBUTYRIC ACID AND GLUTAMIC ACID IN BRAIN HOMOGENATES. J Neurochem. 1964 Mar;11:131–145. doi: 10.1111/j.1471-4159.1964.tb06124.x. [DOI] [PubMed] [Google Scholar]